Evaluating Iso-Mukaadial Acetate and Ursolic Acid Acetate as Plasmodium falciparum Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
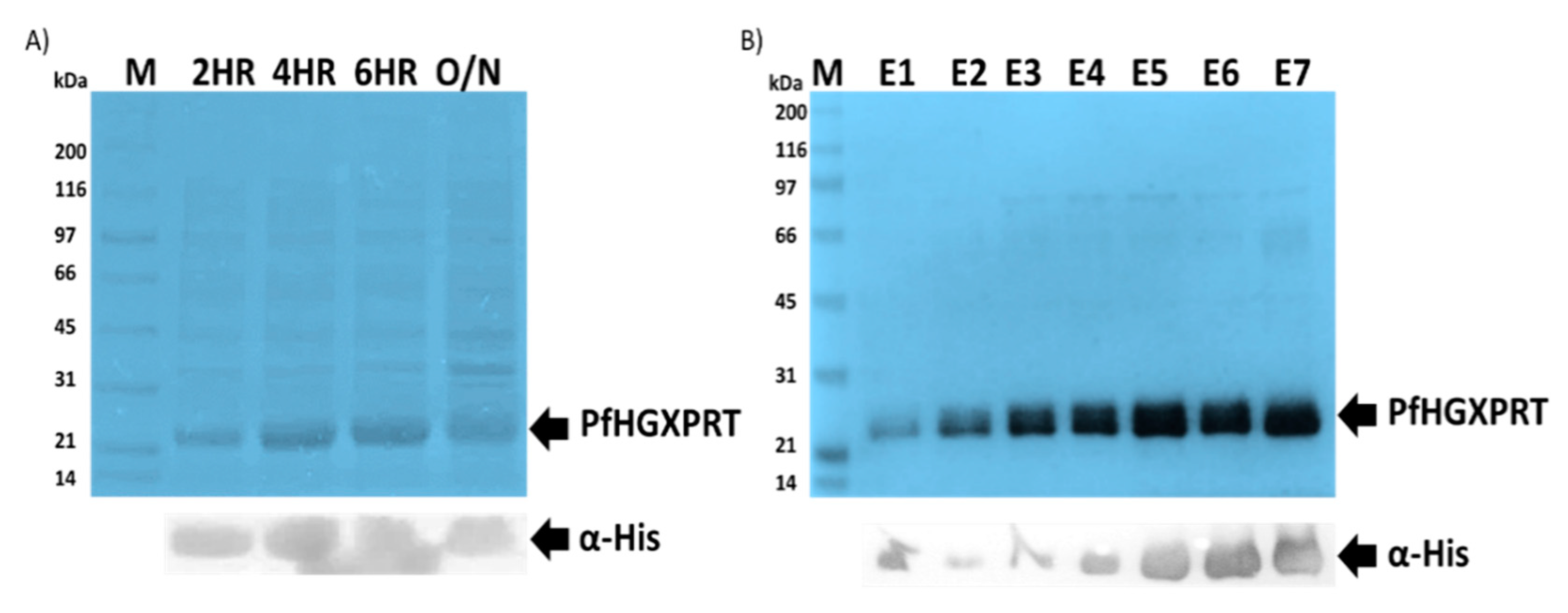
2.1. Recombinant Expression and Purification of PfHGXPRT
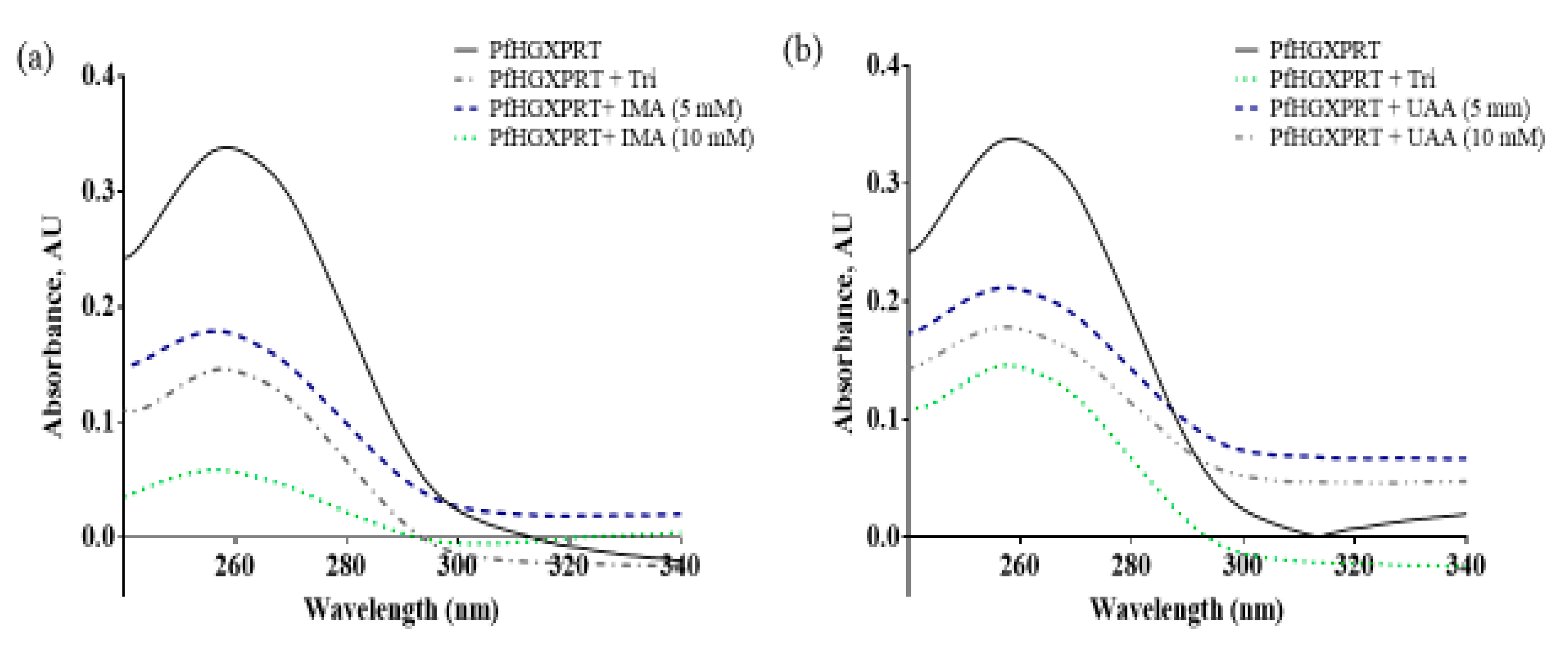
2.2. UV-Vis Spectrometer Analysis
2.3. Binding Affinity Analysis Using MicroScale Thermophoresis
2.4. Computational Details
2.5. Preparation of the Targeted Protein
2.6. Ligand Preparation
2.7. Molecular Docking Studies
2.8. Absorption, Distribution, Metabolism and Excretion/Toxicity (ADME/T) Prediction
2.9. Binding Free Energy Calculations
2.10. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Successful Expression and Purification Studies of Recombinant PfHGXPRT
3.2. Iso-Mukaadial Acetate and Ursolic Acid Acetate Binds to PfHGXPRT
3.3. MST Binding Analysis of Ursolic Acid Acetate and Iso-Mukaadial Acetate against PfHGXPRT
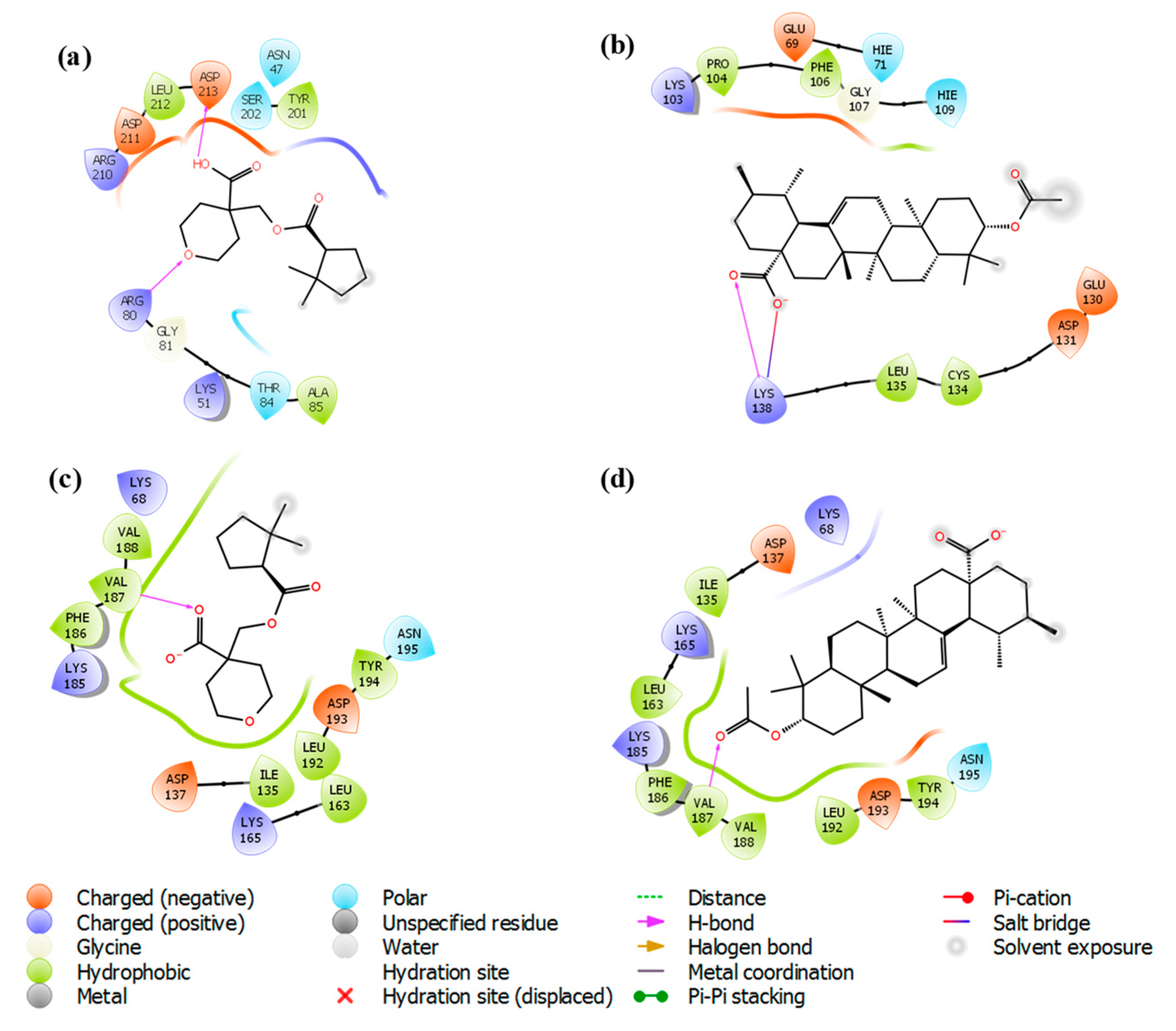
3.4. Molecular Docking Study
3.5. Binding Free Energy Analysis
3.6. Predicted ADME/T Properties
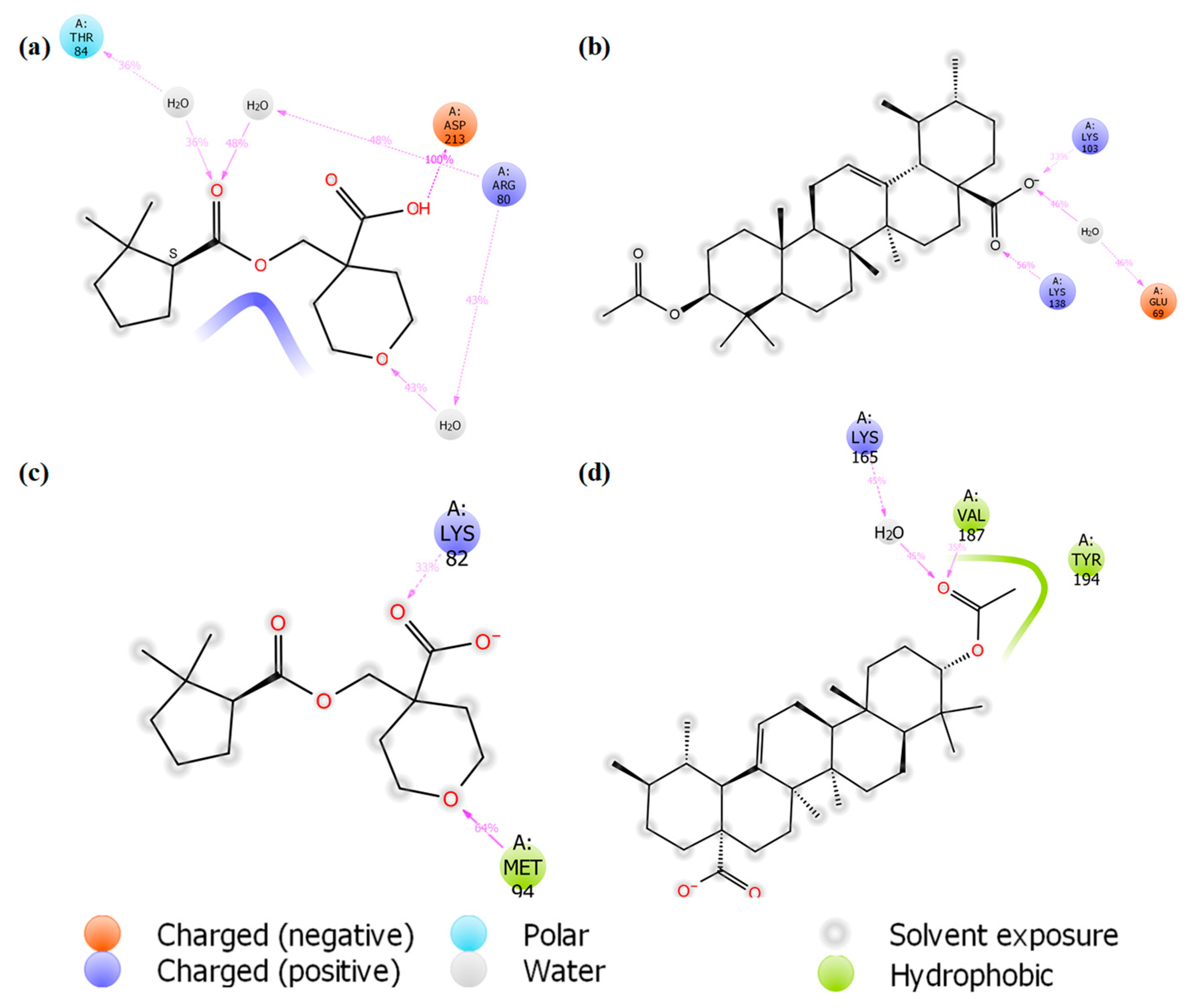
3.7. MD Simulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Final Mile for 21 Countries; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bush, P. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Nyaba, Z.N.; Murambiwa, P.; Opoku, A.R.; Mukaratirwa, S.; Shode, F.O.; Simelane, M.B.C. Isolation, characterization, and biological evaluation of a potent anti-malarial drimane sesquiterpene from Warburgia salutaris stem bark. Malar. J. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Simelane, M.; Shonhai, A.; Shode, F.; Smith, P.; Singh, M.; Opoku, A. Anti-Plasmodial Activity of Some Zulu Medicinal Plants and of Some Triterpenes Isolated from Them. Molecules 2013, 18, 12313–12323. [Google Scholar] [CrossRef]
