Synthesis of Hollow Silica Nanocubes with Tuneable Size and Shape, Suitable for Light Scattering Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
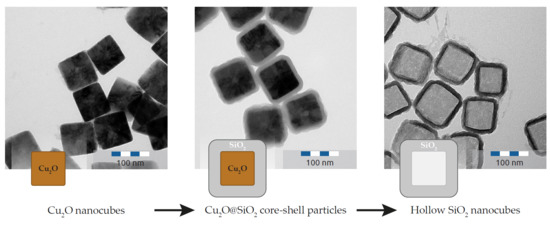
2.2. Synthesis of Cuprous Oxide Nanocubes
2.3. Silica Coated Nanocubes
2.4. Dissolution of the Cuprous Oxide Core
2.5. Transmission Electron Microscopy Sample Preparation
2.6. Infrared Spectroscopy Sample Preparation
2.7. Elemental Analysis
3. Results and Discussion
3.1. Synthesis Method
3.2. Particle Size
3.3. Silica Coating and Hollow Silica Nanocubes
3.4. Particle shape
3.5. Colloidal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damasceno, P.F.; Engel, M.; Glotzer, S.C. Predictive self-assembly of polyhedra into complex structures. Science 2012, 337, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Vrij, A.; Tuinier, R. Structure of Concentrated Colloidal Dispersions. In Fundamentals of Interface and Colloids Science Volume IV Particulate Colloids; Lyklema, J., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2005; p. 692. ISBN 9780124605299. [Google Scholar]
- Meijer, J.M.; Pal, A.; Ouhajji, S.; Lekkerkerker, H.N.W.; Philipse, A.P.; Petukhov, A.V. Observation of solid-solid transitions in 3D crystals of colloidal superballs. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Soni, V.; Ashton, D.J.; Pine, D.J.; Philipse, A.P.; Chaikin, P.M.; Dijkstra, M.; Sacanna, S.; Irvine, W.T.M. Shape-sensitive crystallization in colloidal superball fluids. Proc. Natl. Acad. Sci. USA 2015, 112, 5286–5290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lu, F.; Van Der Lelie, D.; Gang, O. Continuous phase transformation in nanocube assemblies. Phys. Rev. Lett. 2011, 107, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Batten, R.D.; Stillinger, F.H.; Torquato, S. Phase behavior of colloidal superballs: Shape interpolation from spheres to cubes. Phys. Rev. E 2010, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M. Entropy-Driven Phase Transitions in Colloids: From spheres to anisotropic particles. In Advances in Chemical Physics; Rice, S.A., Dinner, A.R., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; Volume 156, pp. 35–71. ISBN 9781118949702. [Google Scholar]
- Castillo, S.I.R. Cubic colloids: Synthesis, functionalization and applications. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, January 2015. [Google Scholar]
- Philipse, A.P.; Vrij, A. Determination of static and dynamic interactions between monodisperse, charged silica spheres in an optically matching, organic solvent. J. Chem. Phys. 1988, 88, 6459–6470. [Google Scholar] [CrossRef]
- Yang, H.; Min, Y.; Kim, Y.J.; Jeong, U. Preparation of Cu2O@SiO2 particles and their evolution to hollow SiO2 particles. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 420, 30–36. [Google Scholar] [CrossRef]
- Wang, Z.; Snyder, M.A.; Fan, W.; Tsapatsis, M. Hollow cubic silica shells and assembled porous coatings. Scr. Mater. 2010, 62, 504–507. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, K.; Lin, M.; Hou, B.; Zhong, L.; Zhu, Y.; Wei, W.; Sun, Y. Controlled fabrication of iron oxide/mesoporous silica core-shell nanostructures. J. Phys. Chem. C 2013, 117, 21529–21538. [Google Scholar] [CrossRef]
- Han, Y.S.; Jeong, G.Y.; Lee, S.Y.; Moon, K.H.; Kim, H.K. Synthesis of cubic type hollow silica particles. Mater. Lett. 2009, 63, 1278–1280. [Google Scholar] [CrossRef]
- Kong, L.; Liu, X.; Bian, X.; Wang, C. Silica nanocubes with a hierarchically porous structure. RSC Adv. 2012, 2, 2887–2894. [Google Scholar] [CrossRef]
- Musa, A.O.; Akomolafe, T.; Carter, M.J. Production of cuprous oxide, a solar cell material, by thermal oxidation and a study of its physical and electrical properties. Sol. Energy Mater. Sol. Cells 1998, 51, 305–316. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Hara, M.; Shinohara, K.; Tanaka, A. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 2, 357–358. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Gao, F.; Lu, Q. Morphology effect on antibacterial activity of cuprous oxide. Chem. Commun. 2009, 1076. [Google Scholar] [CrossRef] [PubMed]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O.I. Novel NO2 gas sensor based on cuprous oxide thin films. Sens. Actuators B Chem. 2006, 113, 468–476. [Google Scholar] [CrossRef]
- Huang, W.C.; Lyu, L.M.; Yang, Y.C.; Huang, M.H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-scale synthesis of Cu2O nanocubes and subsequent oxidation to CuO hollow nanostructures for lithium-ion battery anode materials. Adv. Mater. 2009, 21, 803–807. [Google Scholar] [CrossRef]
- Carbó-Argibay, E.; Bao, X.Q.; Rodríguez-Abreu, C.; Fátima Cerqueira, M.; Petrovykh, D.Y.; Liu, L.; Kolen’ko, Y.V. Up-scaling the synthesis of Cu2O submicron particles with controlled morphologies for solar H2 evolution from water. J. Colloid Interface Sci. 2015, 456, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Murphy, C.J. Solution-phase synthesis of Cu2O nanocubes. Nano Lett. 2003, 3, 231–234. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol synthesis of nanoparticles: status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Zhu, P.; Zhou, F.; Zeng, W.; Lu, D.D.; Sun, R.; Wong, C. Copper Salts Mediated Morphological Transformation of Cu2O from Cubes to Hierarchical Flower-like or Microspheres and Their Supercapacitors Performances. Sci. Rep. 2015, 5, 9672. [Google Scholar] [CrossRef] [PubMed]
- Fievet, F.; Lagier, J.P.; Blin, B.; Beaudoin, B.; Figlarz, M. Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid State Ionics 1989, 32–33, 198–205. [Google Scholar] [CrossRef]
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral silver nanocrystals with distinct scattering signatures. Angew. Chemie - Int. Ed. 2006, 45, 4597–4601. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Fu, W.; Yang, H.; Zeng, Y.; Zhang, Y.; Zhao, Q.; Li, Y.; Zhou, X.; Leng, Y.; Li, M.; et al. Low Temperature Synthesis of Cu2O Crystals: Shape Evolution and Growth Mechanism. Cryst. Growth Des. 2010, 10, 99–108. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A. Controlled Growth of Monodispersed Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- van Blaaderen, A.; Kentgens, A.P.M. Particle morphology and chemical microstructure of colloidal silica spheres made from alkoxysilanes. J. Non. Cryst. Solids 1992, 149, 161–178. [Google Scholar] [CrossRef]
- Philipse, A.P.; Vrij, A. Preparation and properties of nonaqueous model dispersions of chemically modified, charged silica spheres. J. Colloid Interface Sci. 1989, 128, 121–136. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Ying, J.Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Buskens, P.; Arfsten, N.; