Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review
Abstract
:1. Introduction
2. Method Principle
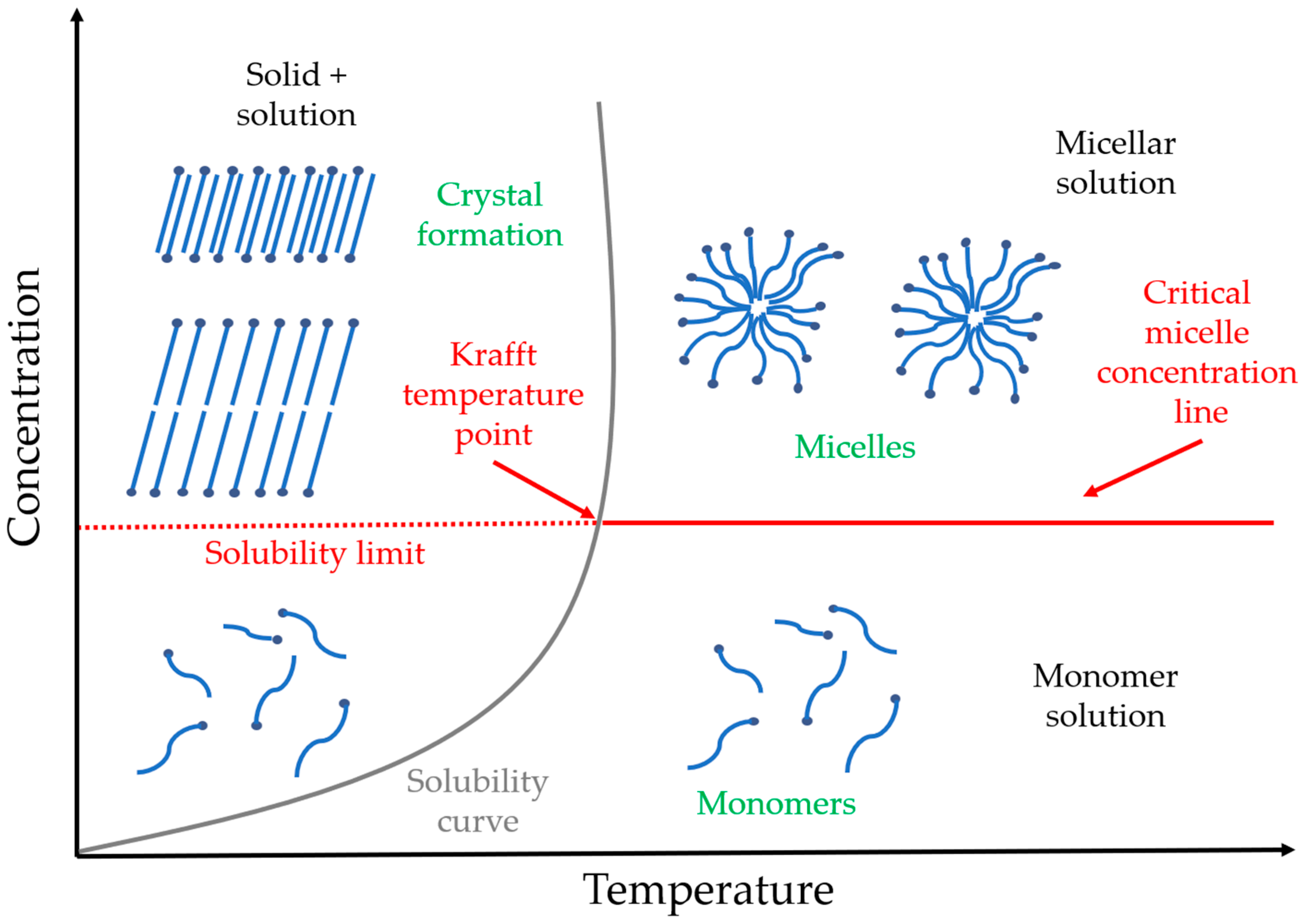
2.1. Surfactants
2.2. Micelle Formation
2.3. pH Level of the Solution
2.4. Salting-Out Effect
2.5. Temperature
3. Application of CPE for Bioactive Compound Extraction in Food Byproducts
3.1. Olive-Based Waste
3.2. Wine-Based Waste
3.3. Pomegranate-Based Waste
3.4. Berry-Based Waste
3.5. Other Food-Based Waste
4. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Gowman, A.C.; Picard, M.C.; Lim, L.-T.; Misra, M.; Mohanty, A.K. Fruit Waste Valorization for Biodegradable Biocomposite Applications: A Review. BioResources 2019, 14, 10047–10092. [Google Scholar] [CrossRef]
- Sogut, E.; Cakmak, H. Utilization of Carrot (Daucus Carota L.) Fiber as a Filler for Chitosan Based Films. Food Hydrocoll. 2020, 106, 105861. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of Bioplastic through Food Waste Valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Shi, K.; Xu, Y.; Hu, H.; Xu, X.; Hu, T.; Zhang, P.; Yao, J.; Pan, S. Trends in Valorization of Citrus By-Products from the Net-Zero Perspective: Green Processing Innovation Combined with Applications in Emission Reduction. Trends Food Sci. Technol. 2023, 137, 124–141. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, D.; Chen, J.; He, Y.; Dai, Y.; Loh, K.-C.; Tong, Y.W. Assessment and Optimization of a Decentralized Food-Waste-to-Energy System with Anaerobic Digestion and CHP for Energy Utilization. Energy Convers. Manag. 2021, 228, 113654. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food Waste Valorisation and Circular Economy Concepts in Insect Production and Processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Karmee, S.K. Liquid Biofuels from Food Waste: Current Trends, Prospect and Limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of Biomass Waste into High Value Chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A Critical Review on Food Waste Management for the Production of Materials and Biofuel. J. Hazard. Mater. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Almomani, F.; Hosseinzadeh-Bandbafha, H.; Aghbashlo, M.; Omar, A.; Joo, S.-W.; Vasseghian, Y.; Karimi-Maleh, H.; Shiung Lam, S.; Tabatabaei, M.; Rezania, S. Comprehensive Insights into Conversion of Microalgae to Feed, Food, and Biofuels: Current Status and Key Challenges towards Implementation of Sustainable Biorefineries. Chem. Eng. J. 2023, 455, 140588. [Google Scholar] [CrossRef]
- Ding, Z.; Ge, Y.; Sar, T.; Kumar, V.; Harirchi, S.; Binod, P.; Sirohi, R.; Sindhu, R.; Wu, P.; Lin, F.; et al. Valorization of Tropical Fruits Waste for Production of Commercial Biorefinery Products—A Review. Bioresour. Technol. 2023, 374, 128793. [Google Scholar] [CrossRef] [PubMed]
- H. Clark, J. Green Biorefinery Technologies Based on Waste Biomass. Green Chem. 2019, 21, 1168–1170. [Google Scholar] [CrossRef]
- Tavares, F.; Costa, G.; Francisco, V.; Liberal, J.; Figueirinha, A.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon Citratus Industrial Waste as a Potential Source of Bioactive Compounds. J. Sci. Food Agric. 2015, 95, 2652–2659. [Google Scholar] [CrossRef]
