Surface and Optical Properties of Gd-Doped ZrO2 Nano Films †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Structure
3.2. Surface Morphology
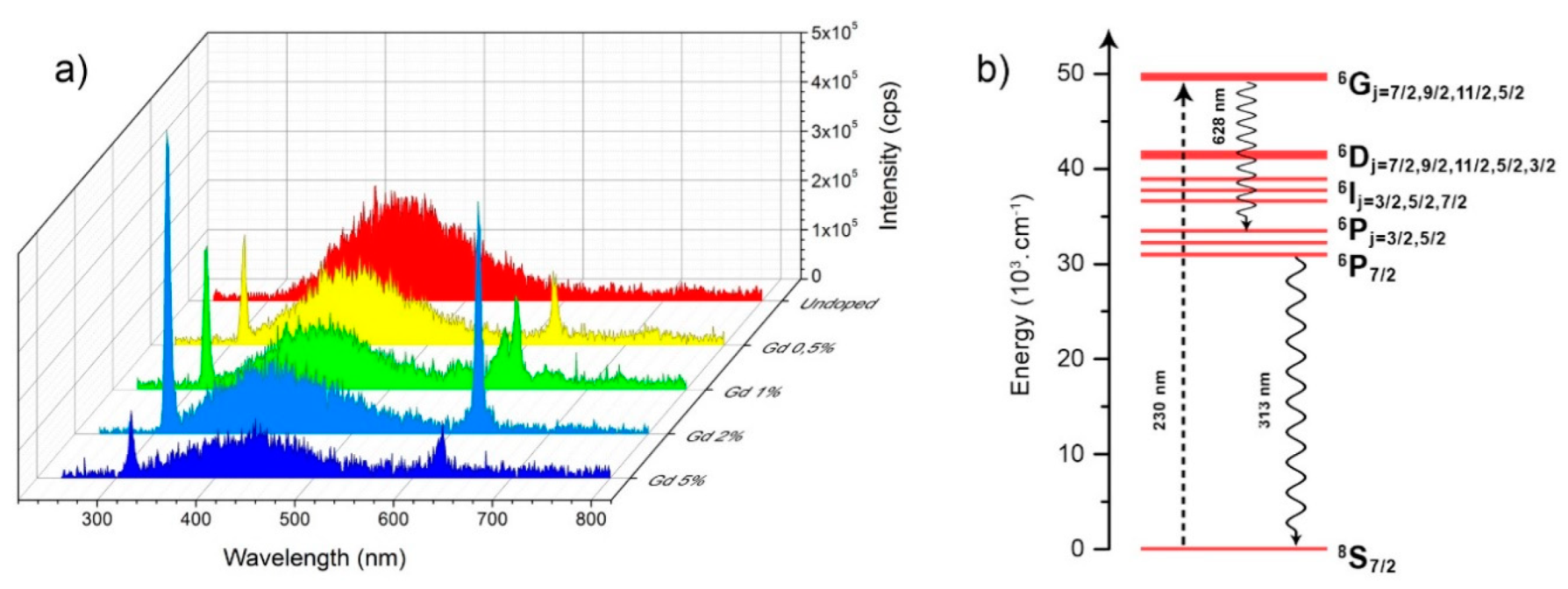
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Izumi, K.; Minami, N.; Uchida, Y. Sol-Gel-Derived Coatings on Steel Sheets. Key Eng. Mater. 1998, 150, 77–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, G.; Shi, Z. Tribological properties of ZrO2 nanofilms coated on stainless steel in a 5% NaCl solution, distilled water and a dry environment. Surf. Coat. Technol. 2018, 350, 128–135. [Google Scholar] [CrossRef]
- Dankeaw, A.; Poungchan, G.; Panapoy, M.; Ksapabutr, B. In-situ one-step method for fabricating three-dimensional grass-like carbon-doped ZrO2 films for room temperature alcohol and acetone sensors. Senors Actuators B Chem. 2017, 242, 202–214. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Kirubaharan, K.; Dharini, T.; Muthaiah, B. Corrosion and biocompatibility behaviour of zirconia coating by EBPVD for biomedical applications. Surf. Coat. Technol. 2018, 334, 336–343. [Google Scholar] [CrossRef]
- Berlin, I.J.; Lekshmy, S.S.; Ganesan, V.; Thomas, P.V.; Joy, K. Effect of Mn doping on the structural and optical properties of ZrO2 thin films prepared by sol–gel method. Thin Solid Films 2014, 550, 199–205. [Google Scholar] [CrossRef]
- Lakshmi, J.S.; Berlin, I.J.; Daniel, G.P.; Thomas, V.P.; Joy, K. Effect of calcination atmosphere on photoluminescence properties of nanocrystalline ZrO2 thin films prepared by sol–gel dip coating method. Phys. B 2011, 406, 3050–3055. [Google Scholar] [CrossRef]
- Salas, P.; Angeles-Chavez, C.; Montoya, J.A.; De la Rosa, E.; Dıaz-Torres, L.A.; Martınez, A.; Romero-Romo, M.A.; Morales, J. Synthesis, characterization and luminescence properties of ZrO2:Yb3+-Er3+ nanophosphor. Opt. Mater. 2005, 27, 1295–1300. [Google Scholar] [CrossRef]
- Lovisa, L.X.; Araújo, V.D.; Tranquilin, R.L.; Longo, E.; Li, M.S.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. White photoluminescence emission from ZrO2 co-doped with Eu3+,Tb3+ and Tm3+. J. Alloys Comp. 2016, 674, 245–251. [Google Scholar] [CrossRef]
- Córdova-Martínez, W.; De la Rosa-Cruz, E.; Díaz-Torres, L.A.; Salas, P.; Montoya, A.; Avendaño, M.; Rodríguez, R.A.; Barbosa-García, O. Nanocrystalline tetragonal zirconium oxide stabilization at low temperatures by using rare earth ions: Sm3+ and Tb3+. Opt. Mater. 2002, 20, 263–271. [Google Scholar] [CrossRef]
- Lazarova, K.; Vasileva, M.; Marinov, G.; Babeva, T. Optical characterization of sol–gel derived Nb2O5 thin films. Opt. Laser Technol. 2014, 58, 114–118. [Google Scholar] [CrossRef]
- Lin, C.K.; Zhang, C.M.; Lin, J. Phase transformation and photoluminescence properties of nanocrystalline ZrO2 powders prepared via the Pechini-type sol-gel process. J. Phys. Chem. C 2007, 111, 3300–3307. [Google Scholar] [CrossRef]
