The Self-Organization of Nanoparticles in Molybdenum Blue Dispersions in the Presence of Organic Reducing Agents †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
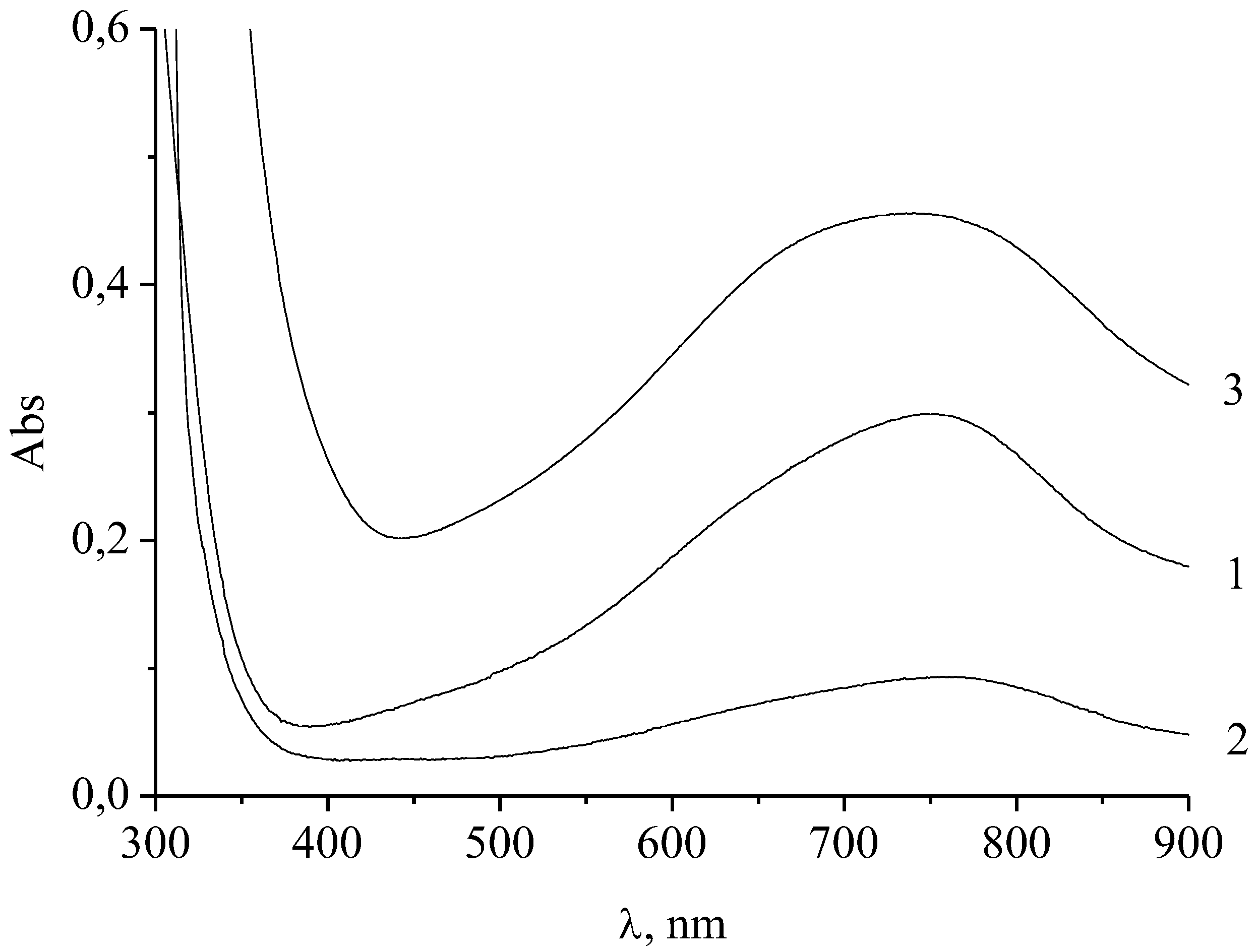
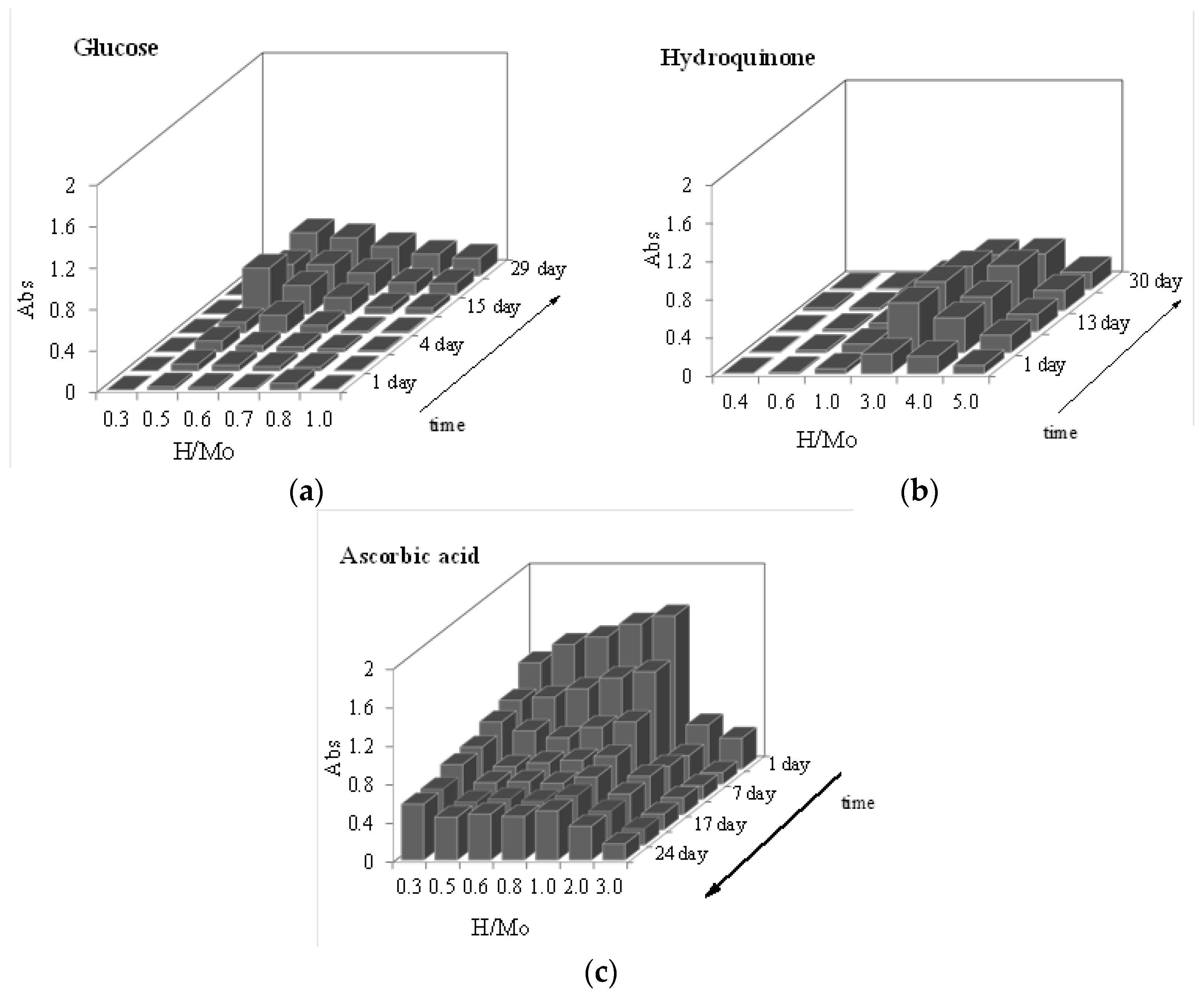
3.1. Properties of Molybdenum Blue Properties
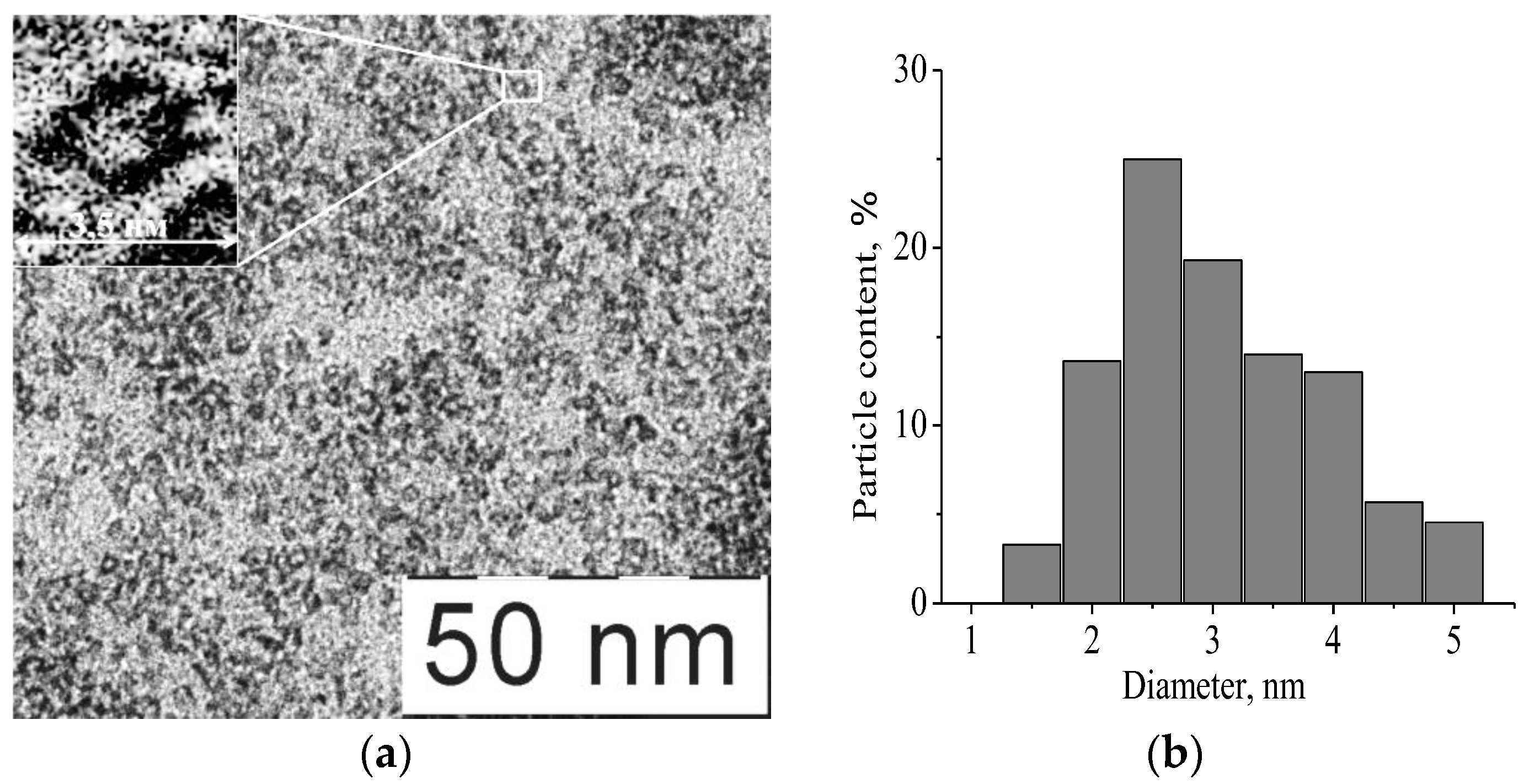
3.2. Characterization of Nanoparticles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schirmer, F.B.; Audrieth, L.F.; Gross, S.T.; Seppi, L.J. The composition and structure of molybdenum blue. J. Am. Chem. Soc. 1942, 64, 2543–2545. [Google Scholar] [CrossRef]
- Liu, X.; Conte, M.; Weng, W.; Knight, D.W. Molybdenum blue nano-rings: An effective catalyst for the partial oxidation of cyclohexane. Catal. Sci. Technol. 2015, 5, 217–227. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Meyer, J.; Krickemeyer, E.; Diemann, E. Molybdenum blue: A 200-year-old mystery unveiled. Angew. Chem. Int. Ed. Engl. 1996, 35, 1206–1208. [Google Scholar] [CrossRef]
- Tang, P.; Jia, X.; Fan, D.; Wang, L.; Hao, J. Surface charges of hedgehog-shaped polyoxomolybdate modified by a cationic surfactant and the inorganic/organic complex. Colloid Surf. A 2008, 312, 18–23. [Google Scholar] [CrossRef]
