Effective Upgrading of Levulinic Acid into Hexyl Levulinate Using AlCl3·6H2O as a Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Reactants, Products, and AlCl3·6H2O
2.3. Esterification Reaction of Levulinic Acid with 1-Hexanol Using AlCl3·6H2O as Catalyst
3. Results
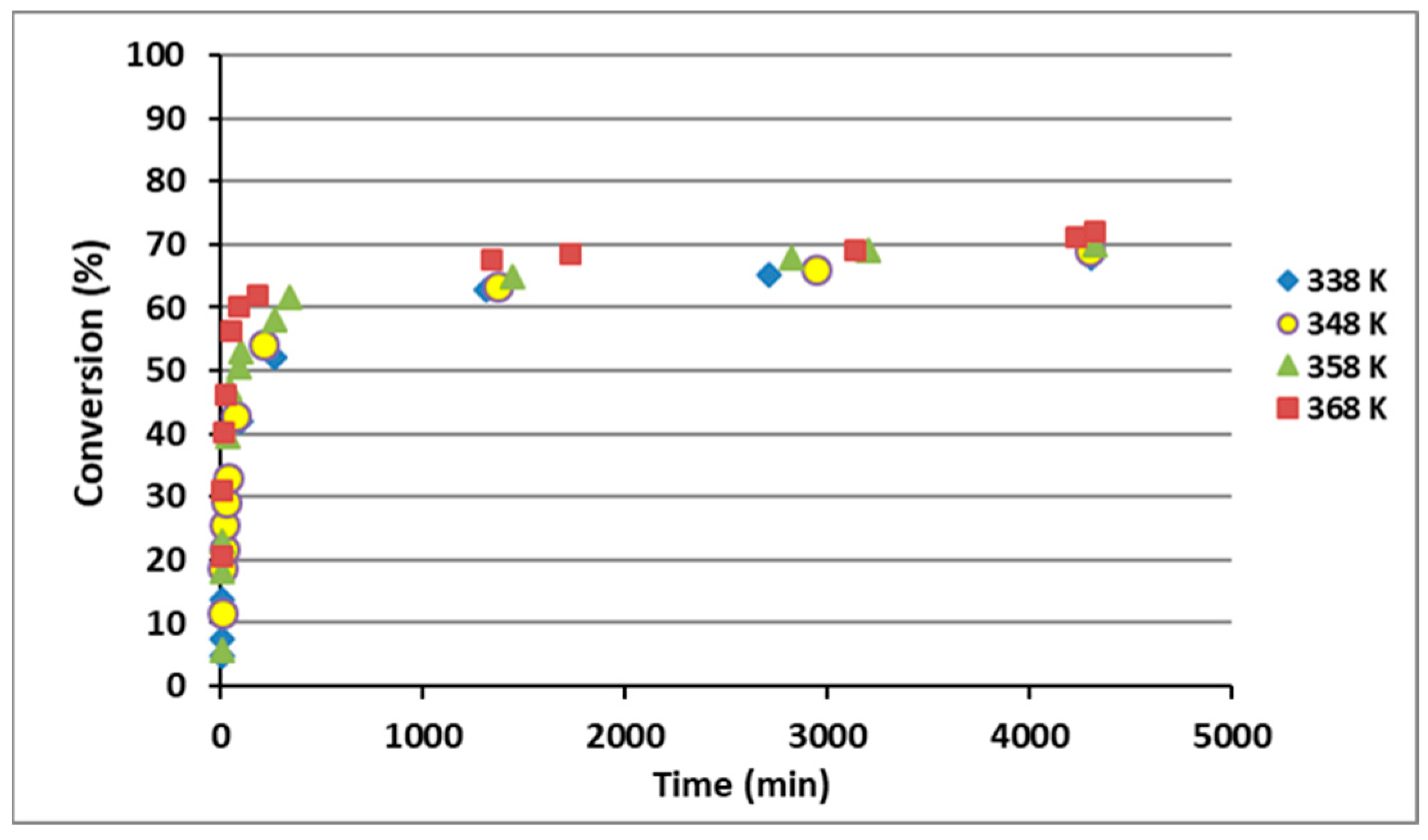
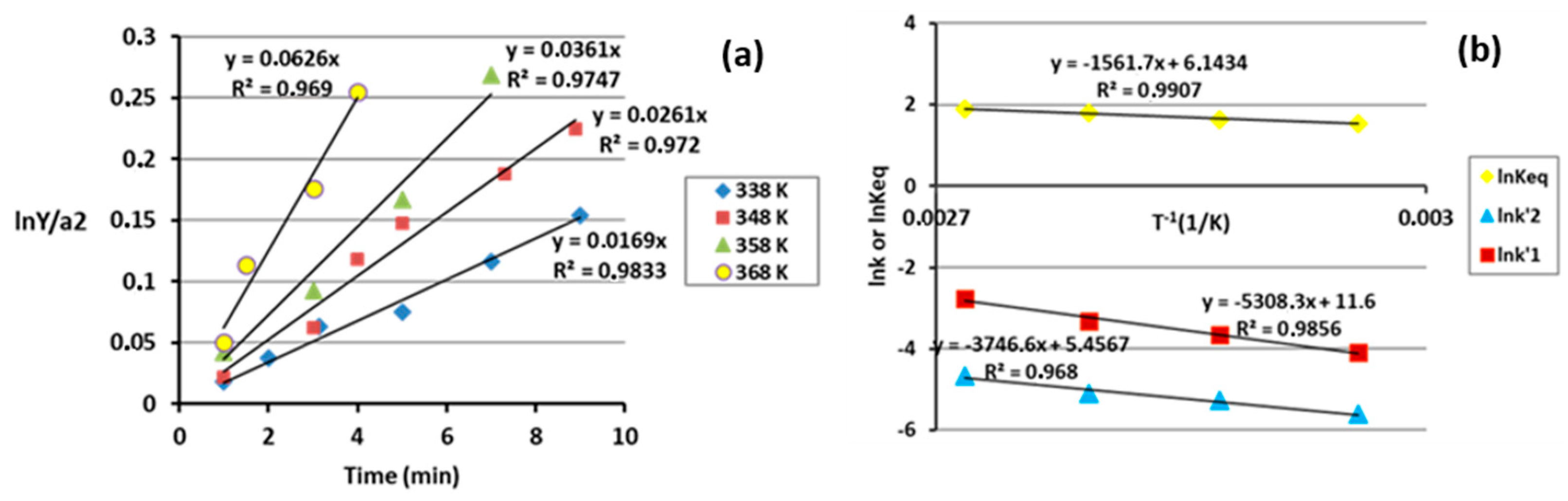
3.1. Kinetic and Thermodynamic Study of the Esterification Reaction of Levulinic Acid with 1-Hexanol
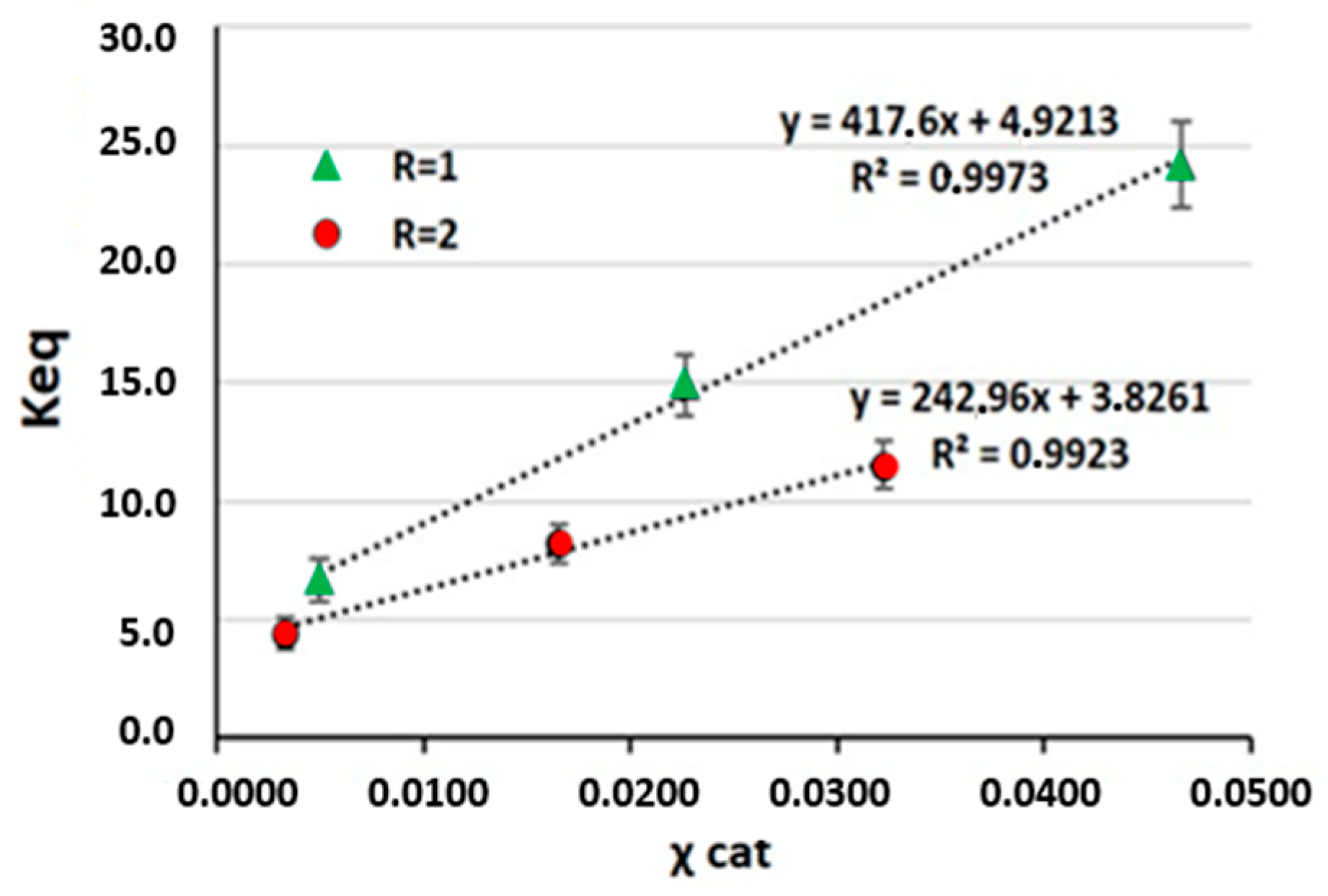
3.2. Effect of the Amounts of 1-Hexanol and AlCl3·6H2O on the Reaction Yield
3.3. Catalyst Recoverability and Reusability
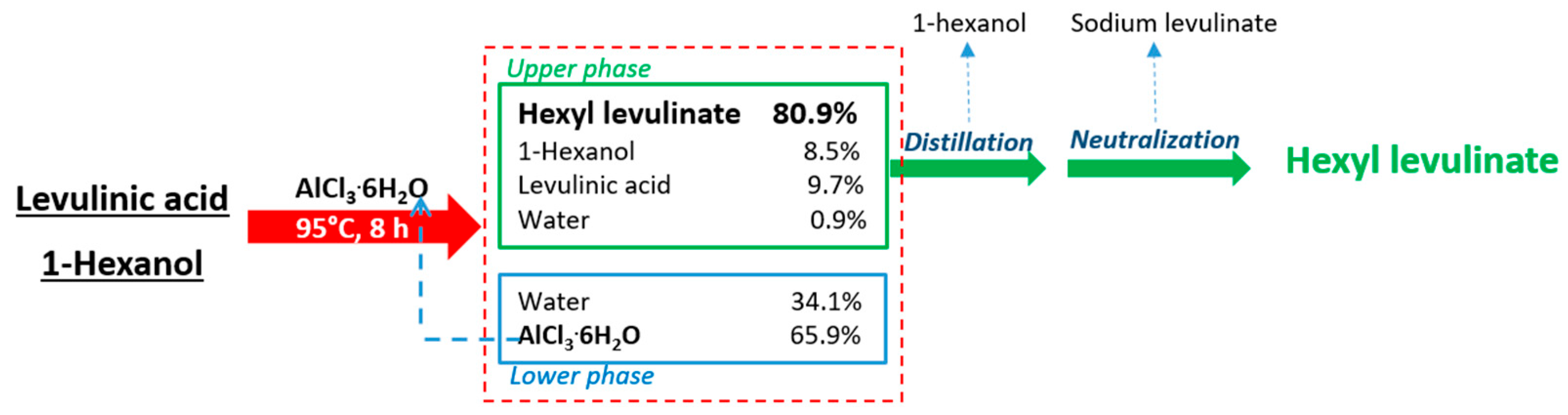
3.4. Energy Demand for the Production and Purification of Hexyl Levulinate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shukla, P.R.; Slade, J.S.; Al Khourdajie, A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Vyas, P.; Fradera, R.; Belkacemi, M.; et al. IPCC 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring Global Carbon Emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, L.; Guo, R.; Cao, X.; Ni, X.; Zhang, W. Accelerating the Energy Transition to Achieve Carbon Neutrality. Resour. Conserv. Recycl. 2022, 177, 105957. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Shahadat Hossain, M.; Maurya, R.; Yang, Y.; Singh, V.; Kumar, D.; Salama, E.S.; Sun, X.; Sindhu, R.; et al. Recent Advances in Lignocellulosic and Algal Biomass Pretreatment and Its Biorefinery Approaches for Biochemicals and Bioenergy Conversion. Bioresour. Technol. 2023, 367, 128281. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass for Biorefinery Applications: A Review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Kumar, V.; Ganeshan, P.; Rajendran, K.; Shekhar Giri, B.; Gómez-García, R.; Li, H.; Zhang, Z.; Sindhu, R.; et al. Sustainable Biorefinery Approaches towards Circular Economy for Conversion of Biowaste to Value Added Materials and Future Perspectives. Fuel 2022, 325, 124846. [Google Scholar] [CrossRef]
