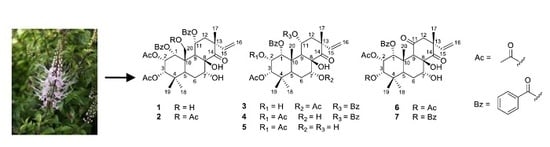
Glucose Uptake Stimulatory and PTP1B Inhibitory Activities of Pimarane Diterpenes from Orthosiphon stamineus Benth
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Isolation and Anti-Diabetes Assay Methods
2.4. Statistical Analysis
3. Results and Discussion
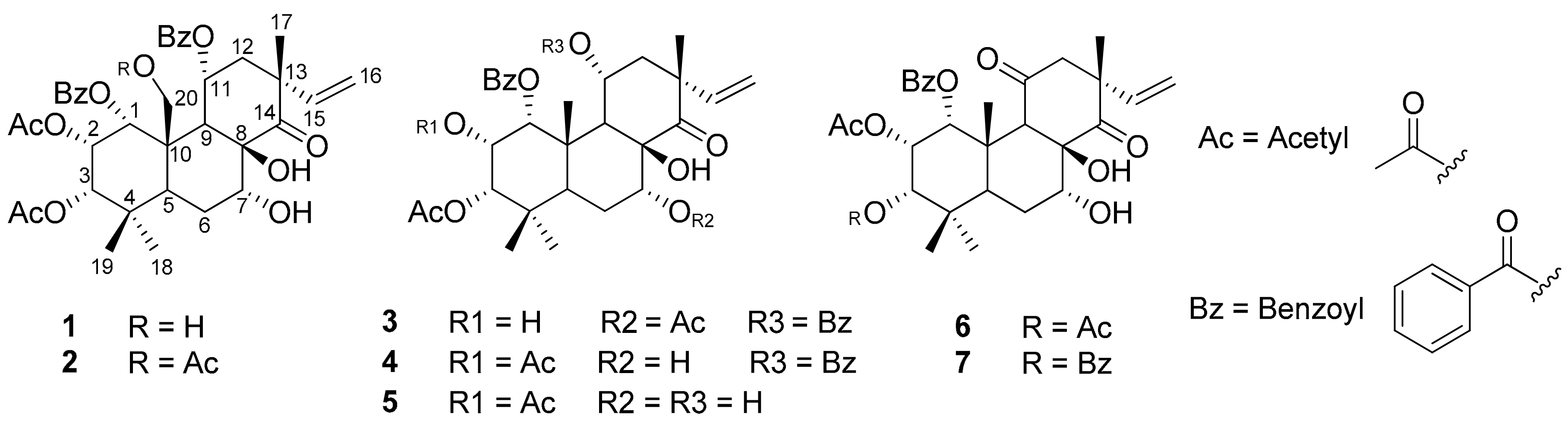
3.1. Structure Elucidation
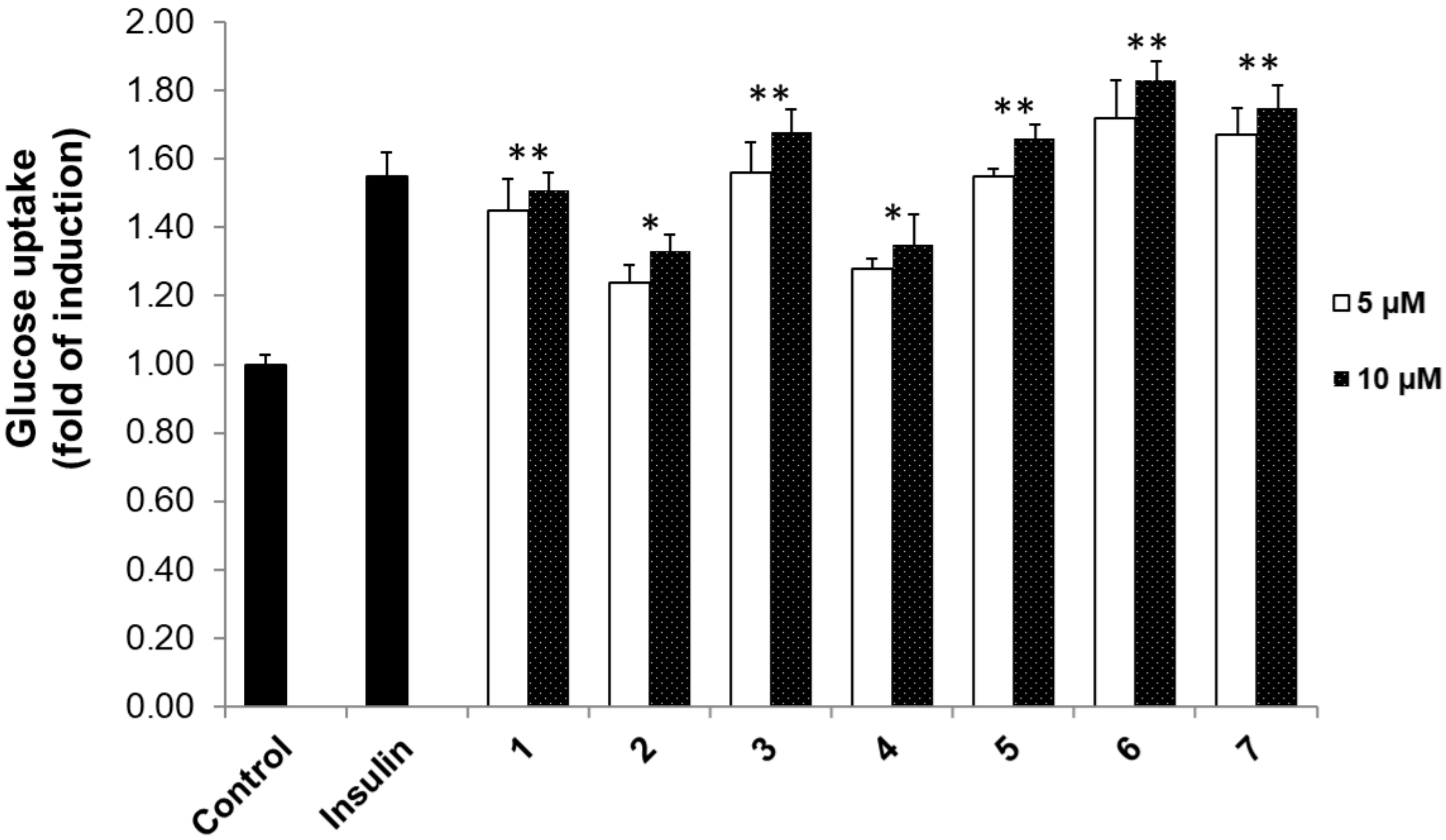
3.2. 2-NBDG Assay Results
3.3. Protein Tyrosine Phosphatase 1B Inhibition Results
3.4. Enzyme Kinetic Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Rahilly, S.; Barroso, I.; Wareham, N.J. Genetic factors in type 2 diabetes: the end of the beginning? Science 2005, 307, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Montalibet, J.; Kennedy, B.P. Therapeutic strategies for targeting PTP1B in diabetes. Drug Discov. Today Ther. Strateg. 2005, 2, 129–135. [Google Scholar] [CrossRef]
- Soulsby, M.; Bennett, A.M. Physiological signaling specificity by protein tyrosine phosphatases. Physiology (Bethesda) 2009, 24, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Panzhinskiy, E.; Ren, J.; Nair, S. Pharmacological Inhibition of Protein Tyrosine Phosphatase 1B: A Promising Strategy for the Treatment of Obesity and Type 2 Diabetes Mellitus. Curr. Med. Chem. 2013, 20, 2609–2625. [Google Scholar] [CrossRef]
- Koren, S.; Fantus, I.G. Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 621–640. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.Y. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov. Today 2007, 12, 373–381. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Zhao, B.T.; Nguyen, D.H.; Le, D.D.; Choi, J.S.; Min, B.S.; Woo, M.H. Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch. Pharm. Res. 2018, 41, 130–161. [Google Scholar] [CrossRef]
