From Regenerated Wood Pulp Fibers to Cationic Cellulose: Preparation, Characterization and Dyeing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
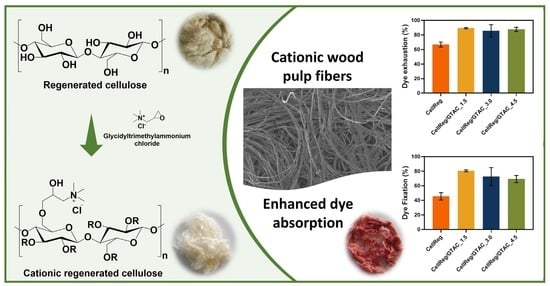
2.2. Cationization of the Regenerated Cellulose Fibers
2.3. Characterization Methods
2.3.1. Elemental Analysis
2.3.2. Fourier Transform Infrared-Attenuated Total Reflection (FTIR-ATR) Spectroscopy
2.3.3. Solid-State Carbon Cross-Polarization/Magic-Angle-Spinning Nuclear Magnetic Resonance (13C CP/MAS NMR) Spectroscopy
2.3.4. X-ray Diffraction (XRD)
2.3.5. Optical Microscopy
2.3.6. Scanning Electron Microscopy (SEM)
2.3.7. Zeta Potential
2.3.8. Contact Angle Measurements
2.3.9. Thermogravimetric Analysis (TGA)
2.3.10. Colorimetric Coordinates and ISO Brightness
2.4. Dyeing Uptake Tests
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition and Structure
3.2. Morphology, Surface Charge and Surface Wettability
3.3. Thermal Stability
3.4. Dye Uptake Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef] [PubMed]
- United Nations Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 3 June 2022).
- Stone, C.; Windsor, F.M.; Munday, M.; Durance, I. Natural or synthetic–How global trends in textile usage threaten freshwater environments. Sci. Total Environ. 2020, 718, 134689. [Google Scholar] [CrossRef] [PubMed]
- Cherrett, N.; Barrett, J.; Clemett, A.; Chadwick, M.; Chadwick, M.J. Ecological Footprint and Water Analysis of Cotton, Hemp and Polyester; Stockholm Environment Institute: Stockholm, Sweden, 2022; ISBN 9197523828. [Google Scholar]
- Henry, B.; Laitala, K.; Grimstad, I. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef]
- EPRS|European Parliamentary Research Service. Environmental Impact of the Textile and Clothing Industry. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2019/633143/EPRS_BRI(2019)633143_EN.pdf (accessed on 3 June 2022).
- Serra, A.; Serra-Parareda, F.; Vilaseca, F.; Delgado-Aguilar, M.; Espinach, F.X.; Tarrés, Q. Exploring the potential of cotton industry byproducts in the plastic composite sector: Macro and micromechanics study of the flexural modulus. Materials 2021, 14, 4787. [Google Scholar] [CrossRef]
- Santiago, A.S.; Neto, C.P.; Vilela, C. Impact of effective alkali and sulfide profiling on Eucalyptus globulus kraft pulping. Selectivity of the impregnation phase and its effect on final pulping results. J. Chem. Technol. Biotechnol. 2008, 83, 242–251. [Google Scholar] [CrossRef]
- Valente, B.F.A.; Silvestre, A.J.D.; Neto, C.P.; Vilela, C.; Freire, C.S.R. Effect of the micronization of pulp fibers on the properties of green composites. Molecules 2021, 26, 5594. [Google Scholar] [CrossRef]
- Salleh, K.M.; Armir, N.A.Z.; Mazlan, N.S.N.; Wang, C.; Zakaria, S. Cellulose and its derivatives in textiles: Primitive application to current trend. In Fundamentals of Natural Fibres and Textiles (The Textile Institute Book Series); Mondal, M.I.H., Ed.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–63. [Google Scholar]
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Modification of textiles for functional applications. In Fundamentals of Natural Fibres and Textiles (The Textile Institute Book Series); Mondal, M.I.H., Ed.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2021; pp. 303–365. ISBN 9780128214831. [Google Scholar]
- Elsayed, S.; Hummel, M.; Sawada, D.; Guizani, C.; Rissanen, M.; Sixta, H. Superbase-based protic ionic liquids for cellulose filament spinning. Cellulose 2021, 28, 533–547. [Google Scholar] [CrossRef]
- Elsayed, S.; Hellsten, S.; Guizani, C.; Witos, J.; Rissanen, M.; Rantama, A.H.; Varis, P.; Wiedmer, S.K.; Sixta, H. Recycling of Superbase-Based Ionic Liquid Solvents for the Production of Textile-Grade Regenerated Cellulose Fibers in the Lyocell Process. ACS Sustain. Chem. Eng. 2020, 8, 14217–14227. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Sosa, F.H.B.; Kilpeläinen, I.; Coutinho, J.A.P. Physico-chemical characterization of aqueous solutions of superbase ionic liquids with cellulose dissolution capability. Fluid Phase Equilib. 2022, 556, 113414. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Ioncell® Technology. Available online: https://ioncell.fi/ (accessed on 6 June 2022).
