Dynamic Interfacial Properties and Foamability of DoTAB/SiO2 Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
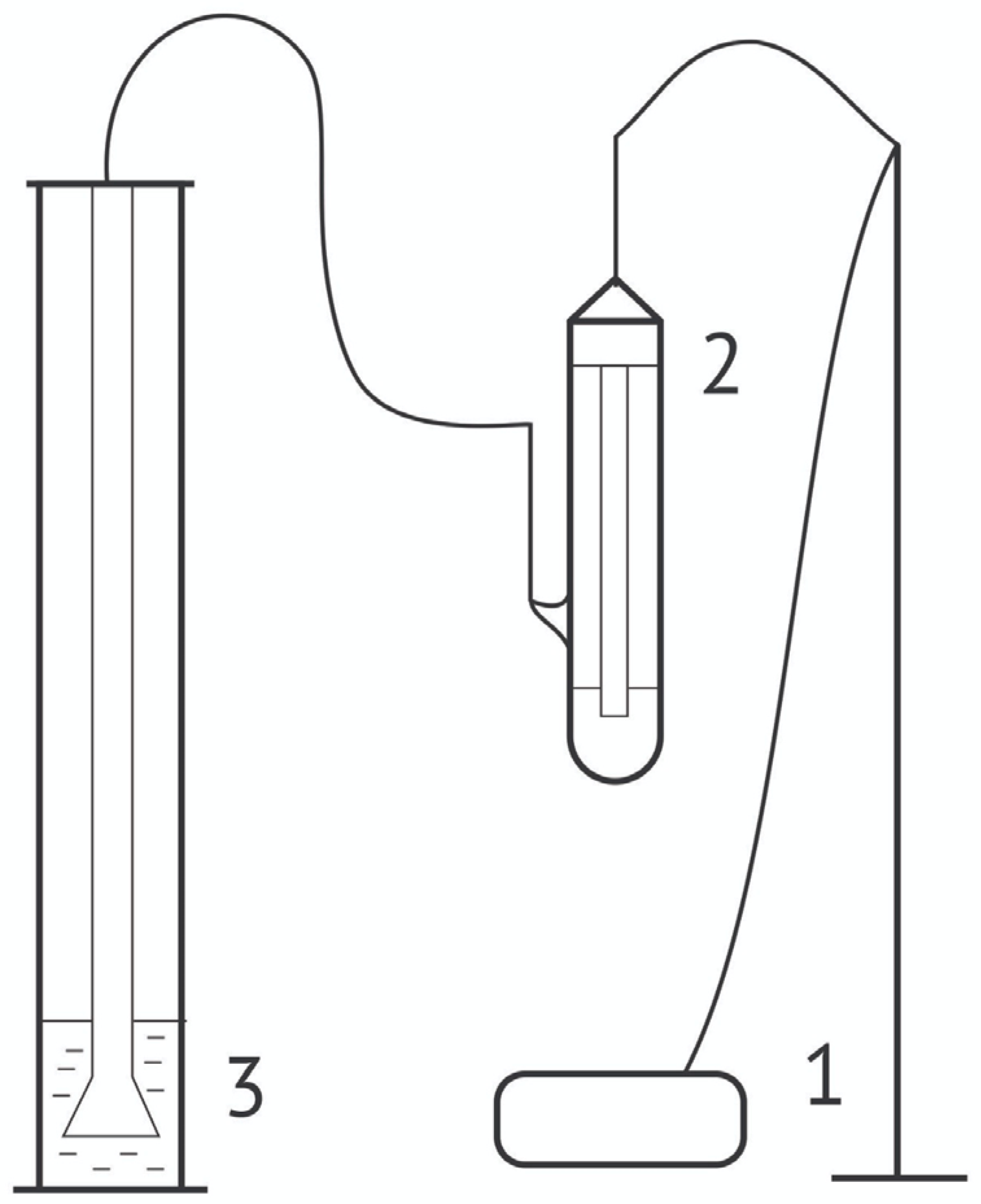
2.2.1. Dynamic Surface Tension and Dilational Visco-Elasticity Measurements
2.2.2. Foamability and Foam Stability Measurements
3. Results
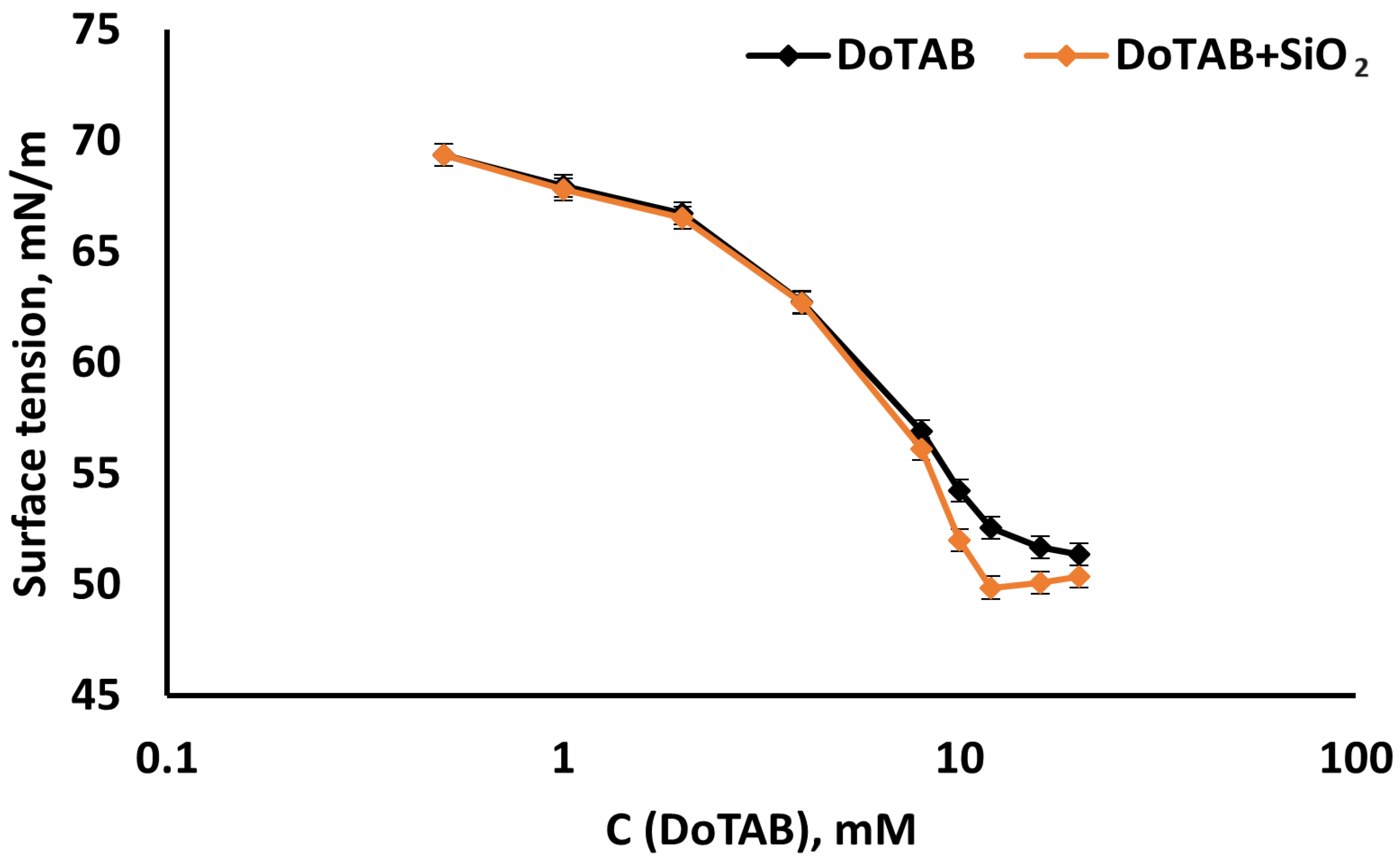
3.1. Equilibrium Surface Tension
3.2. Dynamic Surface Tension
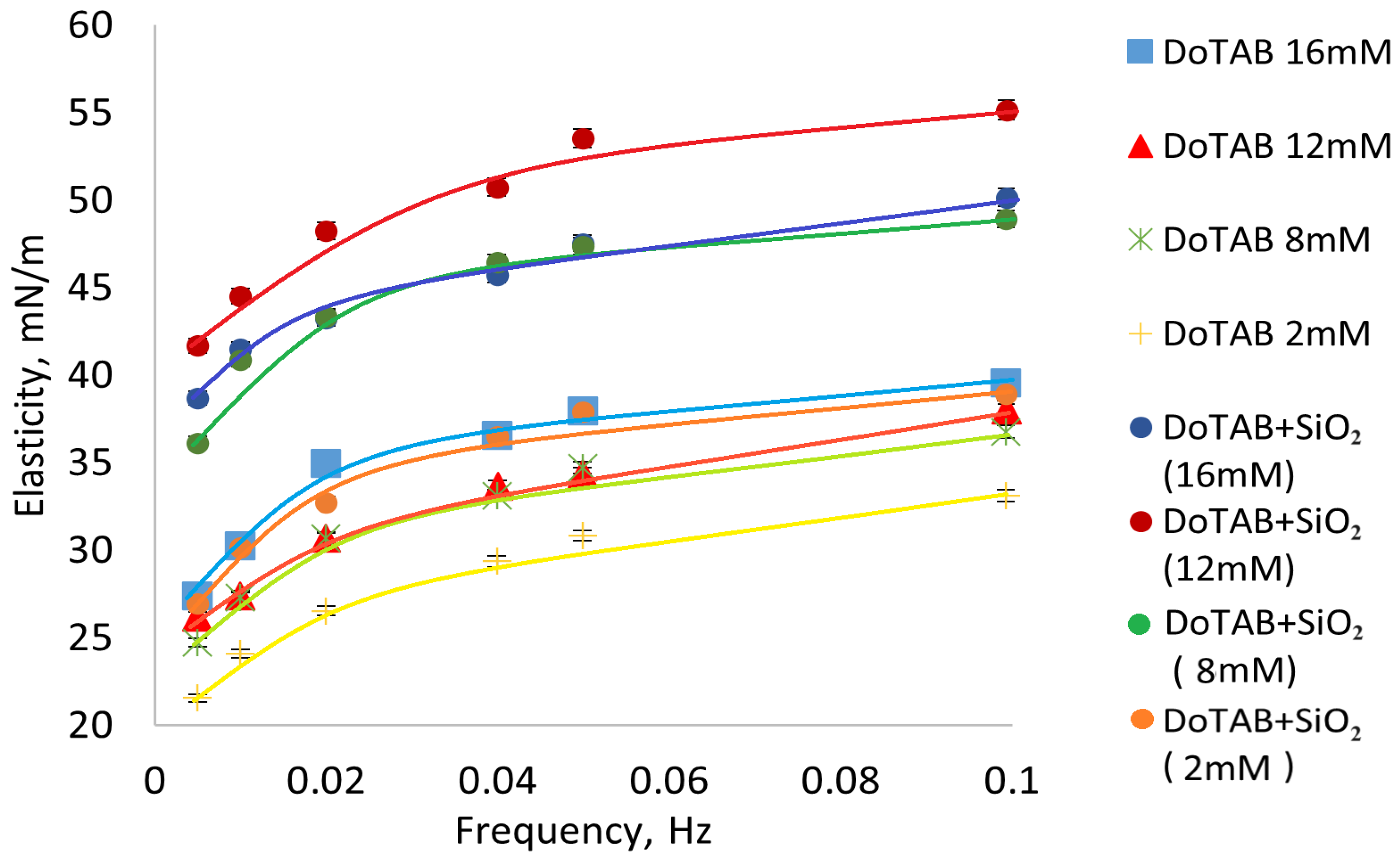
3.3. Elasticity Measurements
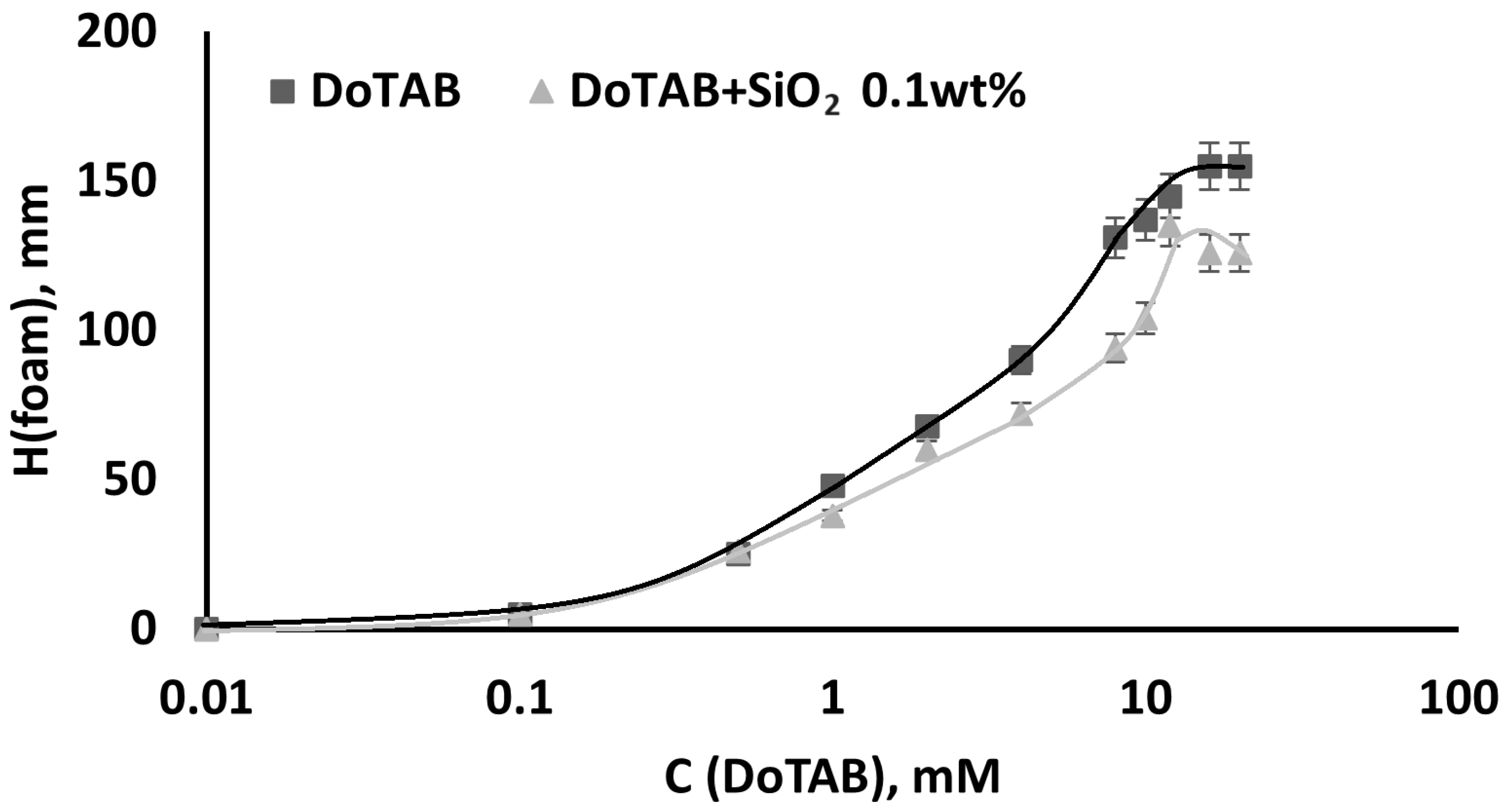
3.4. Foamability and Foam Stability
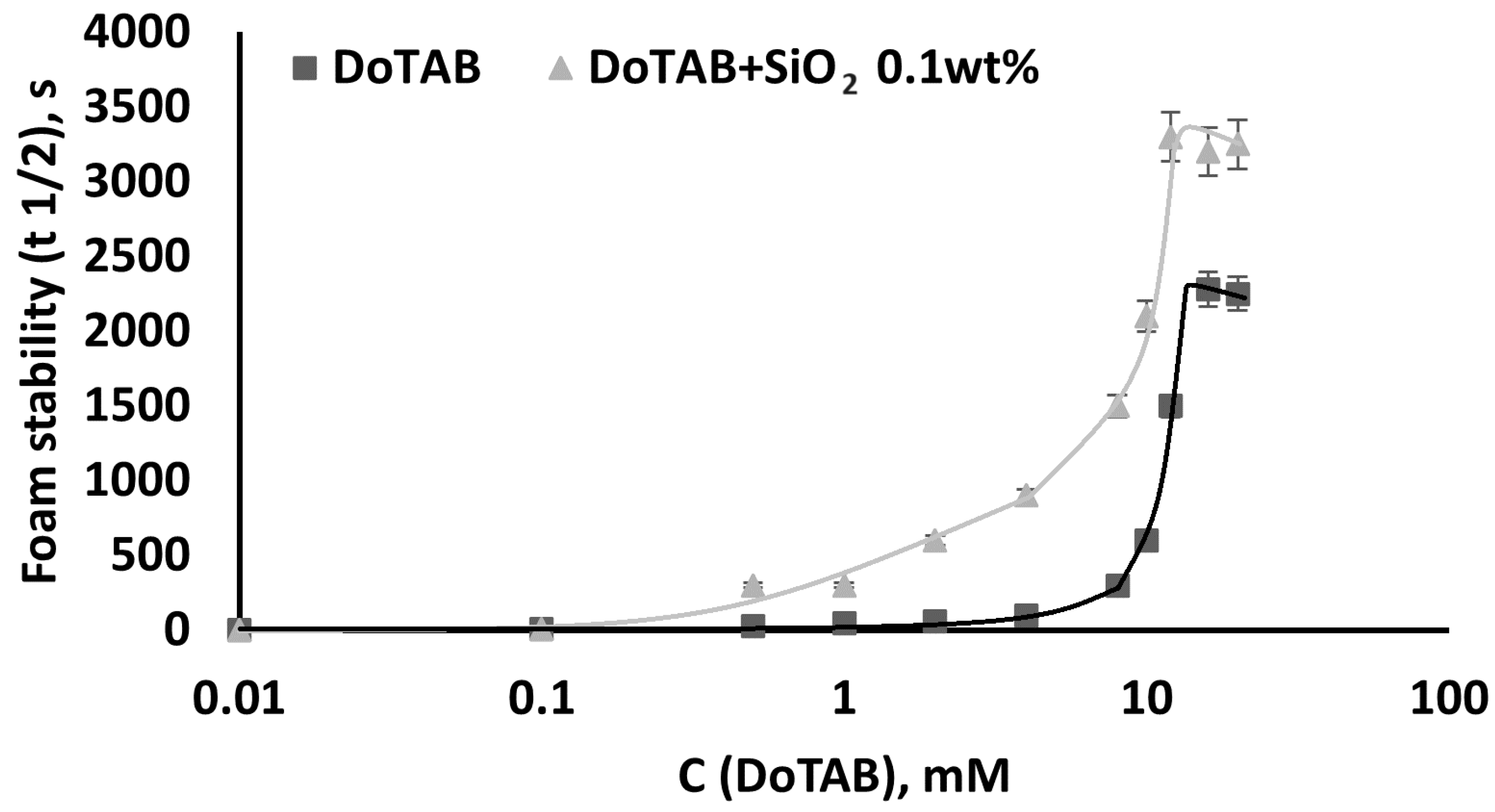
3.4.1. Dependence of Foam Stability of DoTAB/SiO2 Mixed Solutions on Surfactant Concentration
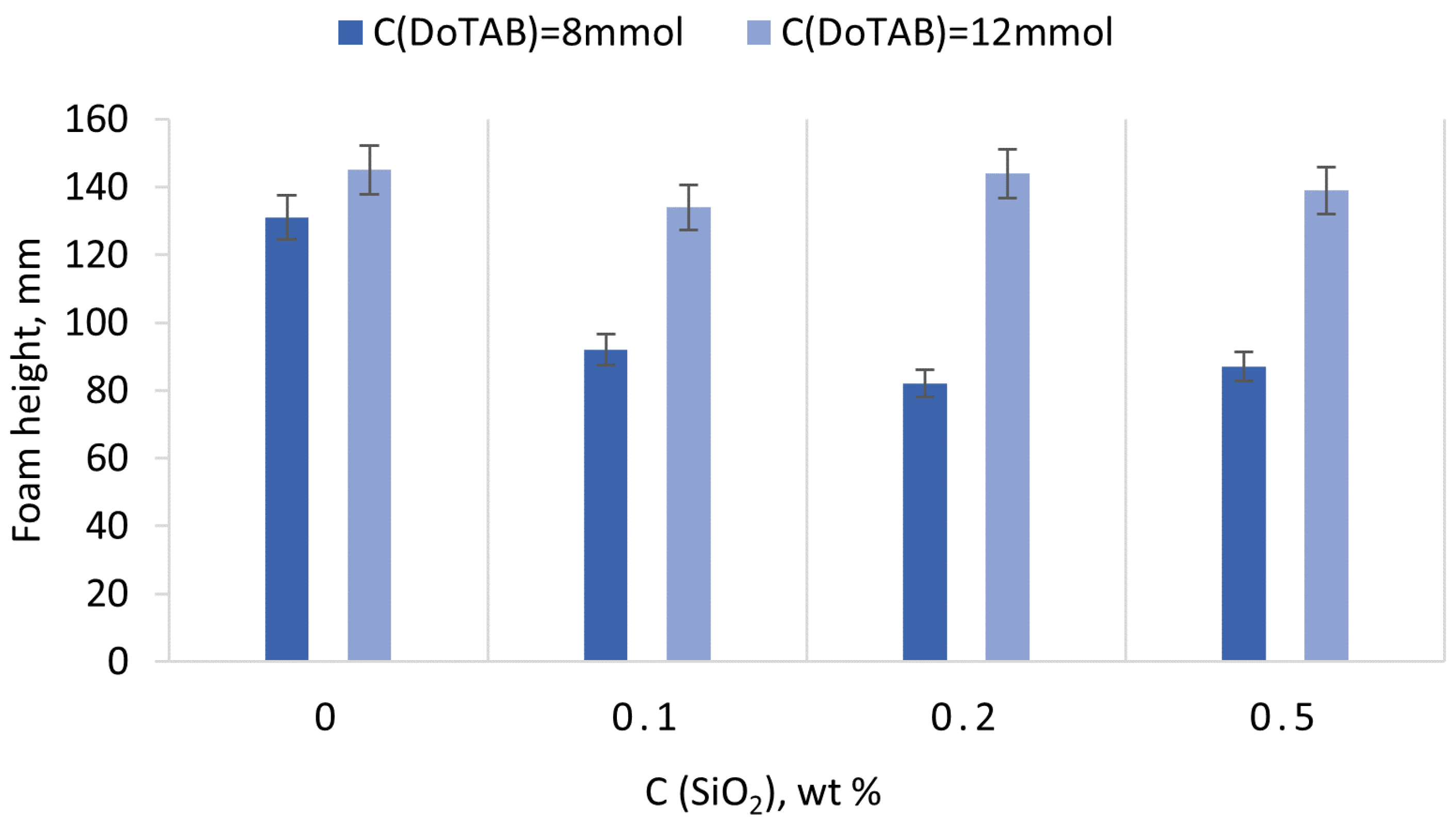
3.4.2. Dependence of Foam Stability of DoTAB/SiO2 Mixed Solutions on Nanoparticle Concentration
4. Conclusions
- (i)
- The equilibrium surface tension isotherms illustrate a notable decrease in surface tension with the addition of SiO2 nanoparticles, particularly at DoTAB concentrations above 6 mM. This suggests that the presence of SiO2 has a surfactant-enhancing effect on reducing surface tension, with the most significant impact observed at DoTAB 12 mM. Dynamic surface tension measurements further revealed the intricate behavior of DoTAB/SiO2 mixtures, emphasizing the importance of surfactant concentration in influencing the adsorption and desorption dynamics. The plateau of elasticity values around DoTAB 12 mM indicated optimal conditions for the adsorption of DoTAB/SiO2 associates.
