Using Chitosan or Chitosan Derivatives in Cancer Therapy
Abstract
:1. Introduction
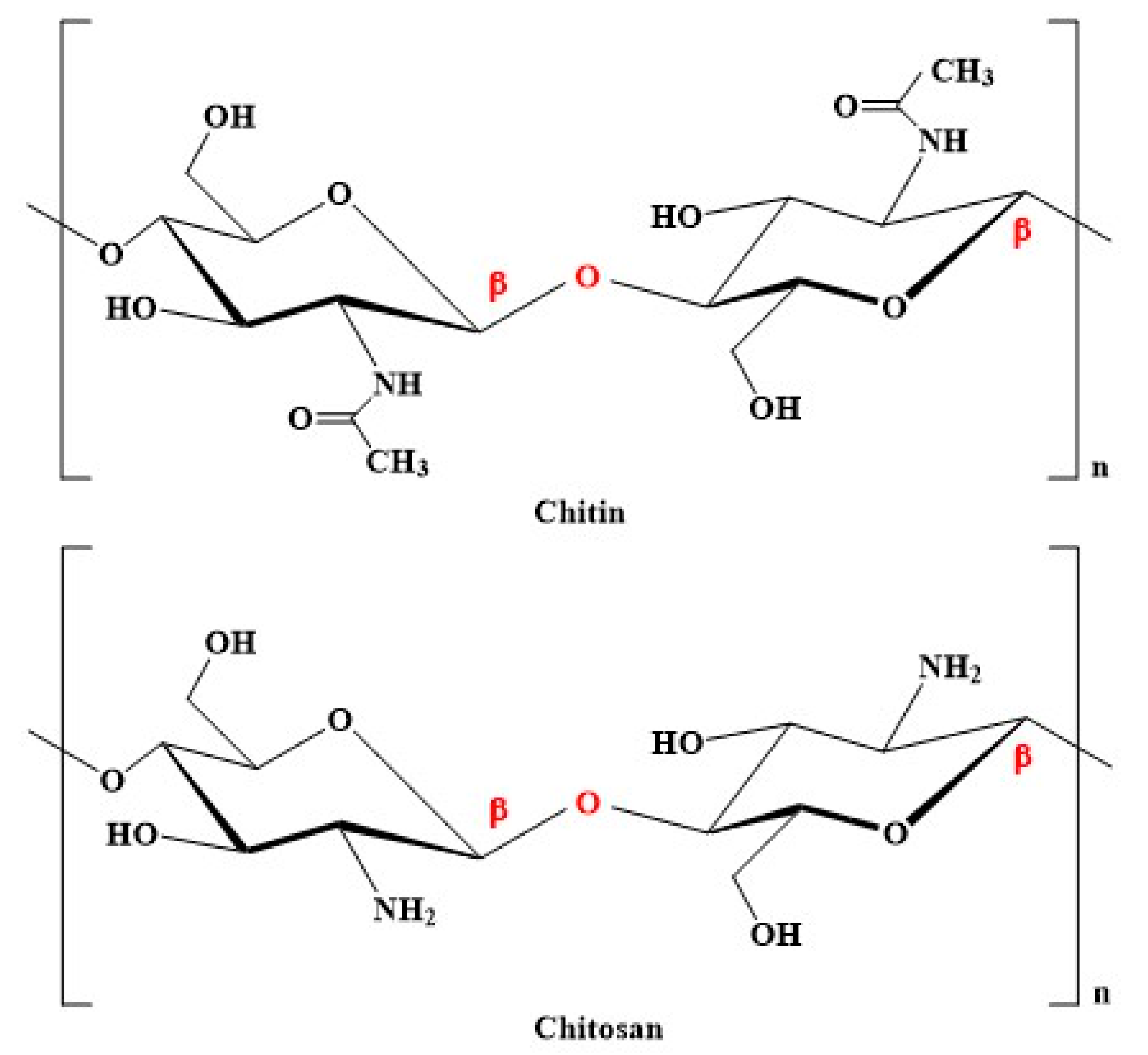
2. Properties of Chitosan and Its Derivatives
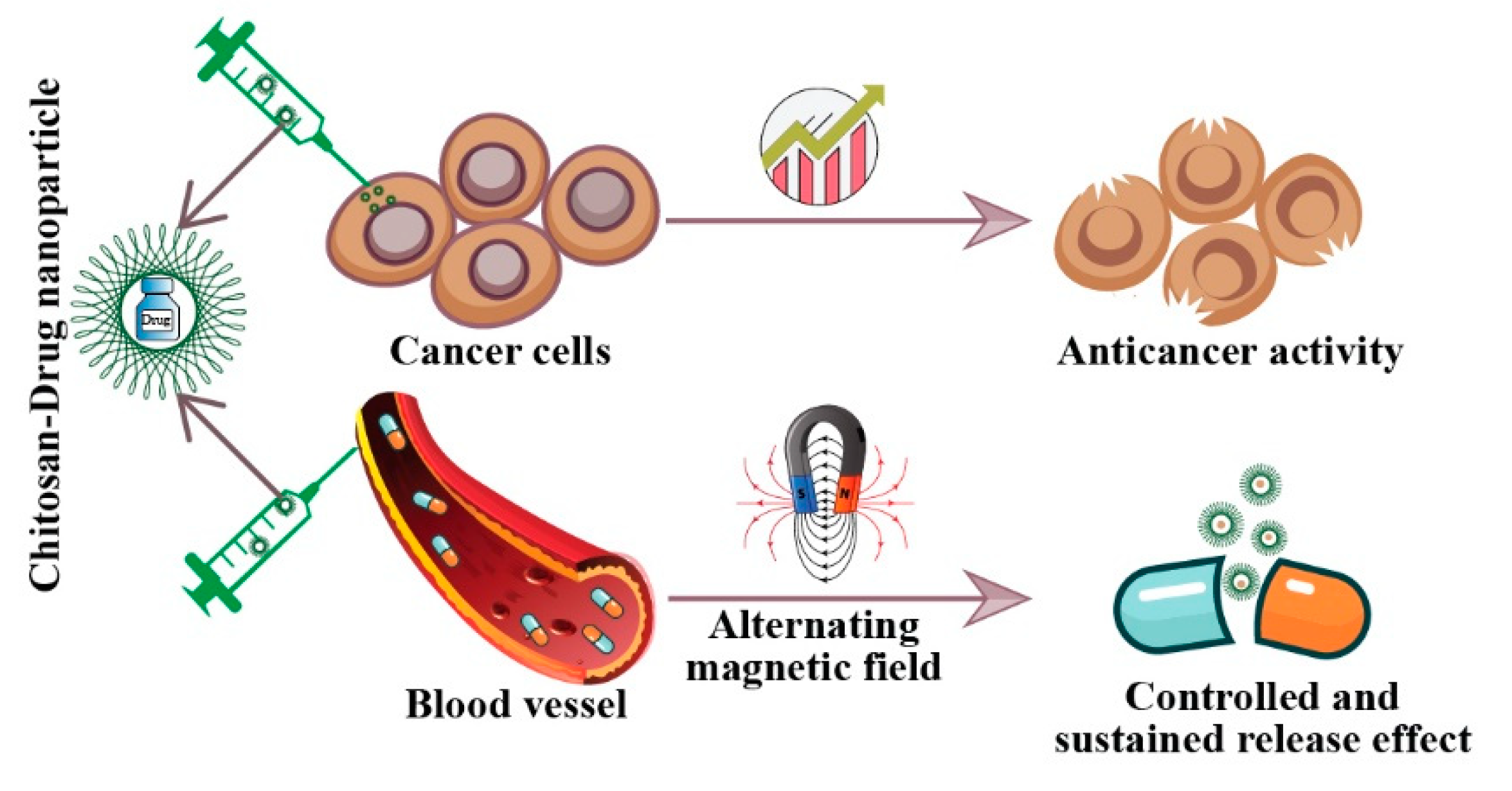
3. Anticancer Activities of Chitosan and Its Derivatives
4. Ligand Decorated Chitosan for Targeted Drug Delivery
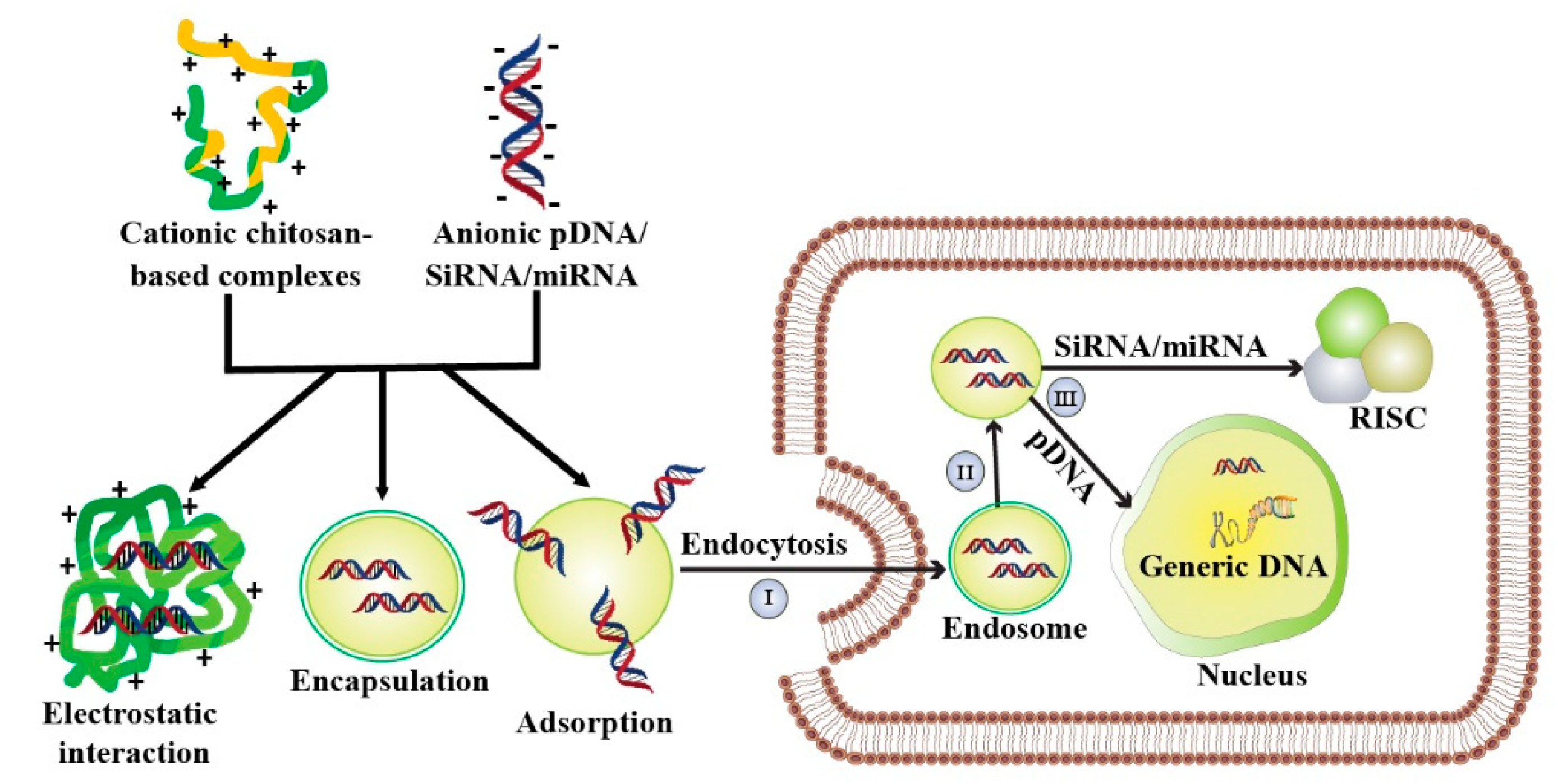
5. Chitosan-Mediated Gene Therapy
| Chitosan Modified Complexes | Nucleic Acids | CytotoxiCity Assay | CytotoxiCity Status | Average Particle Size (nm) | Zeta Potential (mV) | InvestiGated Cell Lines | Transfection Investigated | Ref. |
|---|---|---|---|---|---|---|---|---|
| HPOCP | siRNA, pDNA, | MTT | No cytotoxicity | 100–~ 300 | − | HCT119 | in vitro | [71] |
| FPCPHDs | pDNA | MTT | <10% (HepG2), and 20% (KB) less cytotoxic than control | 51–305 | −0.9–+27.3 | HepG2, KB | in vitro and in vivo | [72] |
| TQCMC- DOPE | pDNA | MTT | 5–40% (L929), 0–70% (HO8910), and 0–60% (HepG2) more cytotoxic than Lipofectamine 2000 | 184.4 | 27.5 | 293T, SGC-7901, PC-3, HO-8910, HepG2, U87, SMMC-7721 | in vitro and in vivo | [77] |
| FA-Au-C-PLGA | pDNA | MTT | 0–10% less cytotoxic than control | 199.4 | 35.7 | HepG2, HEK293, MCF-7 | in vitro | [79] |
| DEMC | pDNA | MTT | 45–70% less cytotoxic than control | 114.24–570.4 | 6.14–16.45 | AsPC-1 | in vitro | [80] |
| C–miRNA | miRNA | MTT | Almost no cytotoxicity | ~80–190 | −20–+20 | MCF-7 | in vitro | [82] |
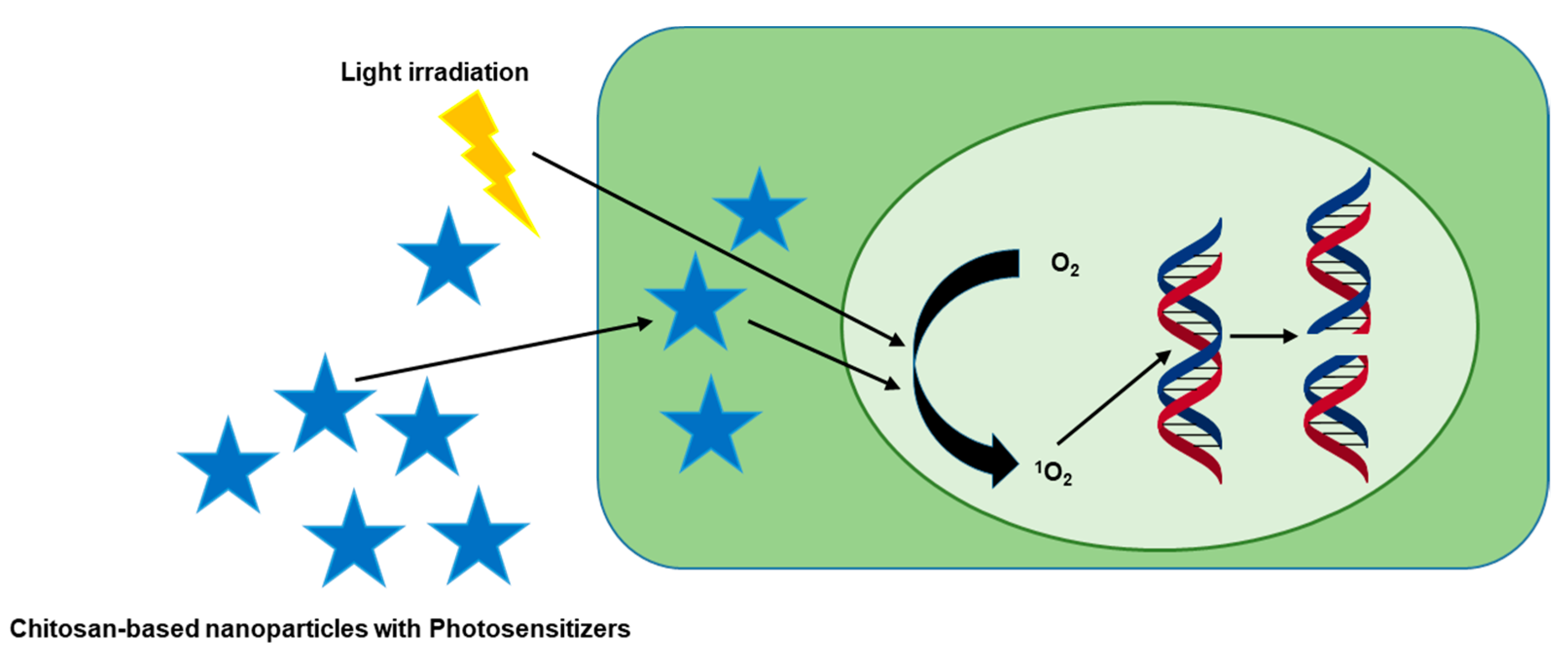
6. Photodynamic Therapy of Chitosan-Based Complexes
7. Delivery of Chemotherapeutic Drugs
7.1. Delivery of Hydrophilic Anticancer Drugs
7.2. Delivery of Hydrophobic Anticancer Drugs
8. Chitosan-Based Surfactant in Cancer Treatment
9. Chitosan-Based Emulsion in Cancer Therapy
10. Chitosan-Based Hydrogel in Cancer Treatment
11. Immunotherapy Using Chitosan
12. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021, 121, 3297–3351. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharm. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal. Transduct Target. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayhan, M.; Hossen, M.; Niloy, M.S.; Bhuiyan, M.H.; Paul, S.; Shakil, M. Biopolymer and Biomaterial Conjugated Iron Oxide Nanomaterials as Prostate Cancer Theranostic Agents: A Comprehensive Review. Symmetry 2021, 13, 974. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Dass, C.R.; Choong, P.F. The use of chitosan formulations in cancer therapy. J. Microencapsul. 2008, 25, 275–279. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan derivatives as promising materials for controlled drug delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmed, S.A.; Alkahtani, S.; Milivojevic, M.; Kandar, C.C.; Dhara, A.K.; Nayak, A.K. Biopolymers for Drug Delivery. In Advanced Biopolymeric Systems for Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–29. [Google Scholar]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Prateeksha; Sharma, V.K.; Liu, X.; Oyarzún, D.A.; Abdel-Azeem, A.M.; Atanasov, A.G.; Hesham, A.E.; Barik, S.K.; Gupta, V.K.; Singh, B.N. Microbial polysaccharides: An emerging family of natural biomaterials for cancer therapy and diagnostics. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Shakil, M.S.; Hasan, M.A.; Uddin, M.F.; Islam, A.; Nahar, A.; Das, H.; Khan, M.N.I.; Dey, B.P.; Rokeya, B.; Hoque, S.M. In Vivo Toxicity Studies of Chitosan-Coated Cobalt Ferrite Nanocomplex for Its Application as MRI Contrast Dye. ACS Appl. Bio Mater. 2020, 3, 7952–7964. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [Green Version]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franconetti, A.; López, Ó.; Fernandez-Bolanos, J.G. Carbohydrates: Potential Sweet Tools Against Cancer. Curr. Med. Chem. 2020, 27, 1206–1242. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Study on different molecular weights of chitosan as an immobilization matrix for a glucose biosensor. PLoS ONE 2013, 8, e70597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Correa-Murrieta, M.A.; Sánchez-Duarte, R.G.; Cruz-Flores, P.; de la Mora-López, G.S. Chitosan. In Nonvitamin and Nonmineral Nutritional Supplements; Seyed Mohammad Nabavi, A.S.S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 485–493. [Google Scholar]