- Du, R.; Li, C.; Lin, W.; Lin, C.S.K.; Yan, J. Domesticating a Bacterial Consortium for Efficient Lignocellulosic Biomass Conversion. Renew. Energy 2022, 189, 359–368. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Wang, Y.; Buvat, J.C.; Pan, Y.; Casson Moreno, V.; Leveneur, S. Production of Levulinic Acid and Alkyl Levulinates: A Process Insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Angelini, A.; Scelsi, E.; Ancona, V.; Aimola, G.; Pastore, C. Performic Acid Pre-Treatment of Poplar Biomasses Grown on a Contaminated Area for Enhanced Enzymatic Digestibility: A Viable Route to Obtain Fine-Products and Recovery of Contaminants. J. Clean. Prod. 2022, 369, 133346. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Galletti, A.M.R. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Ma, J.; Wang, D.; Wang, K.; Zheng, Q.; Song, C.; Guo, X. Fast and Efficient Upgrading of Levulinic Acid into Long-Chain Alkyl Levulinate Fuel Additives with a Tungsten Salt Catalyst at Low Temperature. Sustain. Energy Fuels 2020, 4, 2018–2025. [Google Scholar] [CrossRef]
- Glińska, K.; Lerigoleur, C.; Giralt, J.; Torrens, E.; Bengoa, C. Valorization of Cellulose Recovered from Wwtp Sludge to Added Value Levulinic Acid with a Brønsted Acidic Ionic Liquid. Catalysts 2020, 10, 1004. [Google Scholar] [CrossRef]
- Liu, S.; Wang, K.; Yu, H.; Li, B.; Yu, S. Catalytic Preparation of Levulinic Acid from Cellobiose via Brønsted-Lewis Acidic Ionic Liquids Functional Catalysts. Sci. Rep. 2019, 9, 1810. [Google Scholar] [CrossRef] [Green Version]
- Mohammadbagheri, Z.; Chermahini, N. Direct Production of Hexyl Levulinate as a Potential Fuel Additive from Glucose Catalyzed by Modified Dendritic Fibrous Nanosilica. Renew. Energy 2020, 147, 2229–2237. [Google Scholar] [CrossRef]