- Harlev, E.; Nevo, E.; Mirsky, N.; Ofir, R. Antidiabetic attributes of desert and steppic plants: A review. Planta Med. 2013, 79, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; Kimpe, N.D. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Insulin-mimetic selaginellins from Selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. J. Nat. Prod. 2015, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Hong, B.H.; Yu, Y.S.; Yen, G.C. Antioxidant and Anti-Inflammatory effects of Orthosiphon aristatus and its bioactive compounds. J. Agric. Food Chem. 2010, 58, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Adnyana, I.K.; Kadota, S. Highly-oxygenated isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia and their nitric oxide inhibitory activity. Chem. Pharm. Bull. 2003, 51, 268–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Regional Office for the Western Pacific. In Medicinal plants in Viet Nam; WHO Regional Office for the Western Pacific: Manila, Philippines, 1990; p. 271. [Google Scholar]
- PT Eisai Indonesia. Medicinal herb index in Indonesia, 2nd ed.; PT Eisai Indonesia: Jakarta, Indonesia, 1995. [Google Scholar]
- Hossain, M.A.; Ismail, Z.; Rahman, A.; Kang, S.C. Chemical composition and anti-fungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Ind. Crops Prod. 2008, 27, 328–334. [Google Scholar] [CrossRef]
- Ameer, O.Z.; Salman, I.M.; Asmawi, M.Z.; Ibraheem, Z.O.; Yam, M.F. Orthosiphon stamineus: Traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food 2012, 15, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Ghedira, K.; Goetz, P. Orthosiphon stamineus Benth.: Orthosiphon (Lamiaceae). Phytotherapie 2015, 13, 39–44. [Google Scholar] [CrossRef]