- Huie, C.W. A Review of Modern Sample-Preparation Techniques for the Extraction and Analysis of Medicinal Plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Wang, M.; Morón-Ortiz, Á.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with Dimethyl Sulfoxide on the Recovery of Carotenoids and Other Dietary Valuable Compounds from the Microalgae Spirulina, Chlorella and Phaeodactylum Tricornutum. Food Chem. 2023, 405, 134885. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Boulila, A.; Hassen, I.; Haouari, L.; Mejri, F.; Amor, I.; Casabianca, H.; Hosni, K. Enzyme-Assisted Extraction of Bioactive Compounds from Bay Leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and Recovery of Bioactive Soluble Phenolic Compounds from Brocade Orange (Citrus sinensis) Peels: Effect of Different Extraction Methods Thereon. LWT 2023, 173, 114337. [Google Scholar] [CrossRef]
- Islam, M.R.; Kamal, M.M.; Kabir, M.R.; Hasan, M.M.; Haque, A.R.; Hasan, S.M.K. Phenolic Compounds and Antioxidants Activity of Banana Peel Extracts: Testing and Optimization of Enzyme-Assisted Conditions. Meas. Food 2023, 10, 100085. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Process Optimization of Microwave-Assisted Extraction of Chlorophyll, Carotenoid and Phenolic Compounds from Chlorella vulgaris and Comparison with Conventional and Supercritical Fluid Extraction. Appl. Sci. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Valdés, A.; Mondragon, G.; Garrigós, M.C.; Eceiza, A.; Jiménez, A. Microwave-Assisted Extraction of Cellulose Nanocrystals from Almond (Prunus amygdalus) Shell Waste. Front. Nutr. 2023, 9, 1071754. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Microwave-Assisted Extraction of Polyphenols from Eucalyptus Bark—A First Step for a Green Production of Tannin-Based Coagulants. Water 2023, 15, 317. [Google Scholar] [CrossRef]
- Tsiaka, T.; Lantzouraki, D.Z.; Polychronaki, G.; Sotiroudis, G.; Kritsi, E.; Sinanoglou, V.J.; Kalogianni, D.P.; Zoumpoulakis, P. Optimization of Ultrasound- and Microwave-Assisted Extraction for the Determination of Phenolic Compounds in Peach Byproducts Using Experimental Design and Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2023, 28, 518. [Google Scholar] [CrossRef]
- Hidayah, N.N.; Abidin, S.Z. The Evolution of Mineral Processing in Extraction of Rare Earth Elements Using Solid-Liquid Extraction over Liquid-Liquid Extraction: A Review. Miner. Eng. 2017, 112, 103–113. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Hoff, R.B.; Pizzolato, T.M. Combining Extraction and Purification Steps in Sample Preparation for Environmental Matrices: A Review of Matrix Solid Phase Dispersion (MSPD) and Pressurized Liquid Extraction (PLE) Applications. TrAC Trends Anal. Chem. 2018, 109, 83–96. [Google Scholar] [CrossRef]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Domínguez, M.C. Valorization of Granadilla Waste (Passiflora Ligularis, Juss.) by Sequential Green Extraction Processes Based on Pressurized Fluids to Obtain Bioactive Compounds. J. Supercrit. Fluids 2023, 194, 105833. [Google Scholar] [CrossRef]