- Hocková, D.; Janeba, Z.; Naesens, L.; Edstein, M.D.; Chavchich, M.; Keough, D.T.; Guddat, L.W. Antimalarial activity of prodrugs of N-branched acyclic nucleoside phosphonate inhibitors of 6-oxopurine phosphoribosyltransferases. Bioorg. Med. Chem. 2015, 23, 5502–5510. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Nagappa, L.K.; Prahladarao, V.S.; Balaram, H. Kinetic mechanism of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase. Mol. Biochem. Parasitol. 2015, 204, 111–120. [Google Scholar] [CrossRef]
- Karnawat, V.; Gogia, S.; Balaram, H.; Puranik, M. Differential Distortion of Purine Substrates by Human and Plasmodium falciparum Hypoxanthine-Guanine Phosphoribosyltransferase to Catalyse the Formation of Mononucleotides. ChemPhysChem 2015, 16, 2172–2181. [Google Scholar] [CrossRef]
- Krečmerová, M.; Dračínský, M.; Hocková, D.; Holý, A.; Keough, D.T.; Guddat, L.W. Synthesis of purine N 9-[2-hydroxy-3-O-(phosphonomethoxy)propyl] derivatives and their side-chain modified analogs as potential antimalarial agents. Bioorg. Med. Chem. 2012, 20, 1222–1230. [Google Scholar] [CrossRef]
- Jana, S.; Ganeshpurkar, A.; Singh, S.K. Multiple 3D-QSAR modeling, e-pharmacophore, molecular docking, and in vitro study to explore novel AChE inhibitors. RSC Adv. 2018, 8, 39477–39495. [Google Scholar] [CrossRef] [Green Version]
- Clinch, K.; Crump, D.R.; Evans, G.B.; Hazleton, K.Z.; Mason, J.M.; Schramm, V.L.; Tyler, P.C. Acyclic phosph(on)ate inhibitors of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase. Bioorg. Med. Chem. 2013, 21, 5629–5646. [Google Scholar] [CrossRef] [Green Version]
- Kumalo, H.M.; Bhakat, S.; Soliman, M.E.S. Theory and applications of covalent docking in drug discovery: Merits and pitfalls. Molecules 2015, 20, 1984–2000. [Google Scholar] [CrossRef] [Green Version]
- Msomi, N.Z.; Shode, F.O.; Pooe, O.J.; Mazibuko-mbeje, S.; Simelane, M.B.C. Iso-Mukaadial Acetate from Warburgia salutaris Enhances Glucose Uptake in the L6 Rat Myoblast Cell Line. Biomolecules 2019, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Plach, M.G.; Grasser, K.; Schubert, T. MicroScale Thermophoresis as a Tool to Study Protein-peptide Interactions in the Context of Large Eukaryotic Protein Complexes. Bio-Protocol 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Waddad, A.Y.; Ramharack, P.; Soliman, M.E.S.; Govender, T. Grafted hyaluronic acid N-acetyl-L-methionine for targeting of LAT1 receptor: In-silico, synthesis and microscale thermophoresis studies. Int. J. Biol. Macromol. 2019, 125, 767–777. [Google Scholar] [CrossRef]
- Gayathri, P.; Subbayya, I.N.S.; Ashok, C.S.; Selvi, T.S.; Balaram, H.; Murthy, M.R.N. Crystal structure of a chimera of human and plasmodium falciparum hypoxanthine guanine phosphoribosyltransferases provides insights into oligomerization. Proteins 2008, 73, 1010–1020. [Google Scholar] [CrossRef]
- Rose, P.W.; Prlic, A.; Bi, C.; Bluhm, W.F.; Christie, C.H.; Dutta, S.; Green, R.K.; Goodsell, D.S.; Westbrook, J.D.; Woo, J.; et al. The RCSB Protein Data Bank: Views of structural biology for basic and applied research and education. Nucleic Acids Res. 2015, 43, 345–356. [Google Scholar] [CrossRef]
- Keough, D.T.; Brereton, I.M.; De Jersey, J.; Guddat, L.W. The crystal structure of free human hypoxanthine-guanine phosphoribosyltransferase reveals extensive conformational plasticity throughout the catalytic cycle. J. Mol. Biol. 2005, 351, 170–181. [Google Scholar] [CrossRef]
- Sujay Subbayya, I.N.; Sukumaran, S.; Shivashankar, K.; Balaram, H. Unusual substrate specificity of a chimeric hypoxanthine-guanine phosphoribosyltransferase containing segments from the Plasmodium falciparum and human enzymes. Biochem. Biophys. Res. Commun. 2000, 272, 596–602. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-2. Protein Preparation Wizard; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
- Shelley, J.C.; Cholleti, E.A.; Frye, E.L.L.; Greenwood, J.R.; Timlin, E.M.R. Epik: A software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-2. Prime; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
- Golemis, E.A.; Tew, K.D.; Dadke, D. Protein interaction-targeted drug discovery: Evaluating critical issues. BioTechniques 2002, 32, 636–647. [Google Scholar] [CrossRef]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Markus, K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending Force Field Coverage for Drug-Like Small Molecules. J. Chem. Theory Comput. 2019. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-2. Maestro; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Li, J.; Abel, R.; Zhu, K.; Cao, Y.; Zhao, S.; Friesner, R.A. The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling. Proteins Struct. Funct. Bioinform. 2011, 2794–2812. [Google Scholar] [CrossRef] [Green Version]
- Lyne, P.D.; Lamb, M.L.; Saeh, J.C. Accurate Prediction of the Relative Potencies of Members of a Series of Kinase Inhibitors Using Molecular Docking and MM-GBSA Scoring. J. Med. Chem. 2006, 49, 4805–4808. [Google Scholar] [CrossRef]
- Das, D.; Koh, Y.; Tojo, Y.; Ghosh, A.K.; Mitsuya, H. Prediction of Potency of Protease Inhibitors Using Free Energy Simulations with Polarizable Quantum Mechanics-Based Ligand Charges and a Hybrid Water Model. J. Chem. Inf. Model. 2009, 49, 2851–2862. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger Release 2019-2. Desmond Molecular Dynamics System, D.E. Shaw Research; Schrödinger: New York, NY, USA, 2019. [Google Scholar]
- Schrödinger Release 2019-2. Maestro-Desmond Interoperability Tools; Schrödinger: New York, NY, USA, 2019. [Google Scholar]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces; Springer: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- Zielkiewicz, J. Structural properties of water: Comparison of the SPC, SPCE, TIP4P, and TIP5P models of water. J. Chem. Phys. 2005, 123. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- William, G. Hoover Canonical dynamics: Equilibrium phase-space distributions William. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Zininga, T.; Pooe, O.J.; Makhado, P.B.; Ramatsui, L.; Prinsloo, E.; Achilonu, I.; Dirr, H.; Shonhai, A. Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooe, O.J.; Köllisch, G.; Heine, H.; Shonhai, A. Plasmodium falciparum Heat Shock Protein 70 Lacks Immune Modulatory Activity. Protein Pept. Lett. 2017, 24, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-P.; Barkley, M.D. Conformational effects on tryptophan fluorescence in cyclic hexapeptides. Biophys. J. 2004, 86, 3828–3835. [Google Scholar] [CrossRef] [Green Version]
- Lovric, J. Introducing Proteomics: From Concepts to Sample Separation, Mass Spectrometry and Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]



| Protein and Ligand | KD (µM) | KA (M−1) | Binding Energy (Kcal/mol) |
|---|---|---|---|
| PfHGXPRT + IMA | 0.0833 | 12.0058 | −11.66 |
