Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Materials
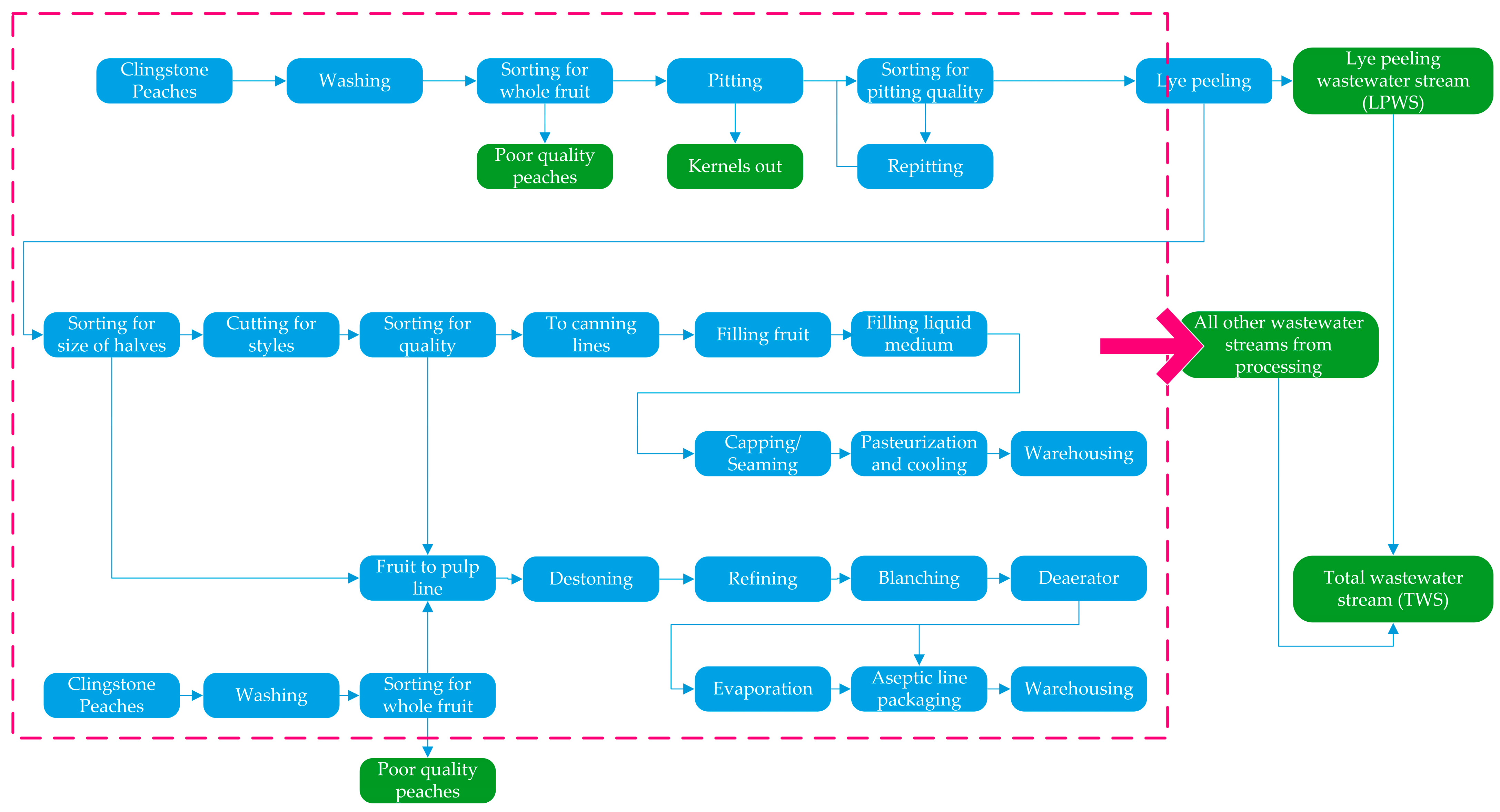
2.2. Peach Waste Streams
2.3. CPE Procedure
2.4. Polyphenol Recovery by CPE
2.5. Determination of Total Polyphenol Content
2.6. Determination of Antiradical Activity
2.7. Statistical Analysis
3. Results and Discussion
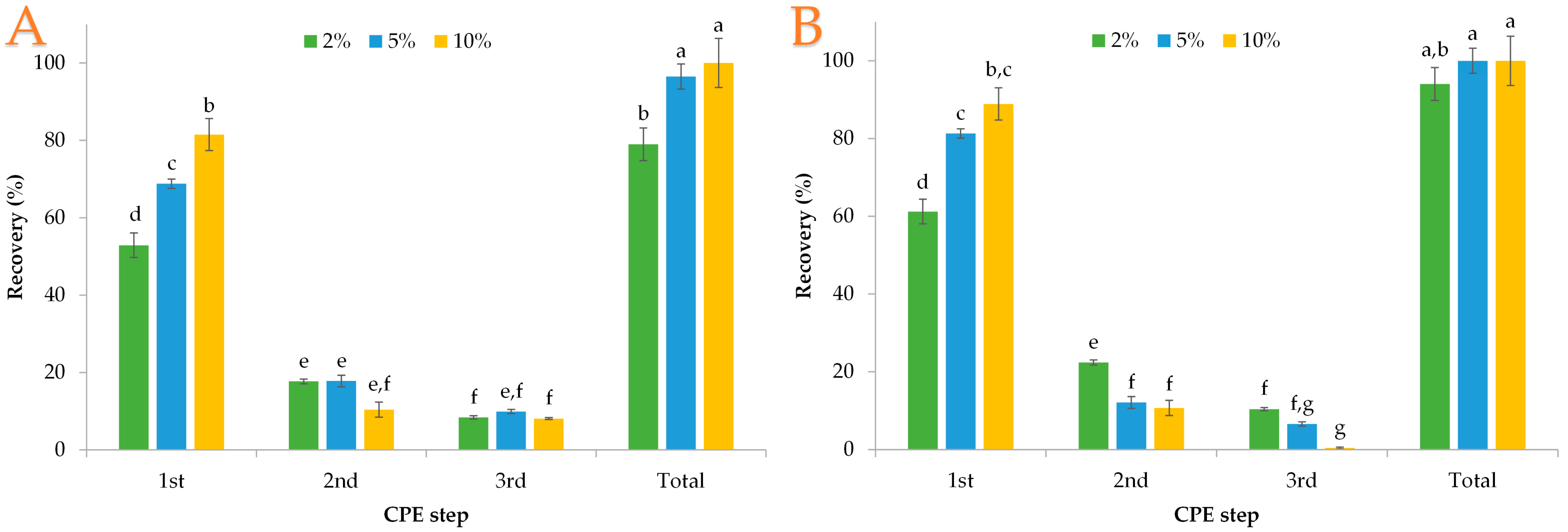
3.1. Polyphenol Extraction with Genapol X-080
3.2. Polyphenol Extraction with PEG 8000
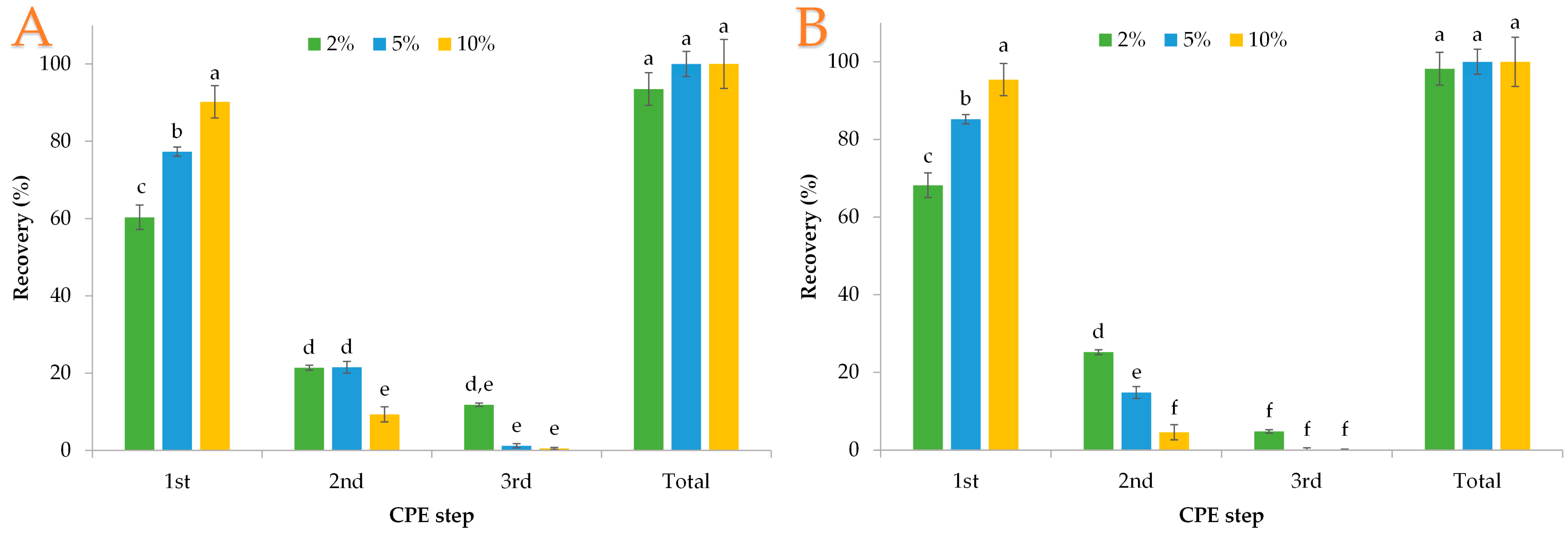
3.3. Polyphenol Extraction with Tween 80
3.4. Polyphenol Extraction with Lecithin
3.5. Total Polyphenol Content and Antiradical Activity of the Recovered Polyphenols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russ, W.; Meyer-Pittroff, R. Utilizing Waste Products from the Food Production and Processing Industries. Crit. Rev. Food Sci. Nutr. 2004, 44, 57–62. [Google Scholar] [CrossRef]
- Breida, M.; Younssi, S.A.; Ouammou, M.; Bouhria, M.; Hafsi, M.; Breida, M.; Younssi, S.A.; Ouammou, M.; Bouhria, M.; Hafsi, M. Pollution of Water Sources from Agricultural and Industrial Effluents: Special Attention to NO3−, Cr(VI), and Cu(II). In Water Chemistry; IntechOpen: London, UK, 2019; ISBN 978-1-78985-558-6. [Google Scholar]
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A Comprehensive Review on Comparison among Effluent Treatment Methods and Modern Methods of Treatment of Industrial Wastewater Effluent from Different Sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef]
- Barbera, M.; Gurnari, G. Wastewater Treatment and Reuse in the Food Industry; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 3-319-68442-6. [Google Scholar]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of Agro-industrial By-products, Effluents and Waste: Concept, Opportunities and the Case of Olive Mill Wastewaters. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on Oxidative Stabilisation of Lard by Natural Antioxidants Recovered from Olive-Oil Mill Wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Waindeskar, V.; Das, S.; Mudliar, S.N. Comparison of Coagulation, Ozone and Ferrate Treatment Processes for Color, COD and Toxicity Removal from Complex Textile Wastewater. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2017, 76, 1001–1010. [Google Scholar] [CrossRef]
- Park, C.; Hong, S.-W.; Chung, T.H.; Choi, Y.-S. Performance Evaluation of Pretreatment Processes in Integrated Membrane System for Wastewater Reuse. Desalination 2010, 250, 673–676. [Google Scholar] [CrossRef]
- Adebayo, F.O.; Obiekezie, S.O. Microorganisms in Waste Management. Res. J. Sci. Technol. 2018, 10, 28. [Google Scholar] [CrossRef]
- Peng, X.; Xu, H.; Yuan, X.; Leng, L.; Meng, Y. Mixed Reverse Micellar Extraction and Effect of Surfactant Chain Length on Extraction Efficiency. Sep. Purif. Technol. 2016, 160, 117–122. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Fan, H.; Zhu, D.; Wu, X.; Huang, X.; Tang, J. Separation and Purification of Alkaloids from Sophora Flavescens Ait. by Focused Microwave-Assisted Aqueous Two-Phase Extraction Coupled with Reversed Micellar Extraction. Ind. Crops Prod. 2016, 86, 231–238. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Freire, M.G.; Coutinho, J.A.P. Aqueous Two-Phase Systems: Towards Novel and More Disruptive Applications. Fluid Phase Equilibria 2020, 505, 112341. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-Based Processes for Wastewater Nutrient Recovery: Technology, Challenges, and Future Direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef]
- Chen, Y.; Du, K.; Li, J.; Bai, Y.; An, M.; Tan, Z.; Chang, Y. A Green and Efficient Method for the Preconcentration and Determination of Gallic Acid, Bergenin, Quercitrin, and Embelin from Ardisia Japonica Using Nononic Surfactant Genapol X-080 as the Extraction Solvent. Int. J. Anal. Chem. 2018, 2018, e1707853. [Google Scholar] [CrossRef] [PubMed]
- Al_Saadi, M.R.; Al-Garawi, Z.S.; Thani, M.Z. Promising Technique, Cloud Point Extraction: Technology & Applications. J. Phys. Conf. Ser. 2021, 1853, 012064. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Racheva, R.; Rahlf, A.F.; Wenzel, D.; Müller, C.; Kerner, M.; Luinstra, G.A.; Smirnova, I. Aqueous Food-Grade and Cosmetic-Grade Surfactant Systems for the Continuous Countercurrent Cloud Point Extraction. Sep. Purif. Technol. 2018, 202, 76–85. [Google Scholar] [CrossRef]
- Yazdi, A.S. Surfactant-Based Extraction Methods. TrAC Trends Anal. Chem. 2011, 30, 918–929. [Google Scholar] [CrossRef]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant Mediated Extraction of Total Phenolic Contents (TPC) and Antioxidants from Fruits Juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Reuse Potential of Vegetable Wastes (Broccoli, Green Bean and Tomato) for the Recovery of Antioxidant Phenolic Acids and Flavonoids. Int. J. Food Sci. Technol. 2017, 52, 98–107. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Kant, R. A Review on Peach (Prunus persica): An Asset of Medicinal Phytochemicals. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2186–2200. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; De La Rosa, L.A.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Phytochemical Composition and Health Aspects of Peach Products. In Dried Fruits; Alasalvar, C., Shahidi, F., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2013; pp. 309–324. ISBN 978-1-118-46466-3. [Google Scholar]
