Thermokinetic Study of Catalytic Pyrolysis of Medium-Density Fiberboards over Beta-Zeolite-Supported Platinum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Thermogravimetric Analysis
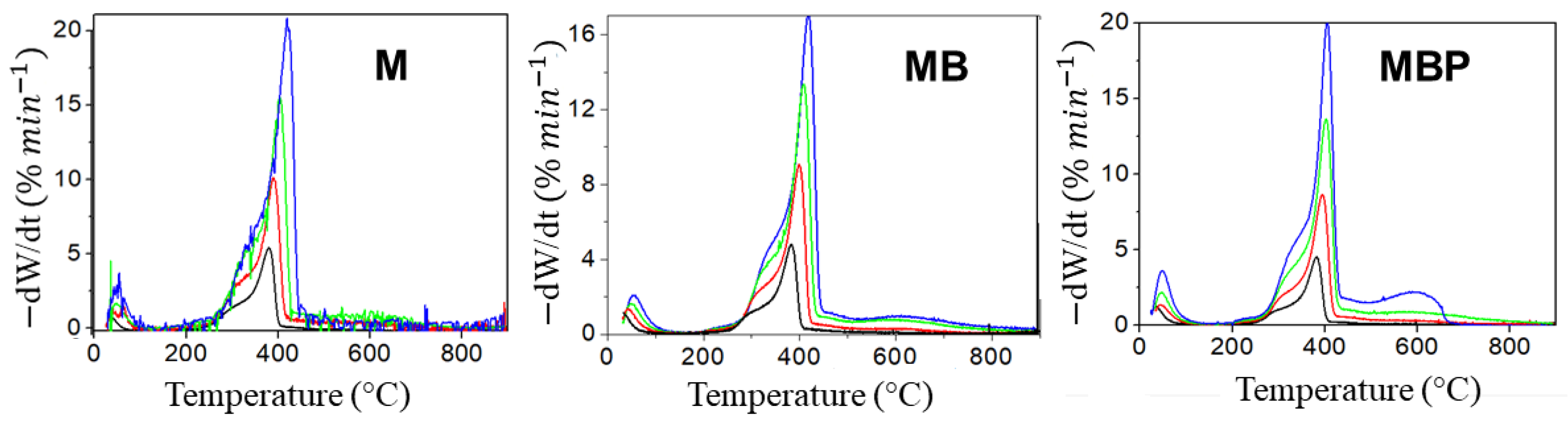
2.4. Thermokinetic Analysis
2.4.1. Flynn-Wall-Ozawa (FWO) Model
2.4.2. Kissinger-Ahakira-Sunose (KAS) Model
2.4.3. Friedman Model
2.4.4. Thermodynamic Parameters
3. Results and Discussion
3.1. Catalyst Characterization
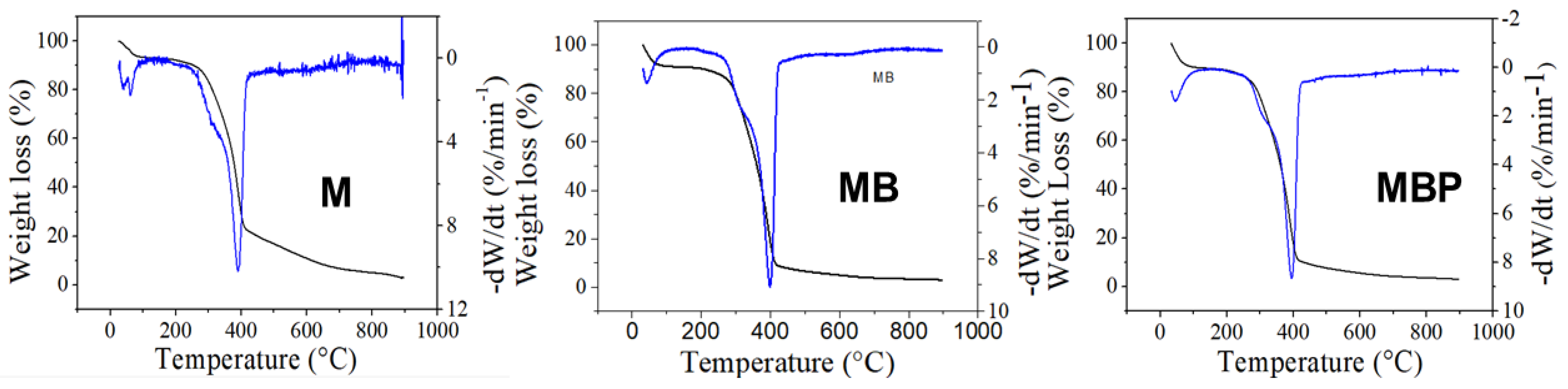
3.2. Thermogravimetry Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Q.-L.; Wu, B.; Pisutpaisal, N.; Wang, Y.-W.; Ma, K.-D.; Dai, L.-C.; Qin, H.; Tan, F.-R.; Maeda, T.; Xu, Y.-S.; et al. Bioenergy from dairy manure: Technologies, challenges and opportunities. Sci. Total Environ. 2021, 790, 148199. [Google Scholar] [CrossRef] [PubMed]
- Okolie, J.A.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Next-generation biofuels and platform biochemicals from lignocellulosic biomass. Int. J. Energy Res. 2021, 45, 14145–14169. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.; Das, P.; Deb, M. A comprehensive study on prospects of economy, environment, and efficiency of palm oil biodiesel as a renewable fuel. J. Clean. Prod. 2020, 286, 124981. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on Catalytic Hydrodeoxygenation of Pyrolysis Oil and Its Model Compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Seo, M.W.; Lee, S.H.; Nam, H.; Lee, D.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent advances of thermochemical conversion processes for biorefinery. Bioresour. Technol. 2021, 343, 126109. [Google Scholar] [CrossRef]
- Rangel, M.D.C.; Mayer, F.M.; Carvalho, M.D.S.; Saboia, G.; de Andrade, A.M. Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass 2023, 3, 31–63. [Google Scholar] [CrossRef]