- (ii)
- The surface visco-elasticity measured during drop oscillations corroborated the trends observed in dynamic surface tension, emphasizing the dynamics of large associates in DoTAB/SiO2 mixtures compared to individual DoTAB. The frequency-dependent behavior highlighted the unique interfacial characteristics of these mixtures.
- (iii)
- Foamability was found to be dependent on the DoTAB concentration, with higher concentrations yielding superior foamability. Importantly, the addition of SiO2 did not significantly influence foamability but markedly improved foam stability, even at low nanoparticle concentrations (0.1 wt% SiO2). The stable foams observed at low SiO2 concentrations suggest a cost-effective strategy for enhancing foam stability. The stability of foam in DoTAB 12 mM solutions remained consistently high across varying SiO2 concentrations, emphasizing the significance of the CAC region. This points to an optimal concentration range for nanoparticle/surfactant mixtures, where the interaction within the CAC region leads to the formation of stable associates with superior surface activity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Liu, Q.; Ye, H.; Yang, L.; Luo, D.; Peng, B. Nanoparticles as foam stabilizer: Mechanism, control parameters and application in foam flooding for enhanced oil recovery. J. Pet. Sci. Eng. 2021, 202, 108561. [Google Scholar] [CrossRef]
- Hunter, T.; Pugh, R.; Franks, G.; Jameson, G. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- Stocco, A.; Rio, E.; Binks, B.P.; Langevin, D. Aqueous foams stabilized solely by particles. Soft Matter 2011, 7, 1260–1267. [Google Scholar] [CrossRef]
- Issakhov, M.; Shakeel, M.; Pourafshary, P.; Aidarova, S.; Sharipova, A. Hybrid surfactant-nanoparticles assisted CO2 foam flooding for improved foam stability: A review of principles and applications. Pet. Res. 2022, 7, 186–203. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, P.; Xu, M.B.; Pu, L.; Zhang, Y. Synergistic improvement of foam stability with SiO2 nanoparticles (SiO2-NPs) and different surfactants. Arab. J. Chem. 2023, 16, 104394. [Google Scholar]
- Jia, H.; Huang, W.; Han, Y.; Wang, Q.; Wang, S.; Dai, J.; Tian, Z.; Wang, D.; Yan, H.; Lv, K. Systematic investigation on the interaction between SiO2 nanoparticles with different surface affinity and various surfactants. J. Mol. Liq. 2020, 304, 112777. [Google Scholar] [CrossRef]
- Suleymani, M.; Ghotbi, C.; Ashoori, S.; Moghadasi, J.; Kharrat, R. Theoretical and experimental study of foam stability mechanism by nanoparticles: Interfacial, bulk, and porous media behaviour. J. Mol. Liq. 2020, 304, 112739. [Google Scholar] [CrossRef]