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-Density Lipoprotein Antioxidant Activity of Phenolic Compounds and Polyphenol Oxidase Activity in Selected Clingstone Peach Cultivars. J. Agric. Food Chem. 2000, 48, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dimou, C.; Karantonis, H.C.; Skalkos, D.; Koutelidakis, A.E. Valorization of Fruits By-Products to Unconventional Sources of Additives, Oil, Biomolecules and Innovative Functional Foods. Curr. Pharm. Biotechnol. 2019, 20, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus Dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Colak Gunes, N.; Gungor, E.; Gunes, M. Solar Process Heat for Sustainable Production of Concentrated Peach Puree. J. Food Process Eng. 2021, 44, e13744. [Google Scholar] [CrossRef]
- Santana, C.M.; Ferrera, Z.S.; Rodríguez, J.J.S. Use of Non-Ionic Surfactant Solutions for the Extraction and Preconcentration of Phenolic Compounds in Water Prior to Their HPLC-UV Detection. Analyst 2002, 127, 1031–1037. [Google Scholar] [CrossRef]
- Sosa Ferrera, Z.; Padrón Sanz, C.; Mahugo Santana, C.; Santana Rodrıíguez, J.J. The Use of Micellar Systems in the Extraction and Pre-Concentration of Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 2004, 23, 469–479. [Google Scholar] [CrossRef]
- Chatzilazarou, A.; Katsoyannos, E.; Gortzi, O.; Lalas, S.; Paraskevopoulos, Y.; Dourtoglou, E.; Tsaknis, J. Removal of Polyphenols from Wine Sludge Using Cloud Point Extraction. J. Air Waste Manag. Assoc. 2010, 60, 454–459. [Google Scholar] [CrossRef]
- Chatzilazarou, A.; Katsoyannos, E.; Lagopoulou, M.; Tsaknis, J. Application of Cloud Point Extraction with the Aid of Genapol X-080 in the Pre-Concentration of Lycopene and Total Carotenoids from Red Fleshed Orange. Nutrition 2011, 35, 10. [Google Scholar]
- Katsoyannos, E.; Chatzilazarou, A.; Gortzi, O.; Lalas, S.; Konteles, S.; Tataridis, P. Application of Cloud Point Extraction Using Surfactants in the Isolations of Physical Antioxidants (Phenols) from Olive Mill Wastewater. Fresenius Environ. Bull. 2006, 15, 4. [Google Scholar]
- Tsaknis, J.; Lalas, S. Extraction and Identification of Natural Antioxidant from Sideritis Euboea (Mountain Tea). J. Agric. Food Chem. 2005, 53, 6375–6381. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud Point Extraction of Phenolic Compounds from Pretreated Olive Mill Wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chatzilazarou, A.; Katsoyannos, E.; Papaconstandinou, S.; Dourtoglou, E. Recovery of Natural Antioxidants from Olive Mill Wastewater Using Genapol-X080. J. Am. Oil Chem. Soc. 2008, 85, 133–140. [Google Scholar] [CrossRef]
