Long-Term Chloride Accumulation on Concrete Surface in Marine Atmosphere Zone—Modelling the Influence of Exposure Time and Chloride Availability in Atmosphere
Abstract
:1. Introduction
2. Experimental Work
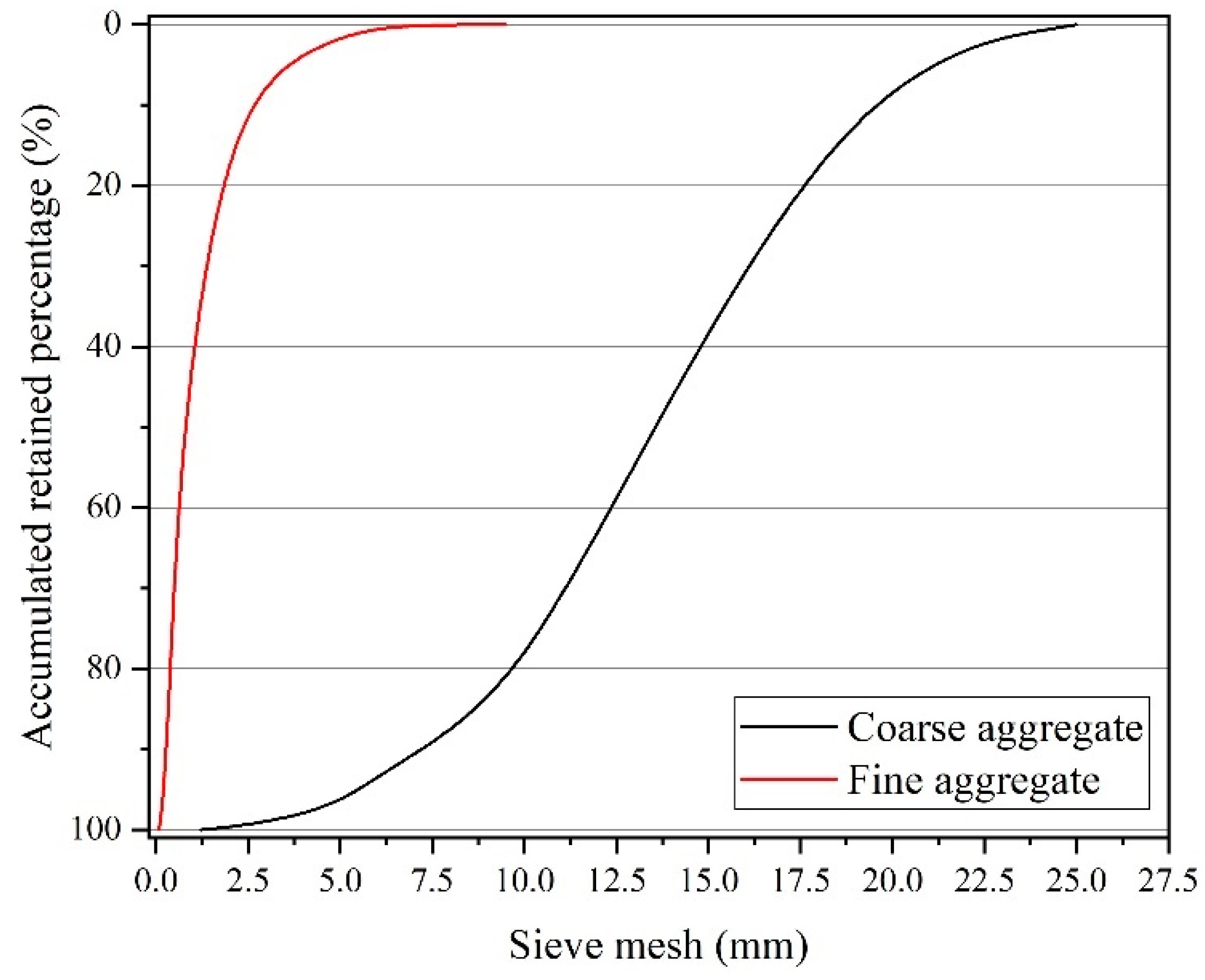
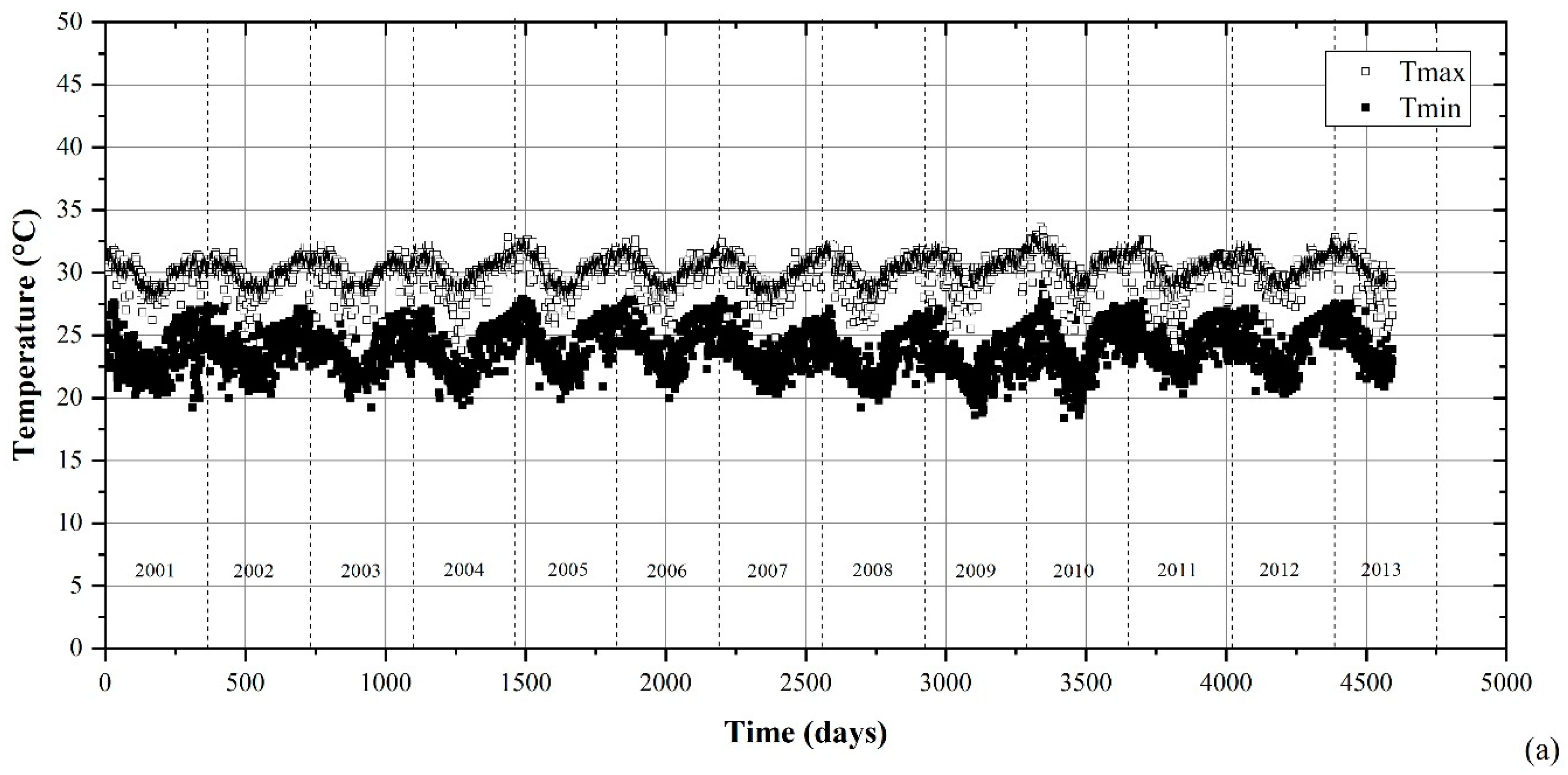
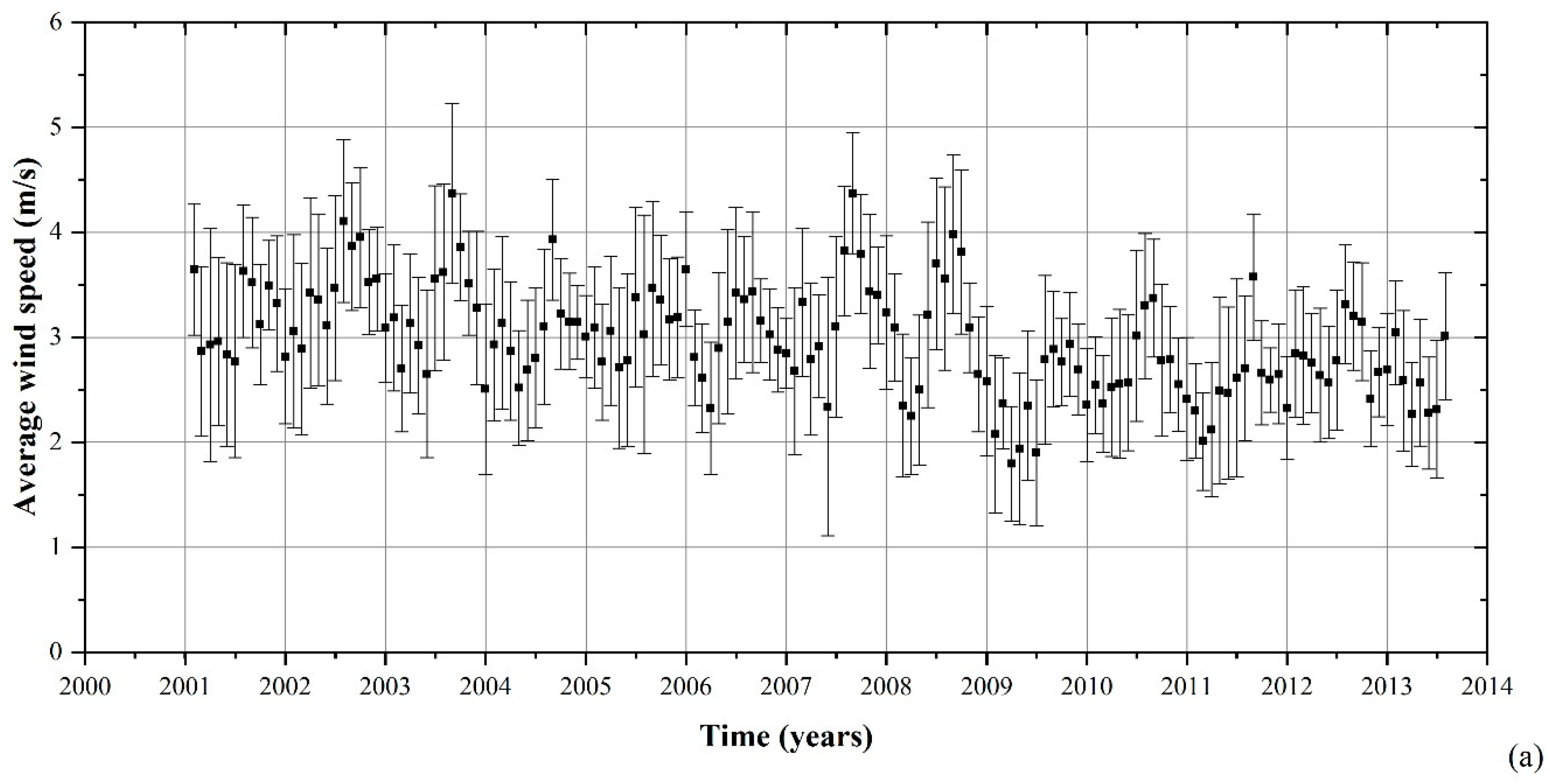
2.1. Environmental Characterization
2.2. Surface Chloride Concentration in Concrete