- Yam, M.F.; Lim, V.; Salman, I.M.; Ameer, O.Z.; Ang, L.F.; Rosidah, N.; Abdulkarim, M.F.; Abdullah, G.Z.; Basir, R.; Sadikun, A.; et al. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules 2010, 15, 4452–4466. [Google Scholar] [CrossRef]
- Yam, M.F.; Ang, L.F.; Salman, I.M.; Ameer, O.Z.; Lim, V.; Ong, L.M.; Ahmad, M.; Asmawil, M.Z.; Basir, R. Orthosiphon stamineus leaf extract protects against ethanol-induced gastropathy in rats. J. Med. Food 2009, 12, 1089–1097. [Google Scholar] [CrossRef]
- Alshawsh, M.A.; Abdulla, M.A.; Ismail, S.; Amin, Z.A. Hepatoprotective effects of Orthosiphon stamineus extract on thioacetamide-induced liver cirrhosis in rats. Evid. Based Complement. Alternat. Med. 2011, 2011. 103039. [Google Scholar] [CrossRef] [Green Version]
- Pauzi, N.; Mohd, K.S.; Halim, N.H.A.; Ismail, Z. Orthosiphon stamineus extracts inhibits proliferation and induces apoptosis in uterine fibroid cells. Asian Pacific J. Cancer Prev. 2018, 19, 2737–2744. [Google Scholar]
- Vogelgesang, B.; Abdul-Malak, N.; Reymermier, C.; Altobelli, C.; Saget, J. On the effects of a plant extract of Orthosiphon stamineus on sebum-related skin imperfections. Int. J. Cosmet. Sci. 2011, 33, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Asmawi, M.Z.; Basir, R. An investigation of the anti-inflammatory and analgesic effects of Orthosiphon stamineus leaf extract. J. Med. Food 2008, 11, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Sriplang, K.; Adisakwattana, S.; Rungsipipat, A.; Yibchokanun, S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007, 109, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Adam, Y.; Somchit, M.N.; Sulaiman, M.R.; Nasaruddin, A.A.; Zuraini, A.; Bustamam, A.A.; Zakaria, Z.A. Diuretic properties of Orthosiphon stamineus Benth. J. Ethnopharmacol. 