| PfHGXPRT + UAA | 2.8396 | 0.3522 | −13.78 |
| Complex | Docking Scores | pIC50 | ΔGbind | Residue Involved H-bond |
|---|---|---|---|---|
| Iso-mukaadial acetate-2VFA | −4.10 | 5.35 | −26.73 | ARG80, ASP213 |
| Ursolic acid acetate-2VFA | −2.96 | 4.19 | −44.46 | LYS138 |
| Principal Descriptors | IMA | UAA | (Range 95% of Drugs) |
|---|---|---|---|
| Solute Molecular Weight | 284.352 | 498.745 | (130.0–725.0) |
| Solute Dipole Moment | 1.465 | 7.944 | (1.0–12.5) |
| Solute Total SASA | 518.477 | 741.364 | (300.0–1000.0) |
| Solute Hydrophobic | 408.528 | 628.533 | (0.0–750.0) |
| Solute Hydrophilic | 109.949 | 104.637 | (7.0–330.0) |
| Solute Carbon Pi SASA | 0 | 8.195 | (0.0–450.0) |
| Solute Weakly Polar SASA | 0 | 0 | (0.0–175.0) |
| Solute Molecular Volume (Å3) | 934.962 | 1522.361 | (500.0–2000.0) |
| Solute vdW Polar SA | 81.308 | 75.126 | (7.0–200.0) |
| Solute Number of Rotatable Bonds | 4 | 2 | (0.0–15.0) |
| Solute as Donor - Hydrogen Bonds | 1 | 1 | (0.0–6.0) |
| Solute as Acceptor - Hydrogen Bonds | 5.7 | 4 | (2.0–20.0) |
| Solute Globularity (Sphere = 1) | 0.892 | 0.863 | (0.75–0.95) |
| Solute Ionization Potential (eV) | 10.803 * | 9.669 | (7.9–10.5) |
| Solute Electron Affinity (eV) | −0.815 | −0.737 | (−0.9–1.7) |
| Predictions for Properties | |||
| QP Polarizability (Å3) | 28.695M | 53.565M | (13.0–70.0) |
| QP log P forhexadecane/gas | 8.065M | 13.173M | (4.0–18.0) |
| QP log P foroctanol/gas | 13.452M | 21.994M | (8.0–35.0) |
| QP log P forwater/gas | 7.911M | 7.234M | (4.0–45.0) |
| QP log P foroctano/water | 2.561 | 7.020 * | (−2.0–6.5) |
| QP log S foraqueous solubility | −3.104 | −7.984 * | (−6.5–0.5) |
| QP log S - conformation independent | −2.765 | −7.823 | (−6.5–0.5) |