- Chen, J.K.; Shen, C.R.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.C.; Chou, C.C.; Li, C.F. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol 2005, 97, 237–245. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Z.W.; Ma, Z.W.; Li, C.; Jia, Y.Y.; Luo, M.; Ma, X.X.; Zhou, S.Y.; Zhang, B.L. Chitosan cross-linked with poly(ethylene glycol)dialdehyde via reductive amination as effective controlled release carriers for oral protein drug delivery. Bioorg Med. Chem. Lett. 2017, 27, 1003–1006. [Google Scholar] [CrossRef]
- Bukzem, A.L.; Signini, R.; Dos Santos, D.M.; Lião, L.M.; Ascheri, D.P. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, A.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Salahuddin, N.; Elbarbary, A.A.; Alkabes, H.A. Quinazolinone Derivatives Loaded Polypyrrole/Chitosan Core–Shell Nanoparticles with Different Morphologies: Antibacterial and Anticancer Activities. Nano 2017, 12, 1750002. [Google Scholar] [CrossRef]
- Salahuddin, N.; Elbarbary, A.A.; Alkabes, H.A. Antibacterial and antitumor activities of 3-amino-phenyl-4 (3H)-quinazolinone/polypyrrole chitosan core shell nanoparticles. Polym. Bull. 2017, 74, 1775–1790. [Google Scholar] [CrossRef]
- Maeda, Y.; Kimura, Y. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J. Nutr. 2004, 134, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.A. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Wong, T.W. Chitosan as Anticancer Compound and Nanoparticulate Matrix for Cancer Therapeutics. Encycl. Mar. Biotechnol. 2020, 3, 1737–1752. [Google Scholar]
- Kumar, S.; Koh, J.; Kim, H.; Gupta, M.K.; Dutta, P.K. A new chitosan-thymine conjugate: Synthesis, characterization and biological activity. Int. J. Biol. Macromol 2012, 50, 493–502. [Google Scholar] [CrossRef]
- Salahuddin, N.; Elbarbary, A.A.; Salem, M.L.; Elksass, S. Antimicrobial and antitumor activities of 1,2,4-triazoles/polypyrrole chitosan core shell nanoparticles. J. Phys. Org. Chem. 2017, 30, e3702. [Google Scholar] [CrossRef]
- Vedham, V.; Divi, R.L.; Starks, V.L.; Verma, M. Multiple infections and cancer: Implications in epidemiology. Technol. Cancer Res. Treat. 2014, 13, 177–194. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef]
- Jiang, M.; Ouyang, H.; Ruan, P.; Zhao, H.; Pi, Z.; Huang, S.; Yi, P.; Crepin, M. Chitosan derivatives inhibit cell proliferation and induce apoptosis in breast cancer cells. Anticancer Res. 2011, 31, 1321–1328. [Google Scholar]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Chen, X.; Tian, J.; Li, L.; Zhao, M.; Jiao, Y.; Zhou, C. Effect of chitooligosaccharides on cyclin D1, bcl-xl and bcl-2 mRNA expression in A549 cells using quantitative PCR. Chin. Sci. Bull. 2011, 56, 1629. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.W.; Li, R.K. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, M.; Low, P.S. Ligand-Targeted Drug Delivery. Chem Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Nandgude, T.; Pagar, R. Plausible role of chitosan in drug and gene delivery against resistant breast cancer cells. Carbohydr. Res. 2021, 506, 108357. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-trimethyl chitosan nanoparticles and CSKSSDYQC peptide: N-trimethyl chitosan conjugates enhance the oral bioavailability of gemcitabine to treat breast cancer. J. Control. Release 2018, 277, 142–153. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, J.; Wang, X.; Wang, J.; Zhang, Y.; Wang, C. Epidermal growth factor receptor expression affects proliferation and apoptosis in non-small cell lung cancer cells via the extracellular signal-regulated kinase/microRNA 200a signaling pathway. Oncol. Lett. 2018, 15, 5201–5207. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Mad2 Checkpoint Gene Silencing Using Epidermal Growth Factor Receptor-Targeted Chitosan Nanoparticles in Non-Small Cell Lung Cancer Model. Mol. Pharm. 2014, 11, 3515–3527. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, K.; Eguchi, H.; Yoshida, T.; Hajiro-Nakanishi, K.; Hayashi, S. Regulation of estrogen receptor alpha gene mediated by promoter B responsible for its enhanced expressionin human breast cancer. Nucleic Acids Res. 1999, 27, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Tang, C.; Yin, C. Estrone-modified pH-sensitive glycol chitosan nanoparticles for drug delivery in breast cancer. Acta Biomater. 2018, 73, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Ahmed, N.; Khan, G.M.; Rehman, A.U. Ligand decorated chitosan as an advanced nanocarrier for targeted delivery: A critical review. Nanomedicine 2019, 14, 1623–1642. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Lameirinhas, N.S.; Guedes, G.; Rodrigues da Silva, G.H.; Oskoei, P.; Spirk, S.; Oliveira, H.; Duarte, I.F.; Vilela, C.; Freire, C.S.R. Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells. Nanomaterials 2021, 11, 2057. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef]
- Mali, S. Delivery systems for gene therapy. Indian J. Hum. Genet. 2013, 19, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadzadeh, R.; Shahim, P.; Akbari, A.J.B. Formulation of a pH-sensitive cancer cell-targeted gene delivery system based on folate–chitosan conjugated nanoparticles. Biotechnol. Appl. Biochem. 2021, 68, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Jiang, H.-L.; Jere, D.; Park, I.-K.; Cho, M.-H.; Nah, J.-W.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726–753. [Google Scholar] [CrossRef]
- Mahato, R.I.; Smith, L.C.; Rolland, A. 4-Pharmaceutical Perspectives of Nonviral Gene Therapy. In Advances in Genetics; Hall, J.C., Dunlap, J.C., Friedmann, T., Giannelli, F., Eds.; Academic Press: Cambridge, MA, USA, 1999; Volume 41, pp. 95–156. [Google Scholar]
- Lundstrom, K.; Boulikas, T. Viral and non-viral vectors in gene therapy: Technology development and clinical trials. Technol. Cancer Res. Treat. 2003, 2, 471–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Carballal, B.; Fernández Fernández, E.; Goycoolea, F.M. Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies. Polymers 2018, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy-an overview. J. Clin. Diagn. Res. JCDR 2015, 9, Ge01–Ge06. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, N.; Mourya, V.K. Chitosan and anionic polymers—Complex formation and applications. In Polysaccharide: Development, Properties and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 333–377. [Google Scholar]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mysore, K.; Flannery, E.; Michel, K.; Severson, D.W.; Zhu, K.Y.; Duman-Scheel, M. Chitosan/interfering RNA nanoparticle mediated gene silencing in disease vector mosquito larvae. J. Vis. Exp. JoVE 2015, 97, 52523. [Google Scholar] [CrossRef] [Green Version]
- Ragelle, H.; Riva, R.; Vandermeulen, G.; Naeye, B.; Pourcelle, V.; Le Duff, C.S.; D’Haese, C.; Nysten, B.; Braeckmans, K.; De Smedt, S.C.; et al. Chitosan nanoparticles for siRNA delivery: Optimizing formulation to increase stability and efficiency. J. Control. Release 2014, 176, 54–63. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Gemeinhart, R.A. Understanding the adsorption mechanism of chitosan onto poly(lactide-co-glycolide) particles. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2008, 70, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Mumper, R.; Wang, J.; Claspell, J.M.; Rolland, A. Novel polymeric condensing carriers for gene delivery. Proc. Natl. Symp. Control. Rel. Bioact. Mater. 1995, 22, 178–179. [Google Scholar]