- Rangel, M.C.; Carvalho, M.D.S.; Mayer, F.M.; Saboia, G.; de Andrade, A.M.; de Oliveira, A.P.S.; Santos, P.L.D. Improving Fast Pyrolysis by Tailoring High-Quality Products Using Catalysts. In Advances in Chemistry Research, 1st ed.; Taylor, J., Ed.; Nova Publishers: New York, NY, USA, 2022; Volume 75, pp. 119–169. [Google Scholar]
- Mayer, F.M.; de Oliveira, A.P.S.; Junior, D.L.d.O.; Agustini, B.C.; da Silva, G.A.; Tanabe, E.H.; Ruiz, D.; Rangel, M.D.C.; Zini, C.A. Influence of Nickel Modified Beta Zeolite in the Production of BTEX During Analytical Pyrolysis of Medium-Density Fiberboard (MDF). Waste Biomass Valoriz. 2021, 13, 1717–1729. [Google Scholar] [CrossRef]
- Carvalho, M.; Oliveira, A.P.; Mayer, F.; Virgens, C.; Rangel, M.D.C. Thermokinetic and Thermodynamic Parameters for Catalytic Pyrolysis of Medium Density Fiber over Ni/Beta Zeolite. Catal. Res. 2022, 2, 38. [Google Scholar] [CrossRef]
- Mayer, F.M.; Teixeira, C.M.; Pacheco, J.G.A.; de Souza, C.T.; Bauer, D.d.V.; Caramão, E.B.; Espíndola, J.d.S.; Trierweiler, J.O.; Lopez, O.W.P.; Zini, C.A. Characterization of analytical fast pyrolysis vapors of medium-density fiberboard (mdf) using metal-modified HZSM-5. J. Anal. Appl. Pyrolysis 2018, 136, 87–95. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Kan, T.; Weldekidan, H.; He, J.; Jahan, S. Investigating the Effect of Mono- and Bimetallic/Zeolite Catalysts on Hydrocarbon Production during Bio-oil Upgrading from Ex Situ Pyrolysis of Biomass. Energy Fuels 2019, 34, 389–400. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Yang, Q.; Williams, L.R.; Yang, H.; Zou, J.; Zeng, K.; Zhu, Y.; Chen, Y.; et al. Influence of physicochemical properties of metal modified ZSM-5 catalyst on benzene, toluene and xylene production from biomass catalytic pyrolysis. Bioresour. Technol. 2019, 278, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Camelo, E.R.; Castro, J.D.S.; das Virgens, C.F. Thermokinetic evaluation of zircon oxide green synthesis mediated by plant extract of Abelmoschus esculentus L. Moench. J. Therm. Anal. Calorim. 2022, 148, 49–62. [Google Scholar] [CrossRef]