3. Results and Discussion
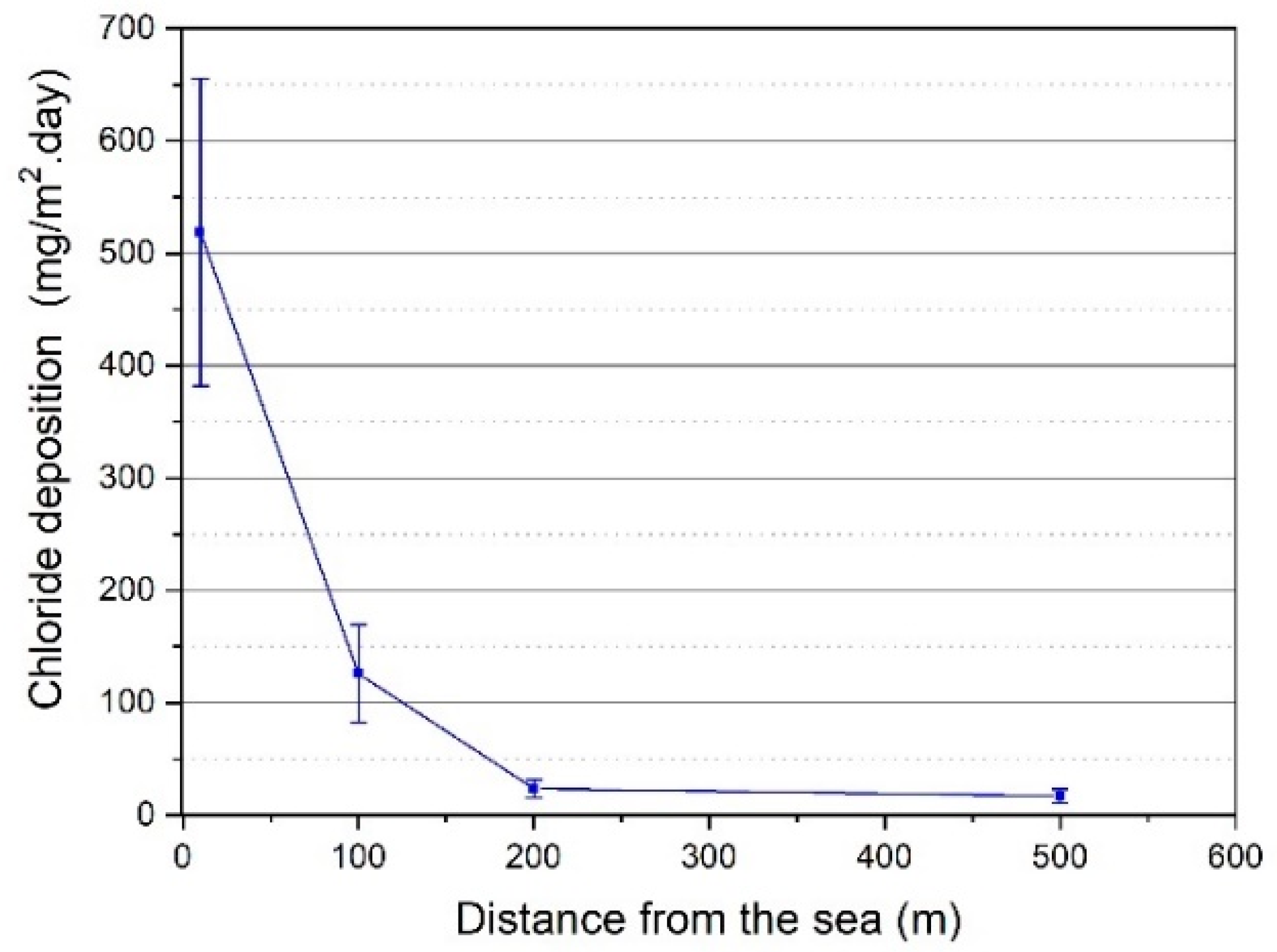
3.1. Environmental Parameters
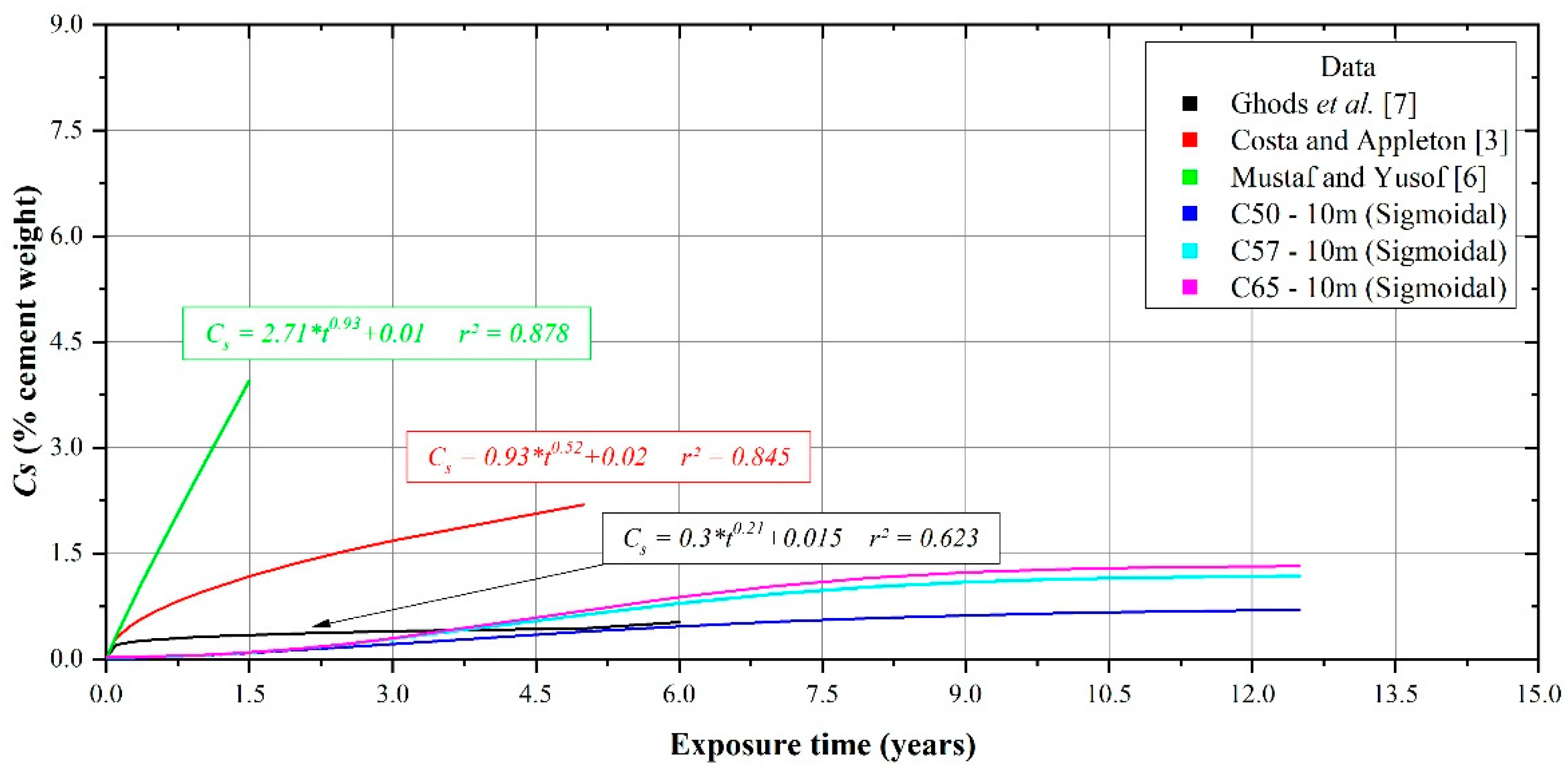
3.2. Surface Chloride Concentration and Its Relationship with Exposure Time
3.3. Surface Chloride Concentration and Its Relationship with Chloride Deposition on a Wet Candle
4. Conclusions
- (1)
- The increase in Cs with exposure time followed three stages: a first short stage characterised by an initial dispersion, followed by a period of increase and then a final period of stabilisation. Considering that the present data focused on the marine atmosphere zone, although the final period of stabilisation tended to reach a maximum, this condition was not fully reached in 12.5 years of exposure, indicating that more time is needed to reach the stabilisation condition in the marine atmosphere zone.
- (2)
- Considering the fact that the concrete porosity at the surface layer may change depending on the w/b of concrete, and that Cs refers to the chloride concentration in the first millimetre of the concrete, the influence of concrete porosity on Cs behaviour occurred in a direct way. Concrete with higher w/b and, thus, higher porosity presented a stronger increase in Cs, independently of the exposure site.
- (3)
- Chloride concentration in the atmosphere plays an important role in Cs behaviour. Higher availability of chlorides means higher Cs values. This leads to different curves for the increase in Cs depending on the availability of chlorides in the atmosphere at different distances from the sea. In terms of the exposure time, this behaviour can be represented by a power function or a sigmoidal function, with a better fit for the latter.