- Melnyk, A.; Namieśnik, J.; Wolska, L. Theory and Recent Applications of Coacervate-Based Extraction Techniques. TrAC Trends Anal. Chem. 2015, 71, 282–292. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A Novel, Green Environment-Friendly Cloud Point Extraction of Polyphenols from Pomegranate Peels: A Comparative Assessment with Ultrasound and Microwave-Assisted Extraction. Sep. Sci. Technol. 2021, 56, 1014–1025. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Moreno-Cordero, B.; Pérez-Pavón, J.L.; García-Pinto, C.; Fernández Laespada, E. Surfactant Cloud Point Extraction and Preconcentration of Organic Compounds Prior to Chromatography and Capillary Electrophoresis. J. Chromatogr. A 2000, 902, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Mortada, W.I.; Hassanien, M.M.; El-Asmy, A.A. Cloud Point Extraction of Some Precious Metals Using Triton X-114 and a Thioamide Derivative with a Salting-out Effect. Egypt. J. Basic Appl. Sci. 2014, 1, 184–191. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-Forming Organic Ligands in Cloud-Point Extraction of Metal Ions: A Review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef]
- Bolan, S.; Padhye, L.P.; Mulligan, C.N.; Alonso, E.R.; Saint-Fort, R.; Jasemizad, T.; Wang, C.; Zhang, T.; Rinklebe, J.; Wang, H.; et al. Surfactant-Enhanced Mobilization of Persistent Organic Pollutants: Potential for Soil and Sediment Remediation and Unintended Consequences. J. Hazard. Mater. 2023, 443, 130189. [Google Scholar] [CrossRef]
- Kori, S. Cloud Point Extraction Coupled with Back Extraction: A Green Methodology in Analytical Chemistry. Forensic Sci. Res. 2021, 6, 19–33. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Xing, W.; Chen, L. Micelle-Mediated Extraction and Cloud Point Preconcentration of Bergenin from Ardisia Japonica. Sep. Purif. Technol. 2013, 110, 57–62. [Google Scholar] [CrossRef]
- Kiathevest, K.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Extraction and Concentration of Anthraquinones from Roots of Morinda citrifolia by Non-Ionic Surfactant Solution. Sep. Purif. Technol. 2009, 66, 111–117. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Extraction and Concentration of Tanshinones in Salvia Miltiorrhiza Bunge by Task-Specific Non-Ionic Surfactant Assistance. Food Chem. 2011, 126, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Paleologos, E.K.; Giokas, D.L.; Karayannis, M.I. Micelle-Mediated Separation and Cloud-Point Extraction. TrAC Trends Anal. Chem. 2005, 24, 426–436. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef]
- Stalikas, C.D. Micelle-Mediated Extraction as a Tool for Separation and Preconcentration in Metal Analysis. TrAC Trends Anal. Chem. 2002, 21, 343–355. [Google Scholar] [CrossRef]
- Ingram, T.; Storm, S.; Glembin, P.; Bendt, S.; Huber, D.; Mehling, T.; Smirnova, I. Aqueous Surfactant Two-Phase Systems for the Continuous Countercurrent Cloud Point Extraction. Chem. Ing. Tech. 2012, 84, 840–848. [Google Scholar] [CrossRef]
- Mohajeri, E.; Noudeh, G.D. Effect of Temperature on the Critical Micelle Concentration and Micellization Thermodynamic of Nonionic Surfactants: Polyoxyethylene Sorbitan Fatty Acid Esters. J. Chem. 2011, 9, 2268–2274. [Google Scholar] [CrossRef]
- Zhao, G.; Khin, C.C.; Chen, S.B.; Chen, B.-H. Nonionic Surfactant and Temperature Effects on the Viscosity of Hydrophobically Modified Hydroxyethyl Cellulose Solutions. J. Phys. Chem. B 2005, 109, 14198–14204. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chatzilazarou, A.; Katsoyannos, E.; Papaconstandinou, S.; Dourtoglou, E. Recovery of Natural Antioxidants from Olive Mill Wastewater Using Genapol-X080. J. Am. Oil Chem. Soc. 2008, 85, 133–140. [Google Scholar] [CrossRef]