- Pithadia, D.; Patel, A.U.; Hatiya, V. 12-Tungstophosphoric Acid Anchored to MCM-22, as a Novel Sustainable Catalyst for the Synthesis of Potential Biodiesel Blend, Levulinate Ester. Renew. Energy 2021, 187, 933–943. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Z.; Chang, X.; Lin, L.; Yan, X.; Sun, Y.; Shi, X.; He, X.; Zhu, J. Performance and Emission Characteristics of a Diesel Engine Running on Optimized Ethyl Levulinate-Biodiesel-Diesel Blends. Energy 2016, 95, 29–40. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Thermo-Chemical Energy Assessment for Production of Energy-Rich Fuel Additive Compounds by Using Levulinic Acid and Immobilized Lipase. Fuel Process. Technol. 2015, 138, 139–146. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Selvaraj, M.; Rajabathar, J.R.; Khoerunnisa, F.; Rigolet, S.; Daou, T.J.; Maireles-Torres, P.; El-Bahy, S.M.; El-Bahy, Z.M.; Ng, E.P. Highly Efficient Non-Microwave Instant Heating Synthesis of Hexyl Levulinate Fuel Additive Enhanced by Sulfated Nanosilica Catalyst. Microporous Mesoporous Mater. 2022, 331, 111645. [Google Scholar] [CrossRef]
- Pastore, C.; D’Ambrosio, V. Intensification of Processes for the Production of Ethyl Levulinate Using AlCl3·6H2O. Energies 2021, 14, 1273. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Ambrosio, V.D.; Pastore, C. A Novel and Efficient Method for the Synthesis of Methyl (R)-10-Hydroxystearate and FAMEs from Sewage Scum. Catalysts 2021, 11, 663. [Google Scholar] [CrossRef]
- Morawala, D.H.; Dalai, A.K.; Maheria, K.C. Synthesis of N-Butyl Levulinate Using Mesoporous Zeolite H-BEA Catalysts with Different Catalytic Characteristics. Catal. Lett. 2020, 150, 1049–1060. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, H.; Zhou, Y.; Bai, Z.; Bao, X. Pure-Phase Zeolite Beta Synthesized from Natural Aluminosilicate Minerals and Its Catalytic Application for Esterification. Appl. Clay Sci. 2016, 126, 1–6. [Google Scholar] [CrossRef]