- (4)
- Regarding the relationship between Cs and the availability of chlorides in the atmosphere, the function that best represented this relationship was , where Cs is the surface chloride concentration, C0 is the initial chloride concentration in concrete, kcs is a coefficient associated with the concrete’s ability to capture chlorides from the atmosphere, Dac is the accumulated deposition of chlorides and n is a coefficient associated with the rate of the increase in Dac over time.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martín-Perez, B.; Lounois, Z. Numerical Modelling of Service Life of Reinforced Concrete Structures. In Proceedings of the 2nd International RILEM Workshop on Life Prediction and Aging Management of Concrete Structures, Paris, France, 5–6 May 2003; pp. 71–79. [Google Scholar]
- Bastidas-Arteaga, E.; Chateauneuf, A.; Sanchez-Silva, M.; Bresso-Lette, P.; Schoefs, F. A comprehensive probabilistic model of chloride ingress in unsaturated concrete. Eng. Struct. 2011, 33, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment—Part II: Prediction of long term chloride penetration. Mater. Struct. 1999, 32, 354–359. [Google Scholar] [CrossRef]
- Yang, L.F.; Cai, R.; Yu, B. Investigation of computational model for surface chloride concentration of concrete in marine atmosphere zone. Ocean. Eng. 2017, 138, 105–111. [Google Scholar] [CrossRef]
- Andrade, C.; Sagrera, J.L.; Sanjuán, M.A. Several years study on chloride ion penetration into concrete exposed to Atlantic Ocean water. In Proceedings of the Second International RILEM Workshop on Testing and Modelling Chloride Ingress into Concrete, Paris, France, 11–12 September 2000. [Google Scholar]
- Mustafa, A.M.; Yusof, C.M. Atmospheric chloride penetration into concrete in semi-tropical marine environment. Cem. Concr. Res. 1994, 4, 661–670. [Google Scholar] [CrossRef]
- Ghods, P.; Chini, M.; Alizadeh, R.; Hoseini, M.; Shekarchi, M.; Ramezanianpour, A.A. The Effect of Different Exposure Conditions on the Chloride Diffusion into Concrete in the Persian Gulf Region. In Proceedings of the 3rd International Conference on Construction Materials on Performance, Innovations and Structural Implications, Vancouver, BC, Canada, 22–24 August 2005. [Google Scholar]
- Uji, K.; Matsuoka, Y.; Maruya, T. Formulation of Equation for Surface Chloride Content due to Permeation of Chloride. In Proceedings of the Corrosion of Reinforcement in Concrete 1990, Belfast, Ireland, 21–24 May 1990; pp. 258–267. [Google Scholar]
- Kassir, M.K.; Ghosn, M. Chloride-induced corrosion of reinforced concrete bridge decks. Cem. Concr. Compos. 2002, 32, 139–143. [Google Scholar] [CrossRef]
- Pack, S.; Jung, M.; Song, H.; Kim, S.H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structure exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Zhou, S. Analytical model for square root increase of surface chloride concentration and decrease of chloride diffusivity. J. Mater. Civ. Eng. (ASCE) 2016, 28, 040151811–040151817. [Google Scholar] [CrossRef]
- Meira, G.R. Chloride Aggressiveness in Marine Atmosphere Zone Connected with Corrosion Problems in Reinforced Concrete Structures. Ph.D. Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 2004. (In Portuguese). [Google Scholar]
- Maheswaran, T.; Sanjayan, J.G. A semi-closed-form solution for chloride diffusion in concrete with time-varying parameters. Cem. Concr. Res. 2004, 56, 359–366. [Google Scholar] [CrossRef]
- Sandberg, P.; Tang, L.; Andersen, A. Recurrent studies of chloride ingress in uncracked marine concrete at various exposure times and elevations. Cem. Concr. Res. 1998, 28, 1489–1503. [Google Scholar] [CrossRef] [Green Version]
- ASTM G-140-02; Standard Test Method for Determining Atmospheric Chloride Deposition Rate by Wet Candle Method. Annual Book of ASTM Standards: Philadelphia, PA, USA, 2014.