- Carreño-Gómez, B.; Duncan, R. Evaluation of the biological properties of soluble chitosan and chitosan microspheres. Int. J. Pharm. 1997, 148, 231–240. [Google Scholar] [CrossRef]
- Richardson, S.W.; Kolbe, H.J.; Duncan, R. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Nam, J.P.; Nah, J.W. Target gene delivery from targeting ligand conjugated chitosan-PEI copolymer for cancer therapy. Carbohydr. Polym. 2016, 135, 153–161. [Google Scholar] [CrossRef]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Cheng, L.; et al. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan—PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef]
- Song, B.; Zhang, W.; Peng, R.; Huang, J.; Nie, T.; Li, Y.; Jiang, Q.; Gao, R. Synthesis and cell activity of novel galactosylated chitosan as a gene carrier. Colloids Surf. B Biointerfaces 2009, 70, 181–186. [Google Scholar] [CrossRef]
- Germershaus, O.; Mao, S.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: Establishment of structure-activity relationships in vitro. J. Control. Release 2008, 125, 145–154. [Google Scholar] [CrossRef]
- Chang, K.-L.; Higuchi, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. Efficient Gene Transfection by Histidine-Modified Chitosan through Enhancement of Endosomal Escape. Bioconjugate Chem. 2010, 21, 1087–1095. [Google Scholar] [CrossRef]
- Kean, T.; Roth, S.; Thanou, M. Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J. Control. Release 2005, 103, 643–653. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Chang, J.; Duan, Y.; Li, Z. Properties and evaluation of quaternized chitosan/lipid cation polymeric liposomes for cancer-targeted gene delivery. Langmuir ACS J. Surf. Colloids 2013, 29, 8683–8693. [Google Scholar] [CrossRef]
- Lee, D.; Lockey, R.; Mohapatra, S. Folate receptor-mediated cancer cell specific gene delivery using folic acid-conjugated oligochitosans. J. Nanosci. Nanotechnol. 2006, 6, 2860–2866. [Google Scholar] [CrossRef]
- Akinyelu, J.; Singh, M. Chitosan Stabilized Gold-Folate-Poly(lactide-co-glycolide) Nanoplexes Facilitate Efficient Gene Delivery in Hepatic and Breast Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 4478–4486. [Google Scholar] [CrossRef]
- Safari, S.; Dorkoosh, F.A.; Soleimani, M.; Zarrintan, M.H.; Akbari, H.; Larijani, B.; Tehrani, M.R. N-Diethylmethyl chitosan for gene delivery to pancreatic cancer cells and the relation between charge ratio and biologic properties of polyplexes via interpolations polynomial. Int. J. Pharm. 2011, 420, 350–357. [Google Scholar] [CrossRef]
- Wang, K.; Kievit, F.M.; Florczyk, S.J.; Stephen, Z.R.; Zhang, M. 3D Porous Chitosan–Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules 2015, 16, 3362–3372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Carballal, B.; Aaldering, L.J.; Ritzefeld, M.; Pereira, S.; Sewald, N.; Moerschbacher, B.M.; Götte, M.; Goycoolea, F.M. Physicochemical and biological characterization of chitosan-microRNA nanocomplexes for gene delivery to MCF-7 breast cancer cells. Sci. Rep. 2015, 5, 13567. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.A.; Rahbek, U.L.; Liu, X.; Damgaard, C.K.; Glud, S.Z.; Andersen, M.Ø.; Hovgaard, M.B.; Schmitz, A.; Nyengaard, J.R.; Besenbacher, F.; et al. RNA Interference in Vitro and in Vivo Using a Novel Chitosan/siRNA Nanoparticle System. Mol. Ther. 2006, 14, 476–484. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chang, Y.T.; Cho, K.C.; Chiu, K.C.; Lien, C.H.; Yeh, C.S.; Chen, S.J. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium Complexes: An Alternative to Platinum Drugs in Colorectal Cancer Treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; de Annunzio, S.R.; Victorelli, F.D.; Frade, M.L.; Ferreira, P.S.; Chorilli, M.; Fontana, C.R. Chitosan-Based Drug Delivery Systems for Optimization of Photodynamic Therapy: A Review. AAPS PharmSciTech 2019, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shen, R.; Bao, L.; Wang, C.; Yuan, H. Chitosan derived glycolipid nanoparticles for magnetic resonance imaging guided photodynamic therapy of cancer. Carbohydr. Polym. 2020, 245, 116509. [Google Scholar] [CrossRef]