- El-wakil, N.A.; Hassan, M.L. Structural Changes of Regenerated Cellulose Dissolved in FeTNa, NaOH/thiourea, and NMMO Systems. J. Appl. Polym. Sci. 2008, 109, 2862–2871. [Google Scholar] [CrossRef]
- Ho, T.T.T.; Zimmermann, T.; Hauert, R.; Caseri, W. Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes. Cellulose 2011, 18, 1391–1406. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, D.M. Chemical modification of cotton to improve fibre dyeability. Color. Technol. 2002, 118, 159–168. [Google Scholar] [CrossRef]
- Correia, J.; Oliveira, F.R.; Valle, R.d.C.S.C.; Valle, J.A.B. Preparation of cationic cotton through reaction with different polyelectrolytes. Cellulose 2021, 28, 11679–11700. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.P.; Surati, D. Enhancing Dyeability and Antibacterial Feature of Cotton Through Nano-chitosan Attachment. J. Inst. Eng. Ser. E 2020, 101, 175–184. [Google Scholar] [CrossRef]
- Björquist, S.; Aronsson, J.; Henriksson, G.; Persson, A. Textile qualities of regenerated cellulose fibers from cotton waste pulp. Text. Res. J. 2018, 88, 2485–2492. [Google Scholar] [CrossRef]
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides: A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- Odabas, N.; Amer, H.; Henniges, U.; Potthast, A.; Rosenau, T. A comparison of methods to quantify cationization of cellulosic pulps. J. Wood Chem. Technol. 2017, 37, 136–147. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Zeng, X.; Sun, Y.; Zhang, X.; Zhou, J.; Zhang, L. Effect of Molecular Weight and Degree of Substitution of Quaternized Cellulose on the Efficiency of Gene Transfection. Bioconjug. Chem. 2010, 21, 1271–1279. [Google Scholar] [CrossRef]
- Salajkov, M.; Berglund, L.A.; Zhou, Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012, 22, 19798–19805. [Google Scholar] [CrossRef]
- ISO 2470-1:2016; Paper, Board and Pulps—Measurement of Diffuse Blue Reflectance Factor—Part 1: Indoor Daylight Conditions (ISO brightness). International Organization for Standardization: Geneva, Switzerland, 2016.
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Bastante, C.C.; Silva, N.H.C.S.; Cardoso, L.C.; Serrano, C.M.; Martínez de la Ossa, E.J.; Freire, C.S.R.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Sarker, S.; Zakaria, M.; Islam, M.R.; Fayazi, R.U.; Acharjya, S. Influence of Process Parameters on Exhaustion, Fixation and Color Strength in Dyeing of Cellulose Fiber with Reactive Dye. Int. J. Text. Sci. Eng. 2020, 3, 127. [Google Scholar] [CrossRef]
- Odabas, N.; Amer, H.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Properties of Cellulosic Material after Cationization in Different Solvents. ACS Sustain. Chem. Eng. 2016, 4, 2295–2301. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Ramalhete, S.; Skuze, W.J.; Soni, R.; MKhimyak, Y.Z.; Edler, K.J.; Scott, J.L. Unravelling cationic cellulose nanofibril hydrogel structure: NMR spectroscopy and small angle neutron scattering analyses. Soft Matter. 2018, 21, 255–263. [Google Scholar] [CrossRef] [PubMed]
- de la Motte, H.; Hasani, M.; Brelid, H.; Westman, G. Molecular characterization of hydrolyzed cationized nanocrystalline cellulose, cotton cellulose and softwood kraft pulp using high resolution 1D and 2D NMR. Carbohydr. Polym. 2011, 85, 738–746. [Google Scholar] [CrossRef]
- Olszewska, A.; Eronen, P.; Johansson, L.-S.; Malho, J.-M.; Ankerfors, M.; Lindstrom, T.; Ruokolainen, J.; Laine, J.; Osterberg, M. The behaviour of cationic NanoFibrillar Cellulose in aqueous media. Cellulose 2011, 18, 1213–1226. [Google Scholar] [CrossRef]
- Yan, L.; Tao, H.; Bangal, P.R.; Chemistry, P. Synthesis and Flocculation Behavior of Cationic Cellulose Prepared in a NaOH/Urea Aqueous Solution. Clean 2009, 37, 39–44. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.-H.; Liu, X.; Wu, M.; Wu, Y.-Y.; Zhang, X.-M.; Sun, R.-C. Fabrication and Characterization of Regenerated Cellulose Films Using Different Ionic Liquids. J. Spectrosc. 2014, 2014, 214057. [Google Scholar] [CrossRef]
- Solin, K.; Beaumont, M.; Rosenfeldt, S.; Orelma, H.; Borghei, M.; Bacher, M.; Opietnik, M.; Rojas, O.J. Self-Assembly of Soft Cellulose Nanospheres into Colloidal Gel Layers with Enhanced Protein Adsorption Capability for Next-Generation Immunoassays. Small 2020, 16, 2004702. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, N.; Pinto, R.J.B.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic nanocellulose-based membranes for organic dye removal. Materials 2019, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and conductive nanocellulose-based films for active and intelligent food packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Onwukamike, K.N.; Tassaing, T.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Detailed Understanding of the DBU/CO2 Switchable Solvent System for Cellulose Solubilization and Derivatization. ACS Sustain. Chem. Eng. 2018, 6, 1496–1503. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C.; Gandini, A.; Desbrieres, J. Novel transparent nanocomposite films based on chitosan and bacterial cellulose. Green Chem. 2009, 11, 2023–2029. [Google Scholar] [CrossRef]
- Stana-Kleinschek, K.; Kreze, T.; Ribitsch, V.; Strnad, S. Reactivity and electrokinetical properties of different types of regenerated cellulose fibres. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 195, 275–284. [Google Scholar] [CrossRef]
- Manian, A.P.; Jaturapiree, A.; Bechtold, T. Salt sorption on regenerated cellulosic fibers: Electrokinetic measurements. Cellulose 2018, 25, 3307–3314. [Google Scholar] [CrossRef] [Green Version]
- Payerl, C.; Bračič, M.; Zankel, A.; Fischer, W.J.; Kaschowitz, M.; Fröhlich, E.; Kargl, R.; Stelzer, F.; Spirk, S. Nonspecific protein adsorption on cationically modified Lyocell fibers monitored by zeta potential measurements. Carbohydr. Polym. 2017, 164, 49–56. [Google Scholar] [CrossRef]
- Wei, D.W.; Wei, H.; Gauthier, A.C.; Song, J.; Jin, Y.; Xiao, H. Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications. J. Bioresour. Bioprod. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Pajorova, J.; Skogberg, A.; Hadraba, D.; Broz, A.; Travnickova, M.; Zikmundova, M.; Honkanen, M.; Hannula, M.; Lahtinen, P.; Tomkova, M.; et al. Cellulose Mesh with Charged Nanocellulose Coatings as a Promising Carrier of Skin and Stem Cells for Regenerative Applications. Biomacromolecules 2020, 21, 4857–4870. [Google Scholar] [CrossRef]
- Skogberg, A.; Ma, A.; Metta, M.; Lahtinen, P.; Kallio, P. Cellulose Nanofiber Alignment Using Evaporation-Induced Droplet-Casting, and Cell Alignment on Aligned Nanocellulose Surfaces. Biomacromolecules 2017, 18, 3936–3953. [Google Scholar] [CrossRef]
- Li, G.; Fu, Y.; Shao, Z.; Zhang, F.; Qin, M. Preparing Cationic Cellulose Derivative in NaOH/Urea Aqueous Solution and its Performance as Filler Modifier. BioResources 2015, 10, 7782–7794. [Google Scholar] [CrossRef]