- Briceño-Ahumada, Z.; Soltero-Martínez, J.F.A.; Castillo, R. Aqueous foams and emulsions stabilized by mixtures of silica na-noparticles and surfactants: A state-of-the-art review. Chem. Eng. J. Adv. 2021, 7, 100116. [Google Scholar] [CrossRef]
- Eftekhari, M.; Schwarzenberger, K.; Javadi, A.; Eckert, K. The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: Electrostatic repulsion or the effect of ionic strength. Phys. Chem. Chem. Phys. 2020, 22, 2238–2248. [Google Scholar] [CrossRef]
- Jangir, K.; Kumar, U.; Suniya, K.N.; Singh, N.; Parihar, K. Enhancing the Aqueous foam Stability Using Nanoparticles: A Review. World J. Chem. Educ. 2022, 10, 84–90. [Google Scholar] [CrossRef]
- Khajehpour, M.; Reza Etminan, S.; Goldman, J.C.; Wassmuth, F.R.; Bryant, S.L. Nanoparticles as Foam Stabilizer for Steam-Foam Process. SPE J. 2018, 23, 2232–2242. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K. Synergistic effects of nanoparticles and surfactants on n-decane-water interfacial tension and bulk foam stability at high temperature. J. Pet. Sci. Eng. 2019, 179, 814–830. [Google Scholar] [CrossRef]
- Yazhgur, P.A.; Noskov, B.A.; Liggieri, L.; Lin, S.-Y.; Loglio, G.; Miller, R.; Ravera, F. Dynamic properties of mixed nanoparti-cle/surfactant adsorption layers. Soft Matter 2013, 9, 3305–3314. [Google Scholar] [CrossRef]
- Ahmadi, A.; Saeedi Dehaghani, A.H.; Saviz, S. Experimental Study of SDS Foam Stability in the Presence of Silica Nanoparticle. J. Chem. Pet. Eng. 2022, 56, 203–213. [Google Scholar]