- Pandya, A.D.; Øverbye, A.; Sahariah, P.; Gaware, V.S.; Høgset, H.; Masson, M.; Høgset, A.; Mælandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-Loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef]
- Jia, H.R.; Jiang, Y.W.; Zhu, Y.X.; Li, Y.H.; Wang, H.Y.; Han, X.; Yu, Z.W.; Gu, N.; Liu, P.; Chen, Z.; et al. Plasma membrane activatable polymeric nanotheranostics with self-enhanced light-triggered photosensitizer cellular influx for photodynamic cancer therapy. J. Control. Release 2017, 255, 231–241. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Winiwarter, S.; Ridderström, M.; Ungell, A.-L.; Andersson, T.; Zamora, I. Use of molecular descriptors for absorption, distribution, metabolism, and excretion predictions. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 531–554. [Google Scholar]
- Liu, Y.; Sun, M.; Wang, T.; Chen, X.; Wang, H. Chitosan-based self-assembled nanomaterials: Their application in drug delivery. View 2021, 2, 20200069. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Q.; Jin, Y.; Qiu, L. High loading of hydrophilic/hydrophobic doxorubicin into polyphosphazene polymersome for breast cancer therapy. Nanomedicine 2014, 10, 349–358. [Google Scholar] [CrossRef]
- Yun, U.J.; Lee, J.H.; Shim, J.; Yoon, K.; Goh, S.H.; Yi, E.H.; Ye, S.K.; Lee, J.S.; Lee, H.; Park, J.; et al. Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab. Investig. 2019, 99, 1157–1172. [Google Scholar] [CrossRef]
- Yousefpour, P.; Atyabi, F.; Vasheghani-Farahani, E.; Movahedi, A.A.; Dinarvand, R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar]
- Zou, Y.; Liu, P.; Liu, C.H.; Zhi, X.T. Doxorubicin-loaded mesoporous magnetic nanoparticles to induce apoptosis in breast cancer cells. Biomed. Pharm. 2015, 69, 355–360. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Kim, S.; Park, J.H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.W.; Kim, I.S.; et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234. [Google Scholar] [CrossRef]
- Wu, C.; Gao, Y.; Liu, Y.; Xu, X. Pure paclitaxel nanoparticles: Preparation, characterization, and antitumor effect for human liver cancer SMMC-7721 cells. Int. J. Nanomed. 2018, 13, 6189–6198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trickler, W.J.; Nagvekar, A.A.; Dash, A.K. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS PharmSciTech 2008, 9, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharm. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, Y.S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Y.; Meng, X. Chitosan Nanolayered Cisplatin-Loaded Lipid Nanoparticles for Enhanced Anticancer Efficacy in Cervical Cancer. Nanoscale Res. Lett. 2016, 11, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [Green Version]
- Zorrilla-Veloz, R.I.; Stelzer, T.; López-Mejías, V. Measurement and Correlation of the Solubility of 5-Fluorouracil in Pure and Binary Solvents. J. Chem. Eng. Data 2018, 63, 3809–3817. [Google Scholar] [CrossRef]
- Raida, M.; Schwabe, W.; Häusler, P.; Van Kuilenburg, A.B.; Van Gennip, A.H.; Behnke, D.; Höffken, K. Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase (DPD) gene within the 5’-splice donor site of intron 14 in patients with severe 5-fluorouracil (5-FU)- related toxicity compared with controls. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2001, 7, 2832–2839. [Google Scholar]
- Rajan, M.; Raj, V.; Al-Arfaj, A.A.; Murugan, A.M. Hyaluronidase enzyme core-5-fluorouracil-loaded chitosan-PEG-gelatin polymer nanocomposites as targeted and controlled drug delivery vehicles. Int. J. Pharm. 2013, 453, 514–522. [Google Scholar] [CrossRef]