- Agarwal, J. Sonia Dyes and dyeing processes for natural textiles and their key sustainability issues. In Fundamentals of Natural Fibres and Textiles (The Textile Institute Book Series); Mondal, M.I.H., Ed.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2021; pp. 339–472. [Google Scholar]
- Akansha, K.; Yadav, A.N.; Manish, K.; Chakraborty, D.; Sachan, S.G. Decolorization and degradation of reactive orange 16 by Bacillus stratosphericus SCA1007. Folia Microbiol. 2022, 67, 91–102. [Google Scholar] [CrossRef]
- Shu, D.; Fang, K.; Liu, X.; Cai, Y.; An, F. High Dye Fixation Pad-Steam Dyeing of Cotton Fabrics with Reactive Dyes Based on Hydrophobic Effect. J. Nat. Fibers 2020, 17, 665–675. [Google Scholar] [CrossRef]
- Khatri, Z.; Mayakrishnan, G.; Hirata, Y.; Wei, K.; Kim, I. Cationic-cellulose nanofibers: Preparation and dyeability with anionic reactive dyes for apparel application. Carbohydr. Polym. 2013, 91, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, G.; Mi, X.; Kang, X.; Zhu, Q.; Yu, Z. A single-step pad-steam cationisation and dyeing process for improving dyeing properties of cotton fabrics. Color. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Gominho, J.; Lopes, C.; Lourenço, A.; Simões, R.; Pereira, H. Eucalyptus globulus stumpwood as a raw material for pulping. BioResources 2014, 9, 4038–4049. [Google Scholar] [CrossRef] [Green Version]




| Sample | Ratio of GTAC/AGU | DS | Zeta Potential (mV) | Contact Angle (°) |
|---|---|---|---|---|
| CellReg | – | – | –15.4 ± 5.0 a | 50.7 ± 6.3 a |
| CellReg/GTAC_1.5 | 1.5:1 | 0.13 ± 0.004 a | 8.4 ± 3.9 b | 65.7 ± 3.4 b |
| CellReg/GTAC_3.0 | 3.0:1 | 0.24 ± 0.006 b | 15.2 ± 3.2 c | 71.5 ± 2.2 c |
| CellReg/GTAC_4.5 | 4.5:1 | 0.33 ± 0.002 c | 26.9 ± 5.5 d | 82.5 ± 3.8 d |
| Sample | L* | a* | b* | ΔE | Brightness (%) | |
|---|---|---|---|---|---|---|
| Before dyeing | CellReg | 83.9 ± 2.0 a | 2.3 ± 0.3 a | 17.5 ± 1.0 a | – | 47.3 ± 3.4 a |
| CellReg/GTAC_1.5 | 86.4 ± 2.6 a | −0.5 ± 0.1 b | 8.6 ± 0.6 b | 9.7 | 61.7 ± 4.4 b | |
| CellReg/GTAC_3.0 | 81.1 ± 2.5 b | −0.7 ± 0.1 b | 7.7 ± 1.5 b | 10.7 | 51.7 ± 3.0 c | |
| CellReg/GTAC_4.5 | 76.0 ± 2.4 c | −0.2 ± 0.1 c | 3.8 ± 0.6 c | 16.0 | 22.1 ± 2.1 d | |
| After dyeing | CellReg | 70.1 ± 1.4 a | 27.6 ± 0.7 a | 16.5 ± 0.5 a | 28.0 | 28.4 ± 0.4 a |
| CellReg/GTAC_1.5 | 57.1 ± 1.7 b | 34.3 ± 1.0 b | 14.6 ± 0.7 b | 45.9 | 16.7 ± 0.8 b | |
| CellReg/GTAC_3.0 | 45.9 ± 1.7 c | 27.4 ± 1.8 a | 8.6 ± 1.2 c | 45.1 | 11.3 ± 0.7 c | |
| CellReg/GTAC_4.5 | 43.5 ± 1.6 c | 27.7 ± 2.1 a | 10.0 ± 1.2 c | 43.1 | 9.6 ± 0.6 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, B.; Matos, F.S.; Valente, B.F.A.; Von Weymarn, N.; Kamppuri, T.; Freire, C.S.R.; Silvestre, A.J.D.; Vilela, C. From Regenerated Wood Pulp Fibers to Cationic Cellulose: Preparation, Characterization and Dyeing Properties. Polysaccharides 2022, 3, 609-624. https://doi.org/10.3390/polysaccharides3030036
Pereira B, Matos FS, Valente BFA, Von Weymarn N, Kamppuri T, Freire CSR, Silvestre AJD, Vilela C. From Regenerated Wood Pulp Fibers to Cationic Cellulose: Preparation, Characterization and Dyeing Properties. Polysaccharides. 2022; 3(3):609-624. https://doi.org/10.3390/polysaccharides3030036
Chicago/Turabian StylePereira, Bárbara, Filipe S. Matos, Bruno F. A. Valente, Niklas Von Weymarn, Taina Kamppuri, Carmen S. R. Freire, Armando J. D. Silvestre, and Carla Vilela. 2022. "From Regenerated Wood Pulp Fibers to Cationic Cellulose: Preparation, Characterization and Dyeing Properties" Polysaccharides 3, no. 3: 609-624. https://doi.org/10.3390/polysaccharides3030036