- Kokare, M.B.; Ranjani, V.; Mathpati, C.S. Response Surface Optimization, Kinetic Study and Process Design of n-Butyl Levulinate Synthesis. Chem. Eng. Res. Des. 2018, 137, 577–588. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabrés i Xamena, F.X. Conversion of Levulinic Acid into Chemicals: Synthesis of Biomass Derived Levulinate Esters over Zr-Containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Zhao, W.; Wen, S.; Xiang, Y.; Liu, X. A New Sulfonic Acid-Functionalized Organic Polymer Catalyst for the Synthesis of Biomass-Derived Alkyl Levulinates. Catal. Lett. 2020, 150, 3553–3560. [Google Scholar] [CrossRef]
- Yang, F.; Tang, J. Catalytic Upgrading of Renewable Levulinic Acid to Levulinate Esters Using Perchloric Acid Decorated Nanoporous Silica Gels. ChemistrySelect 2019, 4, 1403–1409. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhanja, P.; Salam, N.; Khatun, R.; Bhaumik, A.; Islam, S.M. Porous Iron-Phosphonate Nanomaterial as an Efficient Catalyst for the CO2 Fixation at Atmospheric Pressure and Esterification of Biomass-Derived Levulinic Acid. Catal. Today 2018, 309, 253–262. [Google Scholar] [CrossRef]
- Kalghatgi, S.G.; Bhanage, B.M. Green Syntheses of Levulinate Esters Using Ionic Liquid 1-Methyl Imidazolium Hydrogen Sulphate [MIM][HSO 4] in Solvent Free System. J. Mol. Liq. 2019, 281, 70–80. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Green Synthesis of Amyl Levulinate Using Lipase in the Solvent Free System: Optimization, Mechanism and Thermodynamics Studies. Catal. Today 2021, 375, 120–131. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Pastore, C. Metal Hydrated-Salts as Efficient and Reusable Catalysts for Pre-Treating Waste Cooking Oils and Animal Fats for an Effective Production of Biodiesel. Renew. Energy 2019, 143, 1193–1200. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; Martinez, G.; Jones, E.; Bertin, L.; Pastore, C. Ethyl Hexanoate Rich Stream from Grape Pomace: A Viable Route to Obtain Fine Chemicals from Agro by-Products. Sep. Purif. Technol. 2023, 309, 123100. [Google Scholar] [CrossRef]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Di Capua, R.; Astuto, F.; Riela, S.; Nacci, A.; Casiello, M.; Testa, M.L.; Liotta, L.F.; et al. Science of the Total Environment Life Cycle Assessment of a System for the Extraction and Transformation of Waste Water Treatment Sludge (WWTS)-Derived Lipids into Biodiesel. Sci. Total Environ. 2023, 883, 163637. [Google Scholar] [CrossRef]
- Banchero, M.; Gozzelino, G. A Simple Pseudo-Homogeneous Reversible Kinetic Model for the Esterification of Different Fatty Acids with Methanol in the Presence of Amberlyst-15. Energies 2018, 11, 1843. [Google Scholar] [CrossRef] [Green Version]
- Hartman, M.; Trnka, O.; Šolcová, O. Thermal Decomposition of Aluminum Chloride Hexahydrate. Ind. Eng. Chem. Res. 2005, 44, 6591–6598. [Google Scholar] [CrossRef]
- Ariba, H.; Wang, Y.; Devouge-Boyer, C.; Stateva, R.P.; Leveneur, S. Physicochemical Properties for the Reaction Systems: Levulinic Acid, Its Esters, and γ-Valerolactone. J. Chem. Eng. Data 2020, 65, 3008–3020. [Google Scholar] [CrossRef]
- Atrashenok, T.R.; Nesterov, N.A.; Zhuk, I.P.; Peshchenko, A.D. Measured Specific Heats of Hexan-1-Ol and 3-Methyl-2-Butanol over Wide Temperature Ranges. J. Eng. Phys. 1991, 61, 1038–1041. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V.; Uchytilová, V.; Finke, M. Enthalpies of Vaporization of Aliphatic C5 and C6 Alcohols. Fluid Phase Equilib. 1985, 20, 111–118. [Google Scholar] [CrossRef]
- Harwardt, A.; Kraemer, K.; Rüngeler, B.; Marquardt, W. Conceptual Design of a Butyl-Levulinate Reactive Distillation Process by Incremental Refinement. Chin. J. Chem. Eng. 2011, 19, 371–379. [Google Scholar] [CrossRef]