- Andrade, C.; Alonso, C. Modelling of Skin Effects on Diffusion Process in Concrete. In Proceedings of the International RILEM Workshop on Chloride Penetration into Concrete; Nilsson, L.O., Ollivier, J.P., Eds.; RILEM: Paris, France, 1995; pp. 182–194. [Google Scholar]
- Castro, P.; Rincón, O.T.; Pazini, E.J. Interpretation of chloride profiles from concrete exposed to tropical marine environments. Cem. Concr. Res. 2001, 31, 529–537. [Google Scholar] [CrossRef]
- RILEM TC 178-TMC. Analysis of total chloride content in concrete. Mater. Struct. 2002, 35, 583–588. [Google Scholar]
- Cole, I.S.; Paterson, D.A.; Ganther, W.D. Holistic model for atmospheric corrosion Part 1—Theoretical framework for production, transportation and deposition of marine salts. Corros. Eng. Sci. Technol. 2003, 38, 129–134. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Padaratz, I.J.; Alonso, C.; Borba, J.C., Jr. Measurements and modelling of marine salt transportation and deposition in a tropical region in Brazil. Atmos. Environ. 2006, 40, 5596–5607. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Padaratz, I.J.; Borba, J.C., Jr. Modelling sea-salt transport and deposition in marine atmosphere zone—A tool for corrosion studies. Corros. Sci. 2008, 50, 2724–2731. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Padaratz, I.J.; Borba, J.C., Jr. Salinity of marine aerosols in a Brazilian coastal area—Influence of wind regime. Atmos. Environ. 2007, 41, 8431–8441. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chiu, H.J.; Chan, Y.W.; Chang, Y.C.; Yang, C.C. The correlation between airborne salt and chlorides cumulated on concrete surface in the marine atmosphere zone in north taiwan. J. Mar. Sci. Technol. 2013, 21, 24–34. [Google Scholar]






| Source of Data | Exposure Time (Years) | Function | Authors | Year |
|---|---|---|---|---|
| Japan | 23–58 | Cs = at0.5 | Uji et al. [8] | 1990 |
| Portugal | 0.5–5.5 | Cs = atb | Costa and Appleton [3] | 1999 |
| United States of America | 2–16 | Cs = a (a (1 − ebt)) | Kassir et al. [9] | 2002 |
| South Korea | 0.7–48.7 | Cs = aLn(bt + 1) + c | Pack et al. [10] | 2010 |
| Data from the literature | 0–3 | Cs = a + bt0.5 | Zhou et al. [11] | 2016 |
| Data from the literature | 0–5 | Cs = a(1 − ebt) | Yang et al. [4] | 2017 |
| Composition (%) | SO3 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Insoluble Residue (IR) | Loss on Ignition (LI) |
| 3.21 | 18.11 | 4.31 | 2.27 | 59.87 | 3.61 | 0.21 | 1.51 | 1.45 | 5.50 | |
| Property | Specific surface (cm2/g) | Specific density (g/cm3) | ||||||||
| 3650 | 3.06 | |||||||||
| Concrete | C50 | C57 | C65 |
|---|---|---|---|
| Mixture | |||
| Cement (kg/m3) | 406 | 356 | 320 |
| Sand (kg/m3) | 769 | 812 | 840 |
| Coarse aggregate (kg/m3) | 947 | 947 | 947 |
| Plasticiser (kg/m3) | 1.22 | 1.06 | - |
| w/b | 0.5 | 0.57 | 0.65 |
| Property | |||
| Slump (mm) | 80 | 80 | 80 |
| Compressive strength (MPa—28 days) | 31 | 27 | 20 |
| Concrete porosity (% volume—90 days) | 11.0 | 12.4 | 13.2 |
| Function | Concrete | Distance from the Sea | |||
|---|---|---|---|---|---|
| 10 m | 100 m | 200 m | 500 m | ||
| Cs (% Cement Weight)—After 50 Years | |||||
| Power function | C65 | 4.94 | 4.52 | 2.58 | 2.33 |
| C57 | 4.54 | 3.44 | 2.72 | 1.97 | |
| C50 | 2.62 | 2.07 | 1.96 | 2.04 | |
| Sigmodal function | C65 | 1.32 | 0.95 | 0.56 | 0.47 |
| C57 | 1.19 | 0.75 | 0.47 | 0.33 | |
| C50 | 0.70 | 0.43 | 0.31 | 0.29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meira, G.R.; Ferreira, P.R.; Andrade, C. Long-Term Chloride Accumulation on Concrete Surface in Marine Atmosphere Zone—Modelling the Influence of Exposure Time and Chloride Availability in Atmosphere. Corros. Mater. Degrad. 2022, 3, 349-362. https://doi.org/10.3390/cmd3030021
Meira GR, Ferreira PR, Andrade C. Long-Term Chloride Accumulation on Concrete Surface in Marine Atmosphere Zone—Modelling the Influence of Exposure Time and Chloride Availability in Atmosphere. Corrosion and Materials Degradation. 2022; 3(3):349-362. https://doi.org/10.3390/cmd3030021
Chicago/Turabian StyleMeira, Gibson Rocha, Pablo Ramon Ferreira, and Carmen Andrade. 2022. "Long-Term Chloride Accumulation on Concrete Surface in Marine Atmosphere Zone—Modelling the Influence of Exposure Time and Chloride Availability in Atmosphere" Corrosion and Materials Degradation 3, no. 3: 349-362. https://doi.org/10.3390/cmd3030021