- Paleologos, E.K.; Giokas, D.L.; Karayannis, M.I. Micelle-Mediated Separation and Cloud-Point Extraction. TrAC Trends Anal. Chem. 2005, 24, 426–436. [Google Scholar] [CrossRef]
- Kori, S. Cloud Point Extraction Coupled with Back Extraction: A Green Methodology in Analytical Chemistry. Forensic Sci. Res. 2021, 6, 19–33. [Google Scholar] [CrossRef]
- He, J.; Zhao, Z.; Shi, Z.; Zhao, M.; Li, Y.; Chang, W. Analysis of Isoflavone Daidzein in Puerariae Radix with Micelle-Mediated Extraction and Preconcentration. J. Agric. Food Chem. 2005, 53, 518–523. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene Glycol (PEG): The Nature, Immunogenicity, and Role in the Hypersensitivity of PEGylated Products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) Based Microemulsions: Preclinical Drug Delivery, Toxicity and Antimicrobial Applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations; Joint FAO/WHO Expert Committee on Food Additives. Meeting (80th: 2015, R., Italy). In Evaluation of Certain Food Additives and Contaminants: Eightieth Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 995; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-120995-3. [Google Scholar]
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the Suitability of Low Hazard Surfactants for the Separation of Phenols and Carotenoids from Red-Flesh Orange Juice and Olive Mill Wastewater Using Cloud Point Extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Katsoyannos, E.; Chatzilazarou, A. Antioxidant Activity and Thermal Stability of Oleuropein and Related Phenolic Compounds of Olive Leaf Extract after Separation and Concentration by Salting-Out-Assisted Cloud Point Extraction. Antioxidants 2014, 3, 229–244. [Google Scholar] [CrossRef]
- Wang, M.; Yan, W.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Progress in the Application of Lecithins in Water-in-Oil Emulsions. Trends Food Sci. Technol. 2021, 118, 388–398. [Google Scholar] [CrossRef]
- European Parliament, Council of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (Text with EEA Relevance); European Parliament, Council of the European Union: Brussels, Belgium, 2008. [Google Scholar]