- Cavalli, R.; Leone, F.; Minelli, R.; Fantozzi, R.; Dianzani, C. New chitosan nanospheres for the delivery of 5-fluorouracil: Preparation, characterization and in vitro studies. Curr. Drug Deliv. 2014, 11, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Thakur, K.; Sharma, G.; Kush, P.; Jain, U.K. Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 137, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Masuguchi, K.; Kawashiri, T.; Tsuji, T.; Watanabe, H.; Akiyoshi, S.; Kubo, M.; Masuda, S.; Egashira, N. Effects of Diluent Volume and Administration Time on the Incidence of Anaphylaxis Following Docetaxel Therapy in Breast Cancer. Biol. Pharm. Bull. 2020, 43, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhou, C.; Liu, Y.; Su, K.; Jia, L.; Chen, L.; Li, M.; Ma, J.; Zhou, W.; Zhang, S.; et al. Genetic associations of docetaxel-based chemotherapy-induced myelosuppression in Chinese Han population. J. Clin. Pharm. 2020, 45, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D. Surfactant Systems: Their Chemistry, Pharmacy and Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 727–738. [Google Scholar] [CrossRef]
- Alshraim, M.O.; Sangi, S.; Harisa, G.I.; Alomrani, A.H.; Yusuf, O.; Badran, M.M. Chitosan-Coated Flexible Liposomes Magnify the Anticancer Activity and Bioavailability of Docetaxel: Impact on Composition. Molecules 2019, 24, 250. [Google Scholar] [CrossRef] [Green Version]
- Scheeren, L.E.; Nogueira, D.R.; Macedo, L.B.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Rolim, C.M. PEGylated and poloxamer-modified chitosan nanoparticles incorporating a lysine-based surfactant for pH-triggered doxorubicin release. Colloids Surf. B Biointerfaces 2016, 138, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-Coated Cinnamon/Oregano-Loaded Solid Lipid Nanoparticles to Augment 5-Fluorouracil Cytotoxicity for Colorectal Cancer: Extract Standardization, Nanoparticle Optimization, and Cytotoxicity Evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Gancarz, R.; Wilk, K.A. Preparation and characterization of sodium alginate/chitosan microparticles containing esculin. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 22–32. [Google Scholar] [CrossRef]
- Zhi, J.; Wang, Y.; Luo, G. Adsorption of diuretic furosemide onto chitosan nanoparticles prepared with a water-in-oil nanoemulsion system. React. Funct. Polym. 2005, 65, 249–257. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Alginate submicron beads prepared through w/o emulsification and gelation with CaCl2 nanoparticles. Food Hydrocoll. 2013, 31, 428–434. [Google Scholar] [CrossRef]
- Rodrıguez, M.; Albertengo, L.; Agulló, E. Emulsification capacity of chitosan. Carbohydr. Polym. 2002, 48, 271–276. [Google Scholar] [CrossRef]
- Schulz, P.; Rodriguez, M.; Del Blanco, L.; Pistonesi, M.; Agullo, E. Emulsification properties of chitosan. Colloid Polym. Sci. 1998, 276, 1159–1165. [Google Scholar] [CrossRef]
- Trickler, W.J.; Khurana, J.; Nagvekar, A.A.; Dash, A.K. Chitosan and glyceryl monooleate nanostructures containing gemcitabine: Potential delivery system for pancreatic cancer treatment. AAPS PharmSciTech 2010, 11, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Natesan, S.; Sugumaran, A.; Ponnusamy, C.; Thiagarajan, V.; Palanichamy, R.; Kandasamy, R. Chitosan stabilized camptothecin nanoemulsions: Development, evaluation and biodistribution in preclinical breast cancer animal mode. Int. J. Biol. Macromol 2017, 104, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.G.; Winter, H.; DuRoss, A.N.; Sahay, G.; Sun, C. Inverse-micelle synthesis of doxorubicin-loaded alginate/chitosan nanoparticles and in vitro assessment of breast cancer cytotoxicity. Colloid Interface Sci. Commun. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Zhou, X.; Wang, X.; Zhai, W.; Li, B.; Du, J.; Li, G.; Sui, X.; Wu, Y.; et al. An orally available PD-1/PD-L1 blocking peptide OPBP-1-loaded trimethyl chitosan hydrogel for cancer immunotherapy. J. Control. Release 2021, 334, 376–388. [Google Scholar] [CrossRef]