- Zhong, J.; Liang, H.; Su, Q.; Zhou, J.; Huang, Y.; Gao, Z.; Tao, Y.; Wang, J. Luminescence properties of NaGd(PO3)4:Eu3+ and energy transfer from Gd3+ to Eu3+. Appl. Phys. B 2010, 98, 139–147. [Google Scholar] [CrossRef]
- Tjioe, M.; Gerritsen, M.J.P.; Juhlin, L.; Van De Kerkhof, P.C.M. Treatment of vitiligo vulgaris with narrow band UV-B (311 nm) for one year and the effect of addition of folic acid and vitamin B12. Acta Derm. Venereol. 2002, 82, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Höningsmann, H. Phototherapy for psoriasis. Clin. Exp. Dermatol. 2001, 26, 343–350. [Google Scholar] [CrossRef]
- Reich, A.; Mędrek, K. Effects of narrow band UVB (311 nm) irradiation on epidermal cells. Int. J. Mol. Sci. 2013, 14, 8456–8466. [Google Scholar] [CrossRef]
- Takada, A.; Matsushita, K.; Horioka, S.; Furuichi, Y.; Sumi, Y. Bactericidal effects of 310 nm ultraviolet light-emitting diode irradiation on oral bacteria. BMC Oral Health 2017, 17, 96. [Google Scholar] [CrossRef]
- Berneburg, M.; Röcken, M.; Benedix, F. Phototherapy with narrowband vs broadband UVB. Acta Derm. Venereol. 2005, 85, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, S.; Nair, G.B.; Dhoble, S.J.; Burghate, D.K. Energy transfer from Pr3+ to Gd3+ ions in BaB8O13 phosphor for phototherapy lamps. Phys. B 2018, 535, 232–236. [Google Scholar] [CrossRef]
- Motloung, S.V.; Motaung, T.E.; Hlatshwayo, T.T.; Koao, L.F.; Malevu, T.D.; Mpelane, S. Associated aspects on structure, morphology and photoluminescence of MgAl2O4:x% Gd3+ nanophosphor prepared via citrate sol-gel method. J. Electron. Mater. 2019, 48, 3947–3957. [Google Scholar] [CrossRef]




| Undoped ZrO2 | Gd 1% | Gd 5% | |
|---|---|---|---|
| Rq at 2 µm measurement | 0.702 | 0.819 | 0.910 |
| Rq at 5 µm measurement | 0.618 | 0.856 | 0.940 |
| % of Gd | Thickness | n | k |
|---|---|---|---|
| Undoped | 106.3 | 1.83 | 0.004 |
| 0.5% | 104.3 | 1.84 | 0.006 |
| 1% | 106.9 | 1.84 | 0.003 |
| 2% | 125.0 | 1.81 | 0.002 |
| 5% | 136.1 | 1.63 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, O.; Stambolova, I.; Vassilev, S.; Lazarova, K.; Simeonova, S. Surface and Optical Properties of Gd-Doped ZrO2 Nano Films. Mater. Proc. 2021, 4, 4. https://doi.org/10.3390/IOCN2020-07841
Dimitrov O, Stambolova I, Vassilev S, Lazarova K, Simeonova S. Surface and Optical Properties of Gd-Doped ZrO2 Nano Films. Materials Proceedings. 2021; 4(1):4. https://doi.org/10.3390/IOCN2020-07841
Chicago/Turabian StyleDimitrov, Ognian, Irina Stambolova, Sasho Vassilev, Katerina Lazarova, and Silvia Simeonova. 2021. "Surface and Optical Properties of Gd-Doped ZrO2 Nano Films" Materials Proceedings 4, no. 1: 4. https://doi.org/10.3390/IOCN2020-07841