- van Nieuwenhuyzen, W. Lecithin and Other Phospholipids. In Surfactants from Renewable Resources; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 191–212. ISBN 978-0-470-68660-7. [Google Scholar]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic Composition and Antioxidant Properties of Different Peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of Common Analytical Methods for the Quantification of Total Polyphenols and Flavanols in Fruit Juices and Ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Torres-Aguirre, G.A.; Núñez-Gastélum, J.A.; de la Rosa, L.A.; Rodrigo-García, J.; Ayala-Zavala, J.F.; Álvarez-Parrilla, E. New approach to the interaction of Folin-Ciocalteu reagent with sugars during total polyphenol quantification. TIP Rev. Espec. Cienc. Quím.-Biológicas 2017, 20, 23–28. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC−DAD−ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Barros, H.R.D.M.; Ferreira, T.A.P.D.C.; Genovese, M.I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Redondo, D.; Gimeno, D.; Calvo, H.; Venturini, M.E.; Oria, R.; Arias, E. Antioxidant Activity and Phenol Content in Different Tissues of Stone Fruits at Thinning and at Commercial Maturity Stages. Waste Biomass Valorization 2021, 12, 1861–1875. [Google Scholar] [CrossRef]
- Manzoor, M.; Anwar, F.; Mahmood, Z.; Rashid, U.; Ashraf, M. Variation in Minerals, Phenolics and Antioxidant Activity of Peel and Pulp of Different Varieties of Peach (Prunus persica L.) Fruit from Pakistan. Molecules 2012, 17, 6491–6506. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Panagiotopoulou, M.; Papadaki, S.; Krokida, M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources 2023, 12, 72. [Google Scholar] [CrossRef]
- Grilo, A.L.; Raquel Aires-Barros, M.; Azevedo, A.M. Partitioning in Aqueous Two-Phase Systems: Fundamentals, Applications and Trends. Sep. Purif. Rev. 2016, 45, 68–80. [Google Scholar] [CrossRef]


| Sample Waste | Phase | TPC (mg GAE/L) | % DPPH• Scavenging |
|---|---|---|---|
| LPWS | Initial | 603.2 ± 21.1 a | 65.4 ± 4.7 a |
| CPE extract | 595.7 ± 20.5 a | 63.9 ± 1.9 a | |
| TWS | Initial | 50.3 ± 1.3 b | 6.1 ± 0.2 b |
| CPE extract | 49.4 ± 1 b | 5.7 ± 0.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291-305. https://doi.org/10.3390/biomass3030018
Giovanoudis I, Athanasiadis V, Chatzimitakos T, Kalompatsios D, Bozinou E, Gortzi O, Nanos GD, Lalas SI. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass. 2023; 3(3):291-305. https://doi.org/10.3390/biomass3030018
Chicago/Turabian StyleGiovanoudis, Ioannis, Vassilis Athanasiadis, Theodoros Chatzimitakos, Dimitrios Kalompatsios, Eleni Bozinou, Olga Gortzi, George D. Nanos, and Stavros I. Lalas. 2023. "Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method" Biomass 3, no. 3: 291-305. https://doi.org/10.3390/biomass3030018