- Müller, A.; Reuter, H.; Dillinger, S. Supramolecular inorganic chemistry: Small guests in small and large hosts. Angew. Chem. Int. Ed. Engl. 1995, 34, 2328–2361. [Google Scholar] [CrossRef]
- Roy, S. Soft-oxometalates beyond crystalline polyoxometalates: Formation, structure and properties. Cryst. Eng. Comm. 2014, 16, 4667–4676. [Google Scholar] [CrossRef]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self-assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.N.; Nazarov, V.V.; Skudin, V.V. Synthesis of membrane catalysts based on Mo2C. Kinet. Catal. 2015, 56, 670–680. [Google Scholar] [CrossRef]
- Müller, A.; Roy, S. En route from the mystery of molybdenum blue via related manipulatable building blocks to aspects of materials science. Coord. Chem. Rev. 2003, 245, 153–166. [Google Scholar] [CrossRef]
- Bielanski, A.; Malecka-Lubanska, A.; Micek-Ilnicka, A.; Muller, A.; Diemann, E. Thermal properties of (NH4)32[Mo138O416H6(H2O)58(CH3COO)6] approximately 250 H2O: On the route to prove the complexity of a nanostructured landscape especially with different type of H2O ligands—Embedded in an ‘ocean’ of water molecules. Inorg. Chim. Acta 2002, 338, 7–12. [Google Scholar] [CrossRef]