2009, 124, 154–158. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Mohamed, A.J.; Asmawi, M.Z.; Sadikun, A.; Ebrika, O.S.; Yam, M.F. Antihyperglycemic effect of Orthosiphon stamineus benth. leaves extract and its bioassay-guided fractions. Molecules 2011, 16, 3787–3801. [Google Scholar] [CrossRef] [Green Version]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Kadota, S. Siphonols A-E: Novel nitric oxide inhibitors from Orthosiphon stamineus of Indonesia. Bioor. Med. Chem. Lett. 2003, 13, 31–35. [Google Scholar] [CrossRef]
- Tezuka, Y.; Stampoulis, P.; Banskota, A.H.; Awale, S.; Tran, K.Q.; Saiki, I.; Kadota, S. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem. Pharm. Bull. 2000, 48, 1711–1719. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.H.; Lee, J.; Jung, D.W.; Williams, D.R. Visualizing sweetness: increasingly diverse applications for fluorescent-tagged glucose bioprobes and their recent structural modifications. Sensors (Basel) 2012, 12, 5005–5027. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.H.; Yang, J.L.; Uddin, M.N.; Park, S.L.; Lim, S.I.; Jung, D.W.; Williams, D.R.; Oh, W.K. Protein tyrosine phosphatase 1B (PTP1B) inhibitors from Morinda citrifolia (Noni) and their insulin mimetic activity. J. Nat. Prod. 2013, 76, 2080–2087. [Google Scholar] [CrossRef]