- Bezerra, M.D.A.; Arruda, M.A.Z.; Ferreira, S.L.C. Cloud Point Extraction as a Procedure of Separation and Pre-Concentration for Metal Determination Using Spectroanalytical Techniques: A Review. Appl. Spectrosc. Rev. 2005, 40, 269–299. [Google Scholar] [CrossRef]
- Sato, N.; Mori, M.; Itabashi, H. Cloud Point Extraction of Cu(II) Using a Mixture of Triton X-100 and Dithizone with a Salting-out Effect and Its Application to Visual Determination. Talanta 2013, 117, 376–381. [Google Scholar] [CrossRef]
- Han, F.; Yin, R.; Shi, X.; Jia, Q.; Liu, H.; Yao, H.; Xu, L.; Li, S. Cloud Point Extraction-HPLC Method for Determination and Pharmacokinetic Study of Flurbiprofen in Rat Plasma after Oral and Transdermal Administration. J. Chromatogr. B 2008, 868, 64–69. [Google Scholar] [CrossRef]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodríguez, J.J. The Use of Micellar Systems in the Extraction and Pre-Concentration of Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Peng, S.X.; Henson, C.; Strojnowski, M.J.; Golebiowski, A.; Klopfenstein, S.R. Automated High-Throughput Liquid−Liquid Extraction for Initial Purification of Combinatorial Libraries. Anal. Chem. 2000, 72, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Cerutti, E.S.; Martinez, L.D. Coupling Cloud Point Extraction to Instrumental Detection Systems for Metal Analysis. Microchim. Acta 2006, 155, 349–364. [Google Scholar] [CrossRef]
- Pandit, N.K.; Kanjia, J.; Patel, K.; Pontikes, D.G. Phase Behavior of Aqueous Solutions Containing Nonionic Surfactant-Polyethylene Glycol Mixtures. Int. J. Pharm. 1995, 122, 27–33. [Google Scholar] [CrossRef]
- Qin, X.Y.; Meng, J.; Li, X.Y.; Zhou, J.; Sun, X.L.; Wen, A.D. Determination of Venlafaxine in Human Plasma by High-Performance Liquid Chromatography Using Cloud-Point Extraction and Spectrofluorimetric Detection. J. Chromatogr. B 2008, 872, 38–42. [Google Scholar] [CrossRef]
- Liu, W.; Bi, K.; Liu, X.; Zhao, J.; Chen, X. Cloud-Point Extraction Combined with LC–MS for Analysis of Memantine in Rat Plasma. Chromatographia 2009, 69, 837–842. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Integrated Process for Sequential Extraction of Bioactive Phenolic Compounds and Proteins from Mill and Field Olive Leaves and Effects on the Lignocellulosic Profile. Foods 2019, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Voulgaris, A.; Katsoulis, K.; Lalas, S.I.; Roussis, I.G.; Gortzi, O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods 2023, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud Point Extraction of Phenolic Compounds from Pretreated Olive Mill Wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Katsoyannos, E.; Chatzilazarou, A.; Gortzi, O.; Lalas, S.; Konteles, S.; Tataridis, P. Application of Cloud Point Extraction Using Surfactants in the Isolation of Physical Antioxidants (Phenols) from Olive Mill Wastewater. Fresenius Environ. Bull. 2006, 15, 1122–1125. [Google Scholar]