- Whitby, C.P.; Fornasiero, D.; Ralston, J.; Liggieri; Ravera, F. Properties of Fatty Amine–Silica Nanoparticle Interfacial Layers at the Hexane–Water Interface. J. Phys. Chem. C 2012, 116, 3050–3058. [Google Scholar] [CrossRef]
- Maestro, A.; Rio, E.; Drenckhan, W.; Langevin, D.; Salonen, A. Foams stabilised by mixtures of nanoparticles and oppositely charged surfactants: Relationship between bubble shrinkage and foam coarsening. Soft Matter 2014, 10, 6975–6983. [Google Scholar] [CrossRef]
- Amankeldi, F.; Issakhov, M.; Pourafshary, P.; Ospanova, Z.; Gabdullin, M.; Miller, R. Foam stabilization by surfactant/SiO2 composite nanofluids. Colloids Interfaces 2023, 7, 57. [Google Scholar] [CrossRef]
- Wang, P.; You, Q.; Han, L.; Deng, W.; Liu, Y.; Fang, J.; Gao, M.; Dai, C. Experimental study on the stabilization mechanisms of CO2 foams by hydrophilic silica nanoparticles. Energy Fuels 2018, 32, 3709–3715. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergy between nanoparticles and surfactants in stabilizing foams for oil recovery. Energy Fuels 2015, 29, 467–479. [Google Scholar] [CrossRef]
- Eftekhari, M.; Schwarzenberger, K.; Karakashev, S.I.; Grozev, N.A.; Eckert, K. Oppositely charged surfactants and nano-particles at the air-water interface: Influence of surfactant to nanoparticle ratio. J. Colloid Interface Sci. 2024, 653, 1388–1401. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Zhang, W.