- Santos, D.B.P.; de Jesus, M.F.; Júnior, J.M.F.; Pires, C.A.D.M. Determination of kinetic parameters for the sisal residue pyrolysis through thermal analysis. J. Ind. Eng. Chem. 2022, 109, 296–305. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Yusup, S.; Lam, M.K.; Chin, B.L.F.; Shahbaz, M.; Yamamoto, A.; Acda, M.N. The effect of industrial waste coal bottom ash as catalyst in catalytic pyrolysis of rice husk for syngas production. Energy Convers. Manag. 2018, 165, 541–554. [Google Scholar] [CrossRef]
- Brown, M.E. (Ed.) Introduction to Thermal Analysis: Techniques and Applications; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217. [Google Scholar] [CrossRef]
- Loiha, S.; Föttinger, K.; Zorn, K.; Klysubun, W.; Rupprechter, G.; Wittayakun, J. Catalytic enhancement of platinum supported on zeolite beta for toluene hydrogenation by addition of palladium. J. Ind. Eng. Chem. 2009, 15, 819–823. [Google Scholar] [CrossRef]
- Aboul-Gheit, A.K.; Aboul-Fotouh, S.M. Insight in cyclohexene hydroconversion process using catalysts containing 0.35% Pt on amorphous and zeolite supports. J. Taiwan Inst. Chem. Eng. 2012, 43, 711–717. [Google Scholar] [CrossRef]
- Grecco, S.; Gomes, L.; Reyes, P.; Oportus, M.; Rangel, M. Effect of platinum on the activity of zeolite-based catalysts. Stud. Surf. Sci. Catal. 2005, 158, 1937–1944. [Google Scholar] [CrossRef]
- Dirken, P.J.; Kentgens, A.P.M.; Nachtegaal, G.H.; van der Eerden, A.M.J.; Jansen, J.B.H. Solid-state MAS NMR study of pentameric aluminosilicate groups with 180 degrees intertetrahedral Al-O-Si angles in zunyite and harkerite. Am. Miner. 1995, 80, 39–45. [Google Scholar] [CrossRef]
- Teh, L.; Setiabudi, H.; Sidik, S.; Annuar, N.; Jalil, A. Synergic role of platinum (Pt) and molybdenum trioxide (MoO3) promoted HBEA zeolite towards n-heptane isomerization. Mater. Chem. Phys. 2021, 263, 124406. [Google Scholar] [CrossRef]