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the Suitability of Low Hazard Surfactants for the Separation of Phenols and Carotenoids from Red-Flesh Orange Juice and Olive Mill Wastewater Using Cloud Point Extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and Fractionation of Phenolic Compounds Extracted from Olive Oil Mill Wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Katsoyannos, E.; Chatzilazarou, A. Antioxidant Activity and Thermal Stability of Oleuropein and Related Phenolic Compounds of Olive Leaf Extract after Separation and Concentration by Salting-Out-Assisted Cloud Point Extraction. Antioxidants 2014, 3, 229–244. [Google Scholar] [CrossRef]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Chatzilazarou, A.; Katsoyannos, E.; Gortzi, O.; Lalas, S.; Paraskevopoulos, Y.; Dourtoglou, E.; Tsaknis, J. Removal of Polyphenols from Wine Sludge Using Cloud Point Extraction. J. Air Waste Manag. Assoc. 2010, 60, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined Densification and Pulsed Electric Field Treatment for Selective Polyphenols Recovery from Fermented Grape Pomace. Innov. Food Sci. Emerg. Technol. 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Sun, F.; Ning, J.; Wang, Y.; Shi, Y.; Li, J.; Li, H.; Li, W. Optimization of Ultrasound-Assisted Cloud Point Extraction of Polyphenols from Pomegranate Peels. J. Biotech Res. 2023, 14, 160–170. [Google Scholar]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of Ultrasound-Assisted Extraction of Polyphenolic Compounds from Pomegranate Peel Using Response Surface Methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- De Araújo Padilha, C.E.; De Azevedo, J.C.S.; De Sousa, F.C.; De Oliveira, S.D.; Souza, D.F.D.S.; De Oliveira, J.A.; De Macedo, G.R.; Dos Santos, E.S. Recovery of Polyphenols from Camu-Camu (Myrciaria dubia H.B.K. McVaugh) Depulping Residue by Cloud Point Extraction. Chin. J. Chem. Eng. 2018, 26, 2471–2476. [Google Scholar] [CrossRef]
- de Azevêdo, J.C.S.; Fujita, A.; de Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. Dried Camu-Camu (Myrciaria dubia H.B.K. McVaugh) Industrial Residue: A Bioactive-Rich Amazonian Powder with Functional Attributes. Food Res. Int. 2014, 62, 934–940. [Google Scholar] [CrossRef]
- Azevêdo, J.C.S.; Borges, K.C.; Genovese, M.I.; Correia, R.T.P.; Vattem, D.A. Neuroprotective Effects of Dried Camu-Camu (Myrciaria dubia HBK McVaugh) Residue in C. Elegans. Food Res. Int. 2015, 73, 135–141. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.-W.; Kou, P.; Liu, Z.-M.; Efferth, T.; Li, Y.-Y.; Fu, Y.-J. Application of Integrative Cloud Point Extraction and Concentration for the Analysis of Polyphenols and Alkaloids in Mulberry Leaves. J. Pharm. Biomed. Anal. 2019, 167, 132–139. [Google Scholar] [CrossRef]
- Cunha-Santos, E.C.E.; Rodrigues-Silva, C.; da Silveira, T.F.F.; Godoy, H.T. Optimization of Phenolic Compounds Extraction of Different Parts of Camu-Camu Fruit from Different Geographic Regions. Plant Foods Hum. Nutr. 2022, 77, 340–344. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Development of a Cloud Point Extraction Technique Based on Lecithin for the Recovery of Carotenoids from Liquid Tomato Wastewater. Waste 2022, 1, 105–114. [Google Scholar] [CrossRef]