-J.; Lai, L.; Mei, P.; Wu, L.-M.; Wang, Y.-Q. Different cationic surfactants-modified silica nanoparticles for Pickering emulsions. J. Mol. Liq. 2019, 291, 111341. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, S.; Zhang, K.; Zhao, M.; Jiao, B.; Dai, C. The stability mechanism of nitrogen foam in porous media with silica nanoparticles modified by cationic surfactants. Langmuir 2018, 34, 8015–8023. [Google Scholar] [CrossRef] [PubMed]
- Mondragon, R.; Julia, J.E.; Barba, A.; Jarque, J.C. Determination of the packing fraction of silica nanoparticles from the rhe-ological and viscoelastic measurements of nanofluids. Chem. Eng. Sci. 2012, 80, 119–127. [Google Scholar] [CrossRef]
- Lindman, B.; Antunes, F.; Aidarova, S.; Miguel, M.; Nylander, T. Polyelectrolyte-surfactant association—From fundamentals to applications. Colloid J. 2014, 76, 585–594. [Google Scholar] [CrossRef]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of Nanoparticles on the Interfacial Properties of Liquid/Liquid and Liquid/Air Surface Layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Yazhgur, P.A.; Liggieri, L.; Lin, S.-Y.; Loglio, G.; Miller, R.; Ravera, F. Dilational rheology of spread and ad-sorbed layers of silica nanoparticles at the liquid-gas interface. Colloid J. 2014, 76, 127–138. [Google Scholar] [CrossRef]