- Kunkeler, P.; Zuurdeeg, B.; van der Waal, J.; van Bokhoven, J.; Koningsberger, D.; van Bekkum, H. Zeolite Beta: The Relationship between Calcination Procedure, Aluminum Configuration, and Lewis Acidity. J. Catal. 1998, 180, 234–244. [Google Scholar] [CrossRef]
- Abraham, A.; Lee, S.-H.; Shin, C.-H.; Hong, S.B.; Prins, R.; van Bokhoven, J.A. Influence of framework silicon to aluminium ratio on aluminium coordination and distribution in zeolite Beta investigated by 27Al MAS and 27Al MQ MAS NMR. Phys. Chem. Chem. Phys. 2004, 6, 3031–3036. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Sanz, J.; Fornés, V.; Corma, A. 29Si and 27Al MAS NMR Study of Zeolite with Different Si/AI Ratios. J. Phys. Chem. 1990, 124, 217–223. [Google Scholar] [CrossRef]
- Stelzer, J.; Paulus, M.; Hunger, M.; Weitkamp, J. Hydrophobic properties of all-silica zeolite beta. Microporous Mesoporous Mater. 1998, 22, 1–8. [Google Scholar] [CrossRef]
- Castro, J.D.S.; da Silva, E.G.P.; Virgens, C.F. Evaluation of models to predict the influence of chemical pretreatment on the peels of Nephelium lappaceum L. based on pyrolysis kinetic parameters obtained using a combined Fraser-Suzuki function and Friedman’s isoconversional method. J. Anal. Appl. Pyrolysis 2020, 149, 104827. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic hydrogenation of cellulose-hemicellulose-lignin and biomass agricultural wastes for synthetic natural gas production. J. Anal. Appl. Pyrolysis 2019, 145, 104753. [Google Scholar] [CrossRef]
- Iqbal, A.; Noreen, N.; Imran, M.; Alves, J.L.F.; da Silva, J.C.G.; Badshah, S.L. Valorization of the biomass of Rhizoclonium hookeri through slow pyrolysis and its thermokinetic investigation for bioenergy potential. Biomass Bioenergy 2023, 168, 106690. [Google Scholar] [CrossRef]
- Carvalho, M.D.S.; das Virgens, C.F.; Carneiro, L.L.; da Silva, E.G.P.; das Chagas, T.P. Prediction of alkaline treatment effect on the slow pyrolysis of the Pachira aquatica Aubl. Fruit bark using artificial neural networks. Braz. J. Dev. 2020, 6, 80216–80235. [Google Scholar] [CrossRef]