| QP log K hsa Serum Protein Binding | −0.231 | 1.771 * | (−1.5–1.5) |
| QP log BB for brain/blood | −0.599 | −0.493 | (−3.0–1.2) |
| No. of Primary Metabolites | 2 | 2 | (1.0–8.0) |
| HERG K+ Channel Blockage: log IC50 | −1.474 | −1.976 | (concern below −5) |
| Apparent Caco-2 Permeability (nm/sec) | 227 | 255 | (<25 poor, >500 great) |
| Apparent MDCK Permeability (nm/sec) | 126 | 143M | (<25 poor, >500 great) |
| QP log Kp for skin permeability | −3.162 | −3.227 | (Kp in cm/hr) |
| Jm, max transdermal transport rate | 0.154 | 0 | (micrograms/cm2-hr) |
| Lipinski Rule of 5 Violations | 0 | 1 | (maximum is 4) |
| Jorgensen Rule of 3 Violations | 0 | 1 | (maximum is 3) |
| % Human Oral Absorption in GI (±20%) | 84 | 100 | (<25% is poor) |
| Qualitative Model for Human Oral Absorption | High | Low | (>80% is high) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opoku, F.; Govender, P.P.; Pooe, O.J.; Simelane, M.B.C. Evaluating Iso-Mukaadial Acetate and Ursolic Acid Acetate as Plasmodium falciparum Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase Inhibitors. Biomolecules 2019, 9, 861. https://doi.org/10.3390/biom9120861
Opoku F, Govender PP, Pooe OJ, Simelane MBC. Evaluating Iso-Mukaadial Acetate and Ursolic Acid Acetate as Plasmodium falciparum Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase Inhibitors. Biomolecules. 2019; 9(12):861. https://doi.org/10.3390/biom9120861
Chicago/Turabian StyleOpoku, Francis, Penny P. Govender, Ofentse J. Pooe, and Mthokozisi B.C. Simelane. 2019. "Evaluating Iso-Mukaadial Acetate and Ursolic Acid Acetate as Plasmodium falciparum Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase Inhibitors" Biomolecules 9, no. 12: 861. https://doi.org/10.3390/biom9120861