- Santini, E.; Krägel, J.; Ravera, F.; Liggieri, L.; Miller, R. Study of the monolayer structure and wettability properties of silica nanoparticles and CTAB using the Langmuir trough technique. Colloids Surf. A 2011, 382, 186–191. [Google Scholar] [CrossRef]
- Liggieri, L.; Santini, E.; Guzman, E.; Maestro, A.; Ravera, F. Wide-frequency dilational rheology investigation of mixed silica nanoparticle—CTAB interfacial layers. Soft Matter 2011, 7, 7699–7709. [Google Scholar] [CrossRef]
- Maestro, A.; Guzman, E.; Santini, E.; Ravera, F.; Liggieri, L.; Ortega, F. Wettability of silica nanoparticle—Surfactant nano-composite interfacial layers. Soft Matter 2012, 8, 837–843. [Google Scholar] [CrossRef]
- Ravera, F.; Ferrari, M.; Liggieri, L.; Loglio, G.; Santini, E.; Zanobini, A. Liquid-liquid interfacial properties of mixed nanoparticle—Surfactant systems. Colloids Surf. A 2008, 323, 99–108. [Google Scholar] [CrossRef]
- Lan, Q.; Yang, F.; Zhang, S.; Liu, S.; Xu, J.; Sun, D. Synergistic effect of silica nanoparticle and cetyltrimethyl ammonium bromide on the stabilization of O/W emulsions. Colloids Surf. A 2007, 302, 126–135. [Google Scholar] [CrossRef]
- Ravera, F.; Loglio, G.; Pandolfini, P.; Santini, E.; Liggieri, L. Determination of the dilational visco-elasticity by the oscillating drop/bubble method in a capillary pressure tensiometer. Colloids Surf. A 2010, 365, 2–13. [Google Scholar] [CrossRef]
- Jaensson, N.O.; Vermant, J. Tensiometry and rheology of complex interfaces, Curr. Opin. Colloid Interface Sci. 2018, 37, 136–150. [Google Scholar]