- Aslan, D.I.; Özoğul, B.; Ceylan, S.; Geyikçi, F. Thermokinetic analysis and product characterization of Medium Density Fiberboard pyrolysis. Bioresour. Technol. 2018, 258, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Al-Balushi, F.A.; Burra, K.G.; Chai, Y.; Wang, M. Co-pyrolysis of waste tyre and pine bark: Study of reaction kinetics and mechanisms. Biomass Bioenergy 2023, 168, 106654. [Google Scholar] [CrossRef]
- Plata, D.B.; Infantes-Molina, A.; Rodríguez-Castellón, E. Study of bifunctionality of Pt/SBA-15 catalysts for HDO of Dibenzofuran reaction: Addition of Mo or use of an acidic support. Appl. Catal. A Gen. 2019, 580, 93–101. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Highly efficient catalytic pyrolysis of biomass vapors upgraded into jet fuel range hydrocarbon-rich bio-oil over a bimetallic Pt–Ni/γ-Al2O3 catalyst. Int. J. Hydrogen Energy 2021, 46, 27922–27940. [Google Scholar] [CrossRef]
- Pattanayak, S.; Hauchhum, L.; Loha, C.; Sailo, L.; Mishra, L. Experimental investigation on pyrolysis kinetics, reaction mechanisms and thermodynamic parameters of biomass and tar in N2 atmosphere. Sustain. Energy Technol. Assess. 2021, 48, 101632. [Google Scholar] [CrossRef]
- Bieniek, A.; Reinmöller, M.; Küster, F.; Gräbner, M.; Jerzak, W.; Magdziarz, A. Investigation and modelling of the pyrolysis kinetics of industrial biomass wastes. J. Environ. Manag. 2022, 319, 115707. [Google Scholar] [CrossRef]
- Gouda, N.; Panda, A.K. Determination of kinetic and thermodynamic parameters of thermal degradation of different biomasses for pyrolysis. Biocatal. Agric. Biotechnol. 2019, 21, 101315. [Google Scholar] [CrossRef]
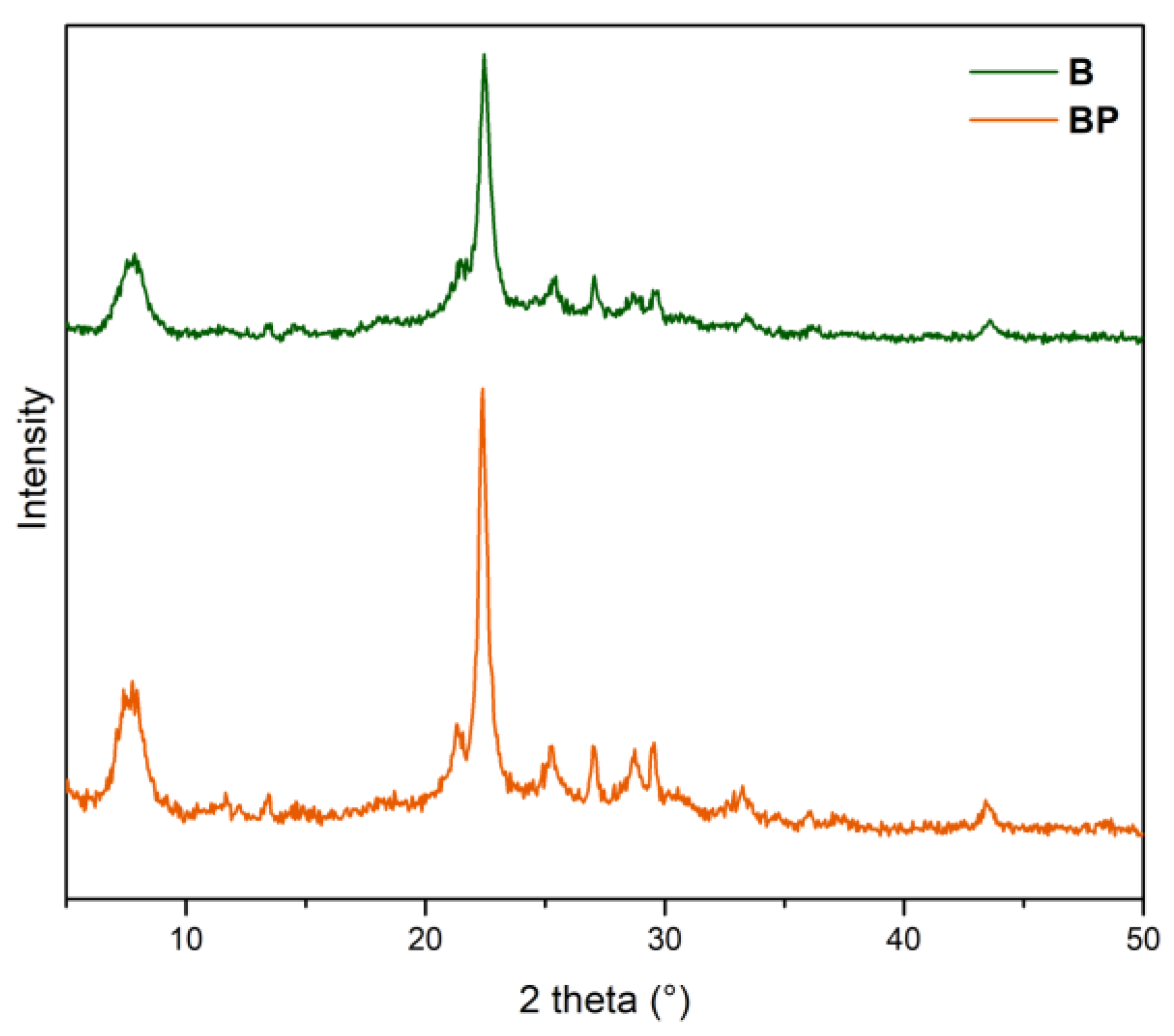
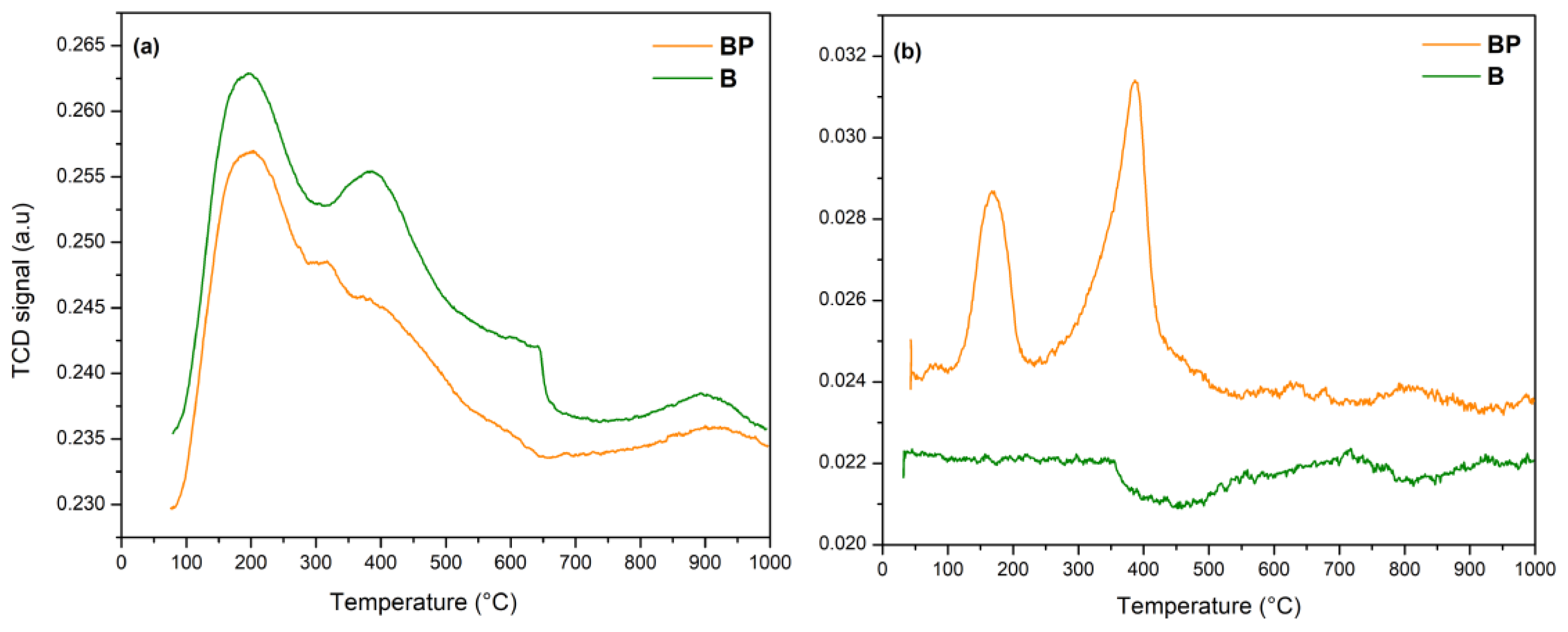


| Catalyst | Peak Number | Temperature (°C) | Hydrogen Amount (μmol·g−1) | Total Hydrogen Amount (μmol·g−1) |
|---|---|---|---|---|
| BP | 1 2 | 166.4 388.1 | 77.5 160.2 | 237.7 |