- Bart, H.J.; Reidetschläger, J.; Schatka, K.; Lehmann, A. Kinetics of Esterification of Levulinic Acid with N-Butanol by Homogeneous Catalysis. Ind. Eng. Chem. Res. 1994, 33, 21–25. [Google Scholar] [CrossRef]
- Pastore, C.; Barca, E.; Del Moro, G.; Lopez, A.; Mininni, G.; Mascolo, G. Recoverable and Reusable Aluminium Solvated Species Used as a Homogeneous Catalyst for Biodiesel Production from Brown Grease. Appl. Catal. A Gen. 2015, 501, 48–55. [Google Scholar] [CrossRef]
- Bährle-Rapp, M. Sodium Levulinate. In Springer Lexikon Kosmetik und Körperpflege; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Cui, L.; Cheng, F.; Zhou, J. Preparation of High Purity AlCl3·6H2O Crystals from Coal Mining Waste Based on Iron(III) Removal Using Undiluted Ionic Liquids. Sep. Purif. Technol. 2016, 167, 45–54. [Google Scholar] [CrossRef]
- Wang, J.; Petit, C.; Zhang, X.; Cui, S. Phase Equilibrium Study of the AlCl3-CaCl2-H2O System for the Production of Aluminum Chloride Hexahydrate from Ca-Rich Flue Ash. J. Chem. Eng. Data 2016, 61, 359–369. [Google Scholar] [CrossRef]
- Ismail, N.I.F.; Sulaiman, S.; Kabbashi, N.A.; Sulaiman, S.Z. Synthesis of Aluminum Chloride Hexahydrate/Polyvinyl Alcohol Catalyst for Biodiesel Production. Mater. Today Proc. 2020, 47, 1273–1279. [Google Scholar] [CrossRef]


| 338 K | 348 K | 358 K | 368 K | |
|---|---|---|---|---|
| Keq | 4.6 | 5.1 | 6.0 | 6.7 |
| kf | 0.0169 | 0.0261 | 0.0361 | 0.0631 |
| kr | 0.0036 | 0.0051 | 0.0060 | 0.0094 |
| Ea (KJ/mol) | Ea−1 (KJ/mol) | ||
|---|---|---|---|
| 43.3 | 29.6 | 13.7 | 53.3 |
| Temperature (K) | Conversion (%) | Phase Distribution (% wt) | Phase Composition (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top Phase | Bottom Phase | ||||||||||||
| Top | Bottom | A | H | C | W | L | A | H | C | W | L | ||
| 338 | 68.3 ± 0.4 | 96.8 | 3.2 | 23.8 | 14.2 | - | 2.4 | 59.6 | - | - | 34.2 | 65.8 | - |