- Li, F.; Xu, L. Coordination assemblies of polyoxomolybdate cluster framework: From labile building blocks to stable functional materials. Dalton Trans. 2011, 40, 4024–4034. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Chandel, S.; Mallick, A.; Sreejith, S.S.; Ghosh, N.; Roy, S. Studying the crystallization of polyoxometalates from colloidal softoxometalates. Cryst. Growth Des. 2018, 18, 4068–4075. [Google Scholar] [CrossRef]
- Myachina, M.A.; Gavrilova, N.N.; Nazarov, V.V. Formation of molybdenum blue particles via the reduction of a molybdate solution with glucose. Russ. J. Phys. Chem. 2018, 92, 2237–2241. [Google Scholar] [CrossRef]
- Bazhenova, M.D.; Gavrilova, N.N.; Nazarov, V.V. Some colloidochemical properties of molybdenum blues synthesized using glucose as a reducing agent. Colloid J. 2015, 77, 1–5. [Google Scholar] [CrossRef]
- Myachina, M.A.; Gavrilova, N.N.; Nazarov, V.V. Formation of molybdenum blue particles via the reduction of a molybdate solution with hydroquinone. Colloid J. 2019, 81, 541–546. [Google Scholar] [CrossRef]
- Liu, T. An unusually slow self-assembly of inorganic ions in dilute aqueous solution. J. Am. Chem. Soc. 2003, 125, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Serein, C. Soluble molybdenum blues “des Pudels Kern”. Acc. Chem. Res. 2000, 33, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Maiti, R.; Schmidtmann, M. Mimicking oxide surfaces: Different types of defects and ligand coordination at well defined positions of a molybdenum oxide based nanoclusters. Chem. Comm. 2001, 20, 2126–2127. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myachina, M.; Gavrilova, N.; Harlamova, D.; Nazarov, V. The Self-Organization of Nanoparticles in Molybdenum Blue Dispersions in the Presence of Organic Reducing Agents. Mater. Proc. 2021, 4, 2. https://doi.org/10.3390/IOCN2020-07895
Myachina M, Gavrilova N, Harlamova D, Nazarov V. The Self-Organization of Nanoparticles in Molybdenum Blue Dispersions in the Presence of Organic Reducing Agents. Materials Proceedings. 2021; 4(1):2. https://doi.org/10.3390/IOCN2020-07895
Chicago/Turabian StyleMyachina, Maria, Natalia Gavrilova, Daria Harlamova, and Victor Nazarov. 2021. "The Self-Organization of Nanoparticles in Molybdenum Blue Dispersions in the Presence of Organic Reducing Agents" Materials Proceedings 4, no. 1: 2. https://doi.org/10.3390/IOCN2020-07895