- Rezakhani, L.; Alizadeh, M.; Alizadeh, A. A three dimensional in vivo model of breast cancer using a thermosensitive chitosan-based hydrogel and 4 T1 cell line in Balb/c. J. Biomed. Mater. Res. A 2021, 109, 1275–1285. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Zhang, Y.; Xu, W.; Xiao, J.; Suo, A. Growth of MCF-7 breast cancer cells and efficacy of anti-angiogenic agents in a hydroxyethyl chitosan/glycidyl methacrylate hydrogel. Cancer Cell Int. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, P.; Shan, W.; Gao, J.; Liang, W. A novel chitosan-based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J. Biomater. Sci. Polym. Ed. 2013, 24, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.; Friehling, E.; Kalpatthi, R.; Siripong, N.; Smith, K. Cost-effectiveness of levofloxacin prophylaxis against bacterial infection in pediatric patients with acute myeloid leukemia. Pediatr. Blood Cancer 2020, 67, e28469. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, 6547. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharm. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Pan, C.; Yang, H.; Lu, Y.; Hu, S.; Wu, Y.; He, Q.; Dong, X. Recent advance of peptide-based molecules and nonpeptidic small-molecules modulating PD-1/PD-L1 protein-protein interaction or targeting PD-L1 protein degradation. Eur J. Med. Chem. 2021, 213, 113170. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Hoefsmit, E.P.; Smyth, M.J.; Blank, C.U.; Teng, M.W.L. The Promise of Neoadjuvant Immunotherapy and Surgery for Cancer Treatment. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5743–5751. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Folate-Targeted mRNA Delivery Using Chitosan-Functionalized Selenium Nanoparticles: Potential in Cancer Immunotherapy. Pharmaceuticals 2019, 12, 164. [Google Scholar] [CrossRef] [Green Version]
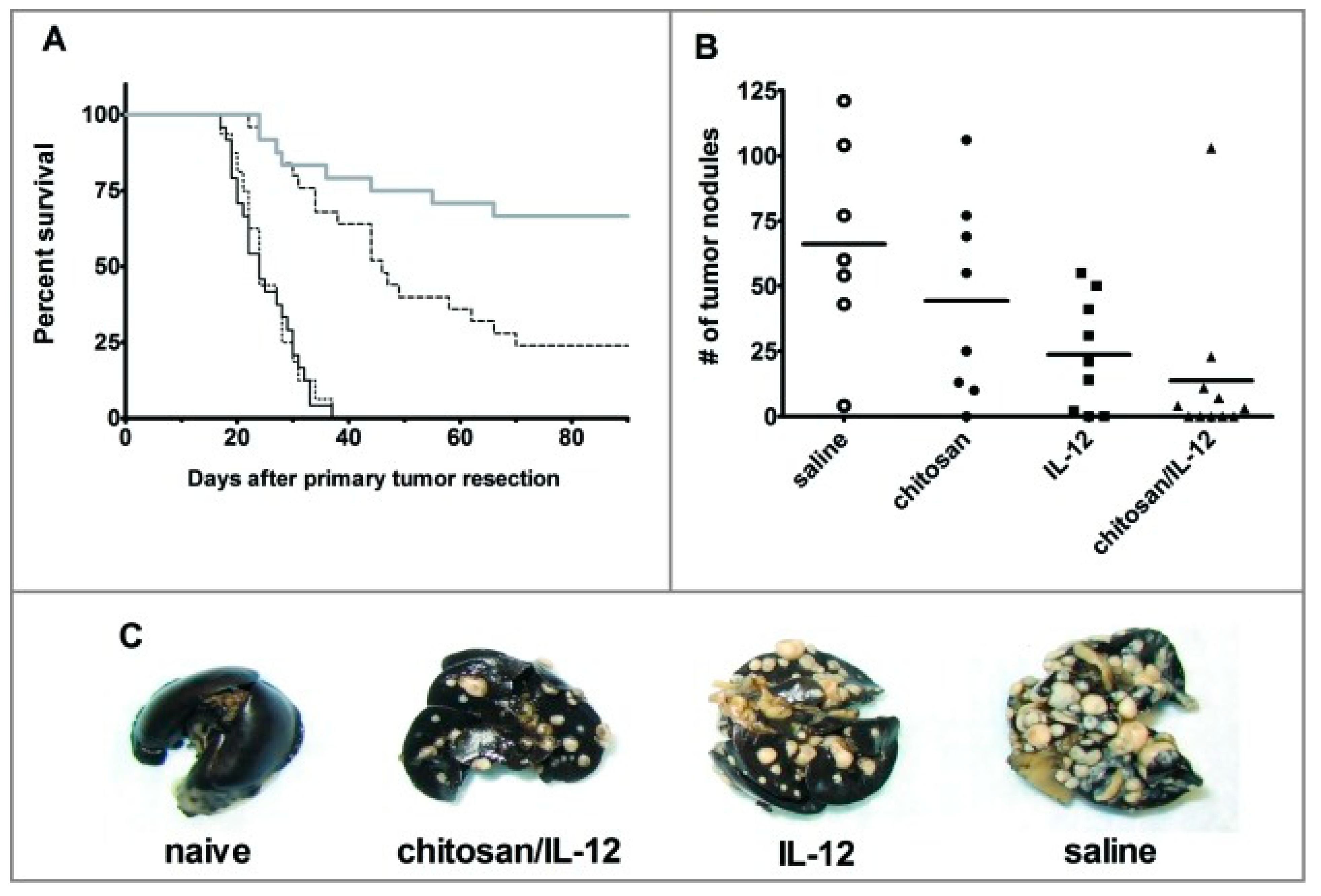
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J. Immunother. 2010, 33, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Vo, J.L.; Yang, L.; Kurtz, S.L.; Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Zaharoff, D.A. Neoadjuvant immunotherapy with chitosan and interleukin-12 to control breast cancer metastasis. Oncoimmunology 2014, 3, e968001. [Google Scholar] [CrossRef] [Green Version]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Zaharoff, D.A.; Hoffman, B.S.; Hooper, H.B.; Benjamin, C.J., Jr.; Khurana, K.K.; Hance, K.W.; Rogers, C.J.; Pinto, P.A.; Schlom, J.; Greiner, J.W. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009, 69, 6192–6199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Kurtz, S.L.; Yang, L.; Katz, M.D.; Zaharoff, D.A. Intravesical chitosan/interleukin-12 immunotherapy induces tumor-specific systemic immunity against murine bladder cancer. Cancer Immunol. Immunother. 2015, 64, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Dodane, V.; Amin Khan, M.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef]
- Smith, S.G.; Baltz, J.L.; Koppolu, B.P.; Ravindranathan, S.; Nguyen, K.; Zaharoff, D.A. Immunological mechanisms of intravesical chitosan/interleukin-12 immunotherapy against murine bladder cancer. Oncoimmunology 2017, 6, e1259050. [Google Scholar] [CrossRef]
- Santos, P.M.; Butterfield, L.H. Dendritic Cell-Based Cancer Vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef]