- Grassino, A.N.; Pedisić, S.; Dragović-Uzelac, V.; Karlović, S.; Ježek, D.; Bosiljkov, T. Insight into High-Hydrostatic Pressure Extraction of Polyphenols from Tomato Peel Waste. Plant Foods Hum. Nutr. 2020, 75, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Panagiotopoulou, M.; Papadaki, S.; Krokida, M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources 2023, 12, 72. [Google Scholar] [CrossRef]

| Category | Properties | Example |
|---|---|---|
| Nonionic | The hydrophilic head is uncharged | Polyoxyethylenes (Genapol X-080, Triton X-100, Tween 80) |
| Anionic | The hydrophilic group contains an anionic moiety, such as carboxylate, sulfonate, or sulfate | Sodium dodecyl sulfate, ammonium lauryl sulfate, sodium laureth sulfate |
| Cationic | The hydrophilic head contains positive groups, such as quaternary ammonium | Cetyl trimethylammonium bromide, methylbenzethonium, benzalkonium |
| Zwitter anionic | cationic, anionic, or neutral, depending on the solution’s pH | 4-(Dodecyldimethyl ammonium) butyrate, erucyl amidopropyl betaine |
| Matrix | Target Bioactive | Surfactant | Extraction Conditions | Extraction Yield (%) | Reference |
|---|---|---|---|---|---|
| Olive mill wastewater | Polyphenols | Tween 80 (10% w/v) | 70 °C, pH 2, 30 min | 75.5 | [63] |
| Polyphenols | Triton X-114 (2% w/v) | 55–60 °C, 20 min | >90 | [65] | |
| Polyphenols, tocopherols | Genapol X-080 (5% w/v) | 55 °C, 20 min | - | [51] | |
| Natural antioxidants, polyphenols, carotenoids | Tween 80 (5–7% w/v) | 55 °C, 30 min | - | [66] | |
| Polyphenols | Lecithin (3% w/v) | 40 °C, pH 3.5, 20 min | 42.2 | [62] | |
| Polyphenols | Lecithin (12.5% w/v) | 80 °C, pH 5.5 | - | [67] | |
| Polyphenols | Triton X-100 (10% w/v) | 90 °C, 30 min | 66.5 | [64] | |
| Olive leaves | Oleuropein, polyphenols | Tween 80 (4% w/v) | 25 °C, pH 2.6, 5 min | 93–100 | [69] |
| Wine sludge | Polyphenols | PEG 8000 (2% w/v), Genapol X-080 (5% w/v) | 55 °C, pH 2.5, 30 min | 98.5 75.8 | [71] |
| Lecithin (5% w/v) | 40 °C, pH 3, 30 min | 76 | [70] | ||
| Pomegranate peel | Polyphenols, flavonoids | Triton X-114 (8% w/v) | 55 °C, pH 4.5, 30 min | 94.48 | [35] |
| Polyphenols, flavonoids | Triton X-114 (10% w/v) | 65 °C, pH 4, 40 min | - | [73] | |
| Camu-camu residue | Polyphenols | Triton X-114 (7% w/v) | 30 °C, 3 h | 95.71 | [75] |
| Mulberry leaves | Polyphenols Alkaloids | Triton X-114 (3% w/v) mixed with 0.05M HCl | 25 °C, 45 min, ultrasonic power 360 W | 89.2 85.9 | [78] |
| Tomato liquid waste | Carotenoids | Lecithin (2% w/v) | 45 °C, pH 3.5, 20 min | 36.3 | [80] |
| Apricot cannery waste | Polyphenols | PEG 8000 (2% w/v) | 55 °C, pH 2.53, 30 min | >92 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Giovanoudis, I.; Lalas, S.I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3, 306-322. https://doi.org/10.3390/biomass3030019
Chatzimitakos T, Athanasiadis V, Mantiniotou M, Kalompatsios D, Bozinou E, Giovanoudis I, Lalas SI. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass. 2023; 3(3):306-322. https://doi.org/10.3390/biomass3030019
Chicago/Turabian StyleChatzimitakos, Theodoros, Vassilis Athanasiadis, Martha Mantiniotou, Dimitrios Kalompatsios, Eleni Bozinou, Ioannis Giovanoudis, and Stavros I. Lalas. 2023. "Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review" Biomass 3, no. 3: 306-322. https://doi.org/10.3390/biomass3030019