- Yu, K.; Yang, J.; Zuo, Y.Y. Droplet Oscillation as an Arbitrary Waveform Generator. Langmuir 2018, 34, 7042–7047. [Google Scholar] [CrossRef]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley: New York, NY, USA, 2004; pp. 185–187. [Google Scholar]



| System | CMC, mmol | , mN·m−1 | × 10−4, mN·m2· kmole−1 | × 109, kmole·m−2 | × 1020, m2 | ||
|---|---|---|---|---|---|---|---|
| kJ·mole−1 | |||||||
| DoTAB | 10.85·10−3 | 54.2 | 16.4 | 6.9 | 24 | 11.3 | 13.7 |
| DoTAB + SiO2 | 11.28·10−3 | 50.8 | 18.8 | 6.1 | 27 | 11.1 | 14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amankeldi, F.; Gabdullin, M.; Issakhov, M.; Ospanova, Z.; Sharipova, A.; Aidarova, S.; Miller, R. Dynamic Interfacial Properties and Foamability of DoTAB/SiO2 Mixtures. Colloids Interfaces 2024, 8, 19. https://doi.org/10.3390/colloids8020019
Amankeldi F, Gabdullin M, Issakhov M, Ospanova Z, Sharipova A, Aidarova S, Miller R. Dynamic Interfacial Properties and Foamability of DoTAB/SiO2 Mixtures. Colloids and Interfaces. 2024; 8(2):19. https://doi.org/10.3390/colloids8020019
Chicago/Turabian StyleAmankeldi, Fariza, Maratbek Gabdullin, Miras Issakhov, Zhanar Ospanova, Altynay Sharipova, Saule Aidarova, and Reinhard Miller. 2024. "Dynamic Interfacial Properties and Foamability of DoTAB/SiO2 Mixtures" Colloids and Interfaces 8, no. 2: 19. https://doi.org/10.3390/colloids8020019