- Han, H.D.; Byeon, Y.; Jang, J.H.; Jeon, H.N.; Kim, G.H.; Kim, M.G.; Pack, C.G.; Kang, T.H.; Jung, I.D.; Lim, Y.T.; et al. In vivo stepwise immunomodulation using chitosan nanoparticles as a platform nanotechnology for cancer immunotherapy. Sci. Rep. 2016, 6, 38348. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.J.; Le, Q.V.; Kim, D.; Kim, Y.B.; Shim, G.; Oh, Y.K. High Molecular Weight Chitosan-Complexed RNA Nanoadjuvant for Effective Cancer Immunotherapy. Pharmaceutics 2019, 11, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, F.; Pinto, M.L.; Pereira, C.L.; Serre, K.; Barbosa, M.A.; Vermaelen, K.; Gärtner, F.; Gonçalves, R.M.; De Wever, O.; Oliveira, M.J. Chitosan/γ-PGA nanoparticles-based immunotherapy as adjuvant to radiotherapy in breast cancer. Biomaterials 2020, 257, 120218. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, R. IR 820 dye encapsulated in polycaprolactone glycol chitosan: Poloxamer blend nanoparticles for photo immunotherapy for breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Du, Q.; Liu, T.; Tan, L.; Niu, M.; Gao, L.; Huang, Z.; Fu, C.; Ma, T.; Meng, X.; et al. In Vivo Magnetic Resonance Imaging and Microwave Thermotherapy of Cancer Using Novel Chitosan Microcapsules. Nanoscale Res. Lett. 2016, 11, 334. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.K.; Sadeghi, M.; Pourbaghi-Masouleh, M.; Tenreiro, C. Scope of nanotechnology-based radiation therapy and thermotherapy methods in cancer treatment. Curr. Cancer Drug Targets 2012, 12, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Niloy, M.S.; Shakil, M.S.; Hossen, M.S.; Alam, M.; Rosengren, R.J. Promise of gold nanomaterials as a lung cancer theranostic agent: A systematic review. Int. Nano Lett. 2021, 11, 93–111. [Google Scholar] [CrossRef]
| Chitosan or Chitosan Derivative | Molecular Weight (M.W.) | Active Moiety | Cancer Type | Cell Line(s) | IC50 | Apoptosis | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chitosan | Low M.W. | N/A | Colon cancer | Ca9–22 | 800 ± 131.45 μg/mL | Yes | Cell cycle arrest in the G1/S phase, apoptosis induction via NF-kB mediated signaling pathways | [32,33] |
| SCS | 38 k Da | Sulfur | Breast cancer | MCF-7, MDA-MB-231 | 35.65 ± 1.44 μM (MCF-7), 36.9 8± 2.36 μM (MDA-MB-231) | Yes | Block cell cycle by inducing apoptosis and blocking FGF-2 medicated phosphorylation ERK | [38] |
| SBCS | 37 k Da | Sulfated benzaldehyde | Breast cancer | MCF-7, MDA-MB-231 | 33.24 ± 1.16 μM (MCF-7), 34.98 ± 1.19 μM (MDA-MB-231) | Yes | Block cell cycle by inducing apoptosis and blocking FGF-2 medicated phosphorylation ERK | [38] |
| CTC | <3 kDa (Chitosan only) | Thymine | Liver cancer | HepG2 | NR | NR | Selectively kills cancer cells | [34] |
| PPC | 12–14 kDa | Pyrrole | Ehrlich ascites carcinoma, breast cancer | EAC, MCF7 | NR | NR | NR | [35,41] |
| CMC | 194.6 kDa | Carboxymethyl | Liver cancer | H22 | NR | NR | Antiangiogenic activity by decreasing VEGF and stimulate immune activity via increase in IFN-γ and TNF-α level | [37] |
| QCOS | 6 to 7 kDa | Amino oligosaccharide | Cervical cancer, colon cancer | HeLa, SW480 | 0.45 mg/mL (HeLa), 0.52 mg/mL SW480 | No | Induce necrosis | [39] |
| SCOS | 6 to 7 kDa | Sufated oligosaccharide | Cervical Cancer, colon cancer | HeLa, SW480 | 0.20 mg/mL (HeLa), 0.50 mg/mL SW480 | No | Induce necrosis | [39] |
| CHEX | NR | Hexose | Cervical cancer, colon cancer | A549 | NR | Yes | Downregulates cyclin D1 and bcl-xl mRNA expression, and induce apoptosis | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795-816. https://doi.org/10.3390/polysaccharides2040048
Shakil MS, Mahmud KM, Sayem M, Niloy MS, Halder SK, Hossen MS, Uddin MF, Hasan MA. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides. 2021; 2(4):795-816. https://doi.org/10.3390/polysaccharides2040048
Chicago/Turabian StyleShakil, Md Salman, Kazi Mustafa Mahmud, Mohammad Sayem, Mahruba Sultana Niloy, Sajal Kumar Halder, Md. Sakib Hossen, Md. Forhad Uddin, and Md. Ashraful Hasan. 2021. "Using Chitosan or Chitosan Derivatives in Cancer Therapy" Polysaccharides 2, no. 4: 795-816. https://doi.org/10.3390/polysaccharides2040048