- Cornish Bowden, A.; Eisenthal, R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot and other methods. Biochem. J. 1974, 139, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; Barr, K.J.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M.; et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Cho, Y.S.; Oh, S.H.; Lee, S.; Min, B.S.; Moon, K.H.; Choi, J.S. Kinetics and molecular docking studies of pimarane-type diterpenes as protein tyrosine phosphatase (PTP1B) inhibitors from Aralia continentalis roots. Arch. Pharm. Res. 2013, 36, 957–965. [Google Scholar] [CrossRef]

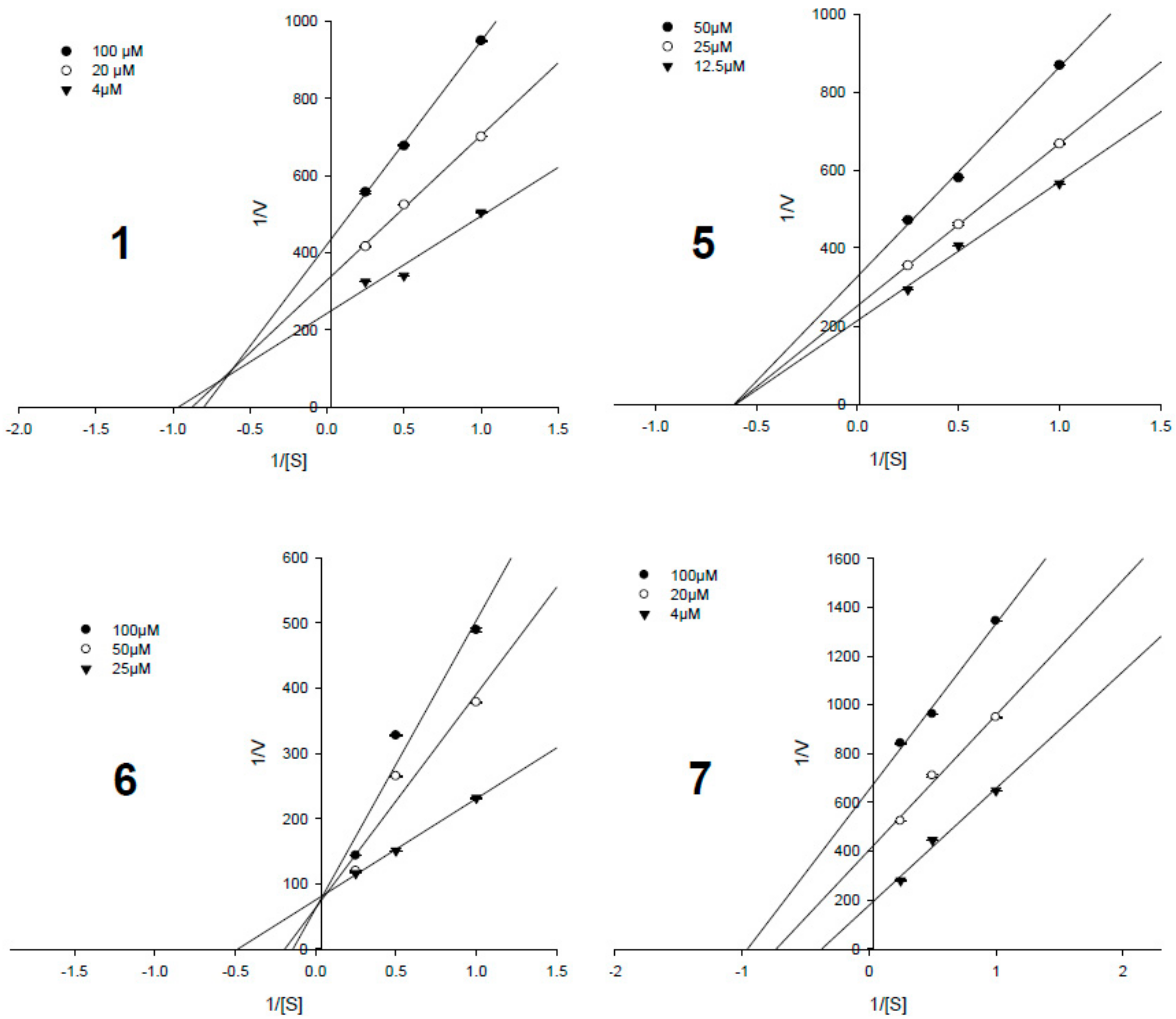

| Compounds | IC50, µM a | Ki Values, µM | Inhibition Type |
|---|---|---|---|
| 1 | 8.18 ± 0.41 | 52.4 ± 0.9 | Mixed-competitive |
| 2 | 24.75 ± 1.12 | - c | - |
| 3 | 9.84 ± 0.33 | 75.6 ± 1.7 | Non-competitive |
| 4 | 27.56 ± 2.99 | - | - |
| 5 | 3.82 ± 0.20 | 23.9 ± 1.2 | Non-competitive |
| 6 | 0.33 ± 0.07 | 1.3 ± 0.6 | Competitive |
| 7 | 1.60 ± 0.17 | 5.5 ± 0.1 | Uncompetitive |
| Ursolic acid b | 3.42 ± 0.26 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, P.H.; Tuan, H.N.; Hoang, D.T.; Vu, Q.T.; Pham, M.Q.; Tran, M.H.; To, D.C. Glucose Uptake Stimulatory and PTP1B Inhibitory Activities of Pimarane Diterpenes from Orthosiphon stamineus Benth. Biomolecules 2019, 9, 859. https://doi.org/10.3390/biom9120859
Nguyen PH, Tuan HN, Hoang DT, Vu QT, Pham MQ, Tran MH, To DC. Glucose Uptake Stimulatory and PTP1B Inhibitory Activities of Pimarane Diterpenes from Orthosiphon stamineus Benth. Biomolecules. 2019; 9(12):859. https://doi.org/10.3390/biom9120859
Chicago/Turabian StyleNguyen, Phi Hung, Huynh Nhu Tuan, Duc Thuan Hoang, Quoc Trung Vu, Minh Quan Pham, Manh Hung Tran, and Dao Cuong To. 2019. "Glucose Uptake Stimulatory and PTP1B Inhibitory Activities of Pimarane Diterpenes from Orthosiphon stamineus Benth" Biomolecules 9, no. 12: 859. https://doi.org/10.3390/biom9120859