| 348 | 69.4 ± 0.4 | 96.8 | 3.2 | 21.6 | 13.6 | - | 2.4 | 62.4 | - | - | 34.2 | 65.8 | - |
| 358 | 71.1 ± 0.5 | 96.7 | 3.3 | 17.4 | 13.2 | - | 2.4 | 63.0 | - | - | 35.3 | 64.7 | - |
| 368 | 72.2 ± 0.5 | 96.5 | 3.5 | 15.7 | 12.6 | - | 2.8 | 69.0 | - | - | 34.3 | 65.5 | - |
| R | Catalyst (% mol) | Conversion (%) | Phase Distribution (% wt) | Phase Composition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Top Phase | Bottom Phase | |||||||||
| Top | Bottom | A | H | W | L | C | W | |||
| 1 | 1 | 72.2 | 96.8 | 3.2 | 15.7 | 12.6 | 2.8 | 69.0 | 34.6 | 65.4 |
| 1 | 5 | 76.5 | 90.1 | 9.9 | 14.0 | 8.3 | 1.8 | 76.4 | 52.9 | 47.1 |
| 1 | 10 | 83.1 | 84.7 | 15.3 | 9.7 | 8.5 | 0.9 | 80.9 | 65.1 | 34.9 |
| 2 | 1 | 85.5 | 97.9 | 2.1 | 5.3 | 37.0 | 3.5 | 54.2 | 35.6 | 64.4 |
| 2 | 5 | 90.8 | 93.1 | 6.9 | 3.4 | 36.0 | 2.2 | 58.7 | 52.6 | 47.4 |
| 2 | 10 | 92.5 | 88.3 | 11.7 | 2.7 | 33.8 | 1.4 | 61.8 | 59.9 | 40.1 |
| System | R | Catalyst (% mol) | χ Cat (mol cat/mol tot) | Conversion (%) | Keq |
|---|---|---|---|---|---|
| S1 | 1 | 1 | 0.0050 | 72.2 | 6.7 |
| S2 | 1 | 5 | 0.0164 | 76.5 | 14.9 |
| S3 | 1 | 10 | 0.0323 | 83.1 | 24.2 |
| S4 | 2 | 1 | 0.0050 | 85.5 | 4.4 |
| S5 | 2 | 5 | 0.0164 | 90.8 | 8.2 |
| S6 | 2 | 10 | 0.0323 | 92.5 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, V.; Pastore, C. Effective Upgrading of Levulinic Acid into Hexyl Levulinate Using AlCl3·6H2O as a Catalyst. Biomass 2023, 3, 266-278. https://doi.org/10.3390/biomass3030016
D’Ambrosio V, Pastore C. Effective Upgrading of Levulinic Acid into Hexyl Levulinate Using AlCl3·6H2O as a Catalyst. Biomass. 2023; 3(3):266-278. https://doi.org/10.3390/biomass3030016
Chicago/Turabian StyleD’Ambrosio, Valeria, and Carlo Pastore. 2023. "Effective Upgrading of Levulinic Acid into Hexyl Levulinate Using AlCl3·6H2O as a Catalyst" Biomass 3, no. 3: 266-278. https://doi.org/10.3390/biomass3030016