| Sample | Stage I (°C) | Stage II (°C) | Stage III (°C) | Biomass Decomposition (%) | Biochar (%) |
|---|---|---|---|---|---|
| M | 25.0–114.0 | 114.0–413.6 | 413.6–>900 | 97.08 | 2.92 |
| MB | 25.0–102.8 | 102.8–416.3 | 416.3–763.7 | 97.06 | 2.94 |
| MBP | 25.0–112.5 | 112.5–412.4 | 412.4–791.0 | 96.98 | 3.02 |
| α | Sample | |||||
|---|---|---|---|---|---|---|
| M | ||||||
| FWO | R2 | KAS | R2 | Friedman | R2 | |
| 0.2 | 120.34 | 0.9272 | 116.80 | 0.9155 | 116.30 | 0.9286 |
| 0.3 | 123.58 | 0.9666 | 119.78 | 0.9607 | 119.39 | 0.9693 |
| 0.4 | 127.44 | 0.9865 | 123.49 | 0.9841 | 123.38 | 0.9894 |
| 0.5 | 128.22 | 0.9926 | 124.06 | 0.9913 | 124.03 | 0.9948 |
| 0.6 | 127.90 | 0.9933 | 123.56 | 0.9920 | 123.82 | 0.9951 |
| 0.7 | 128.68 | 0.9968 | 124.21 | 0.9961 | 124.41 | 0.9980 |
| 0.8 | 100.61 | 0.8475 | 94.49 | 0.8157 | 94.94 | 0.9953 |
| Average | 122.40 | 0.9586 | 118.06 | 0.9501 | 118.04 | 0.9815 |
| MB | ||||||
| 0.2 | 101.32 | 0.9877 | 96.71 | 0.9850 | 96.43 | 0.9861 |
| 0.3 | 104.37 | 0.9887 | 99.58 | 0.9865 | 99.85 | 0.9877 |
| 0.4 | 109.43 | 0.9917 | 104.44 | 0.9899 | 104.99 | 0.9910 |
| 0.5 | 115.55 | 0.9946 | 110.63 | 0.9934 | 111.37 | 0.9944 |
| 0.6 | 118.77 | 0.9954 | 113.86 | 0.9944 | 114.86 | 0.9955 |
| 0.7 | 118.32 | 0.9950 | 113.24 | 0.9938 | 114.29 | 0.9949 |
| 0.8 | 84.39 | 0.9441 | 77.29 | 0.9272 | 78.48 | 0.9953 |
| Average | 107.45 | 0.9853 | 102.25 | 0.9658 | 102.90 | 0.9921 |
| MBP | ||||||
| 0.2 | 87.35 | 0.9400 | 82.26 | 0.9264 | 83.29 | 0.9384 |
| 0.3 | 107.56 | 0.9626 | 102.98 | 0.9553 | 104.56 | 0.9636 |
| 0.4 | 123.16 | 0.9802 | 118.99 | 0.9767 | 121.04 | 0.9820 |
| 0.5 | 138.57 | 0.9875 | 134.94 | 0.9855 | 137.25 | 0.9884 |
| 0.6 | 147.51 | 0.9863 | 144.16 | 0.9842 | 146.77 | 0.9868 |
| 0.7 | 145.48 | 0.9835 | 141.88 | 0.9809 | 144.56 | 0.9830 |
| 0.8 | 60.07 | 0.9867 | 51.52 | 0.9847 | 53.18 | 0.9876 |
| Average | 115.67 | 0.9753 | 110.96 | 0.9705 | 112.95 | 0.9757 |
| M | |||
|---|---|---|---|
| ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K) | |
| 0.2 | 179.25 | 110.79 | −0.10 |
| 0.3 | 181.43 | 117.80 | −0.10 |
| 0.4 | 181.94 | 119.39 | −0.09 |
| 0.5 | 185.28 | 121.36 | −0.10 |
| 0.6 | 188.77 | 123.38 | −0.10 |
| 0.7 | 189.58 | 123.77 | −0.10 |
| 0.8 | 220.58 | 124.03 | −0.15 |
| Average | 189.55 | 120.07 | −0.10 |
| MB | |||
| ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K) | |
| 0.2 | 90.84 | 192.42 | −0.15 |
| 0.3 | 92.62 | 191.00 | −0.15 |
| 0.4 | 94.27 | 187.52 | −0.14 |
| 0.5 | 96.47 | 182.87 | −0.13 |
| 0.6 | 99.40 | 181.60 | −0.12 |
| 0.7 | 102.73 | 184.71 | −0.12 |
| 0.8 | 105.78 | 222.90 | −0.17 |
| Average | 191.86 | 97.45 | −0.14 |
| MBP | |||
| ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K) | |
| 0.2 | 184.04 | 83.29 | −0.15 |
| 0.3 | 185.37 | 104.56 | −0.12 |
| 0.4 | 187.41 | 121.04 | −0.10 |
| 0.5 | 188.86 | 137.25 | −0.08 |
| 0.6 | 190.07 | 146.77 | −0.06 |
| 0.7 | 191.36 | 144.56 | −0.07 |
| 0.8 | 192.81 | 53.18 | −0.21 |
| Average | 188.59 | 112.95 | −0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, M.d.S.; Mayer, F.M.; de Oliveira, A.P.S.; Ruiz, D.; das Virgens, C.F.; Rangel, M.d.C. Thermokinetic Study of Catalytic Pyrolysis of Medium-Density Fiberboards over Beta-Zeolite-Supported Platinum. Biomass 2023, 3, 279-290. https://doi.org/10.3390/biomass3030017
Carvalho MdS, Mayer FM, de Oliveira APS, Ruiz D, das Virgens CF, Rangel MdC. Thermokinetic Study of Catalytic Pyrolysis of Medium-Density Fiberboards over Beta-Zeolite-Supported Platinum. Biomass. 2023; 3(3):279-290. https://doi.org/10.3390/biomass3030017
Chicago/Turabian StyleCarvalho, Mateus da Silva, Francieli Martins Mayer, Ana Paula Stelzer de Oliveira, Doris Ruiz, Cesário Francisco das Virgens, and Maria do Carmo Rangel. 2023. "Thermokinetic Study of Catalytic Pyrolysis of Medium-Density Fiberboards over Beta-Zeolite-Supported Platinum" Biomass 3, no. 3: 279-290. https://doi.org/10.3390/biomass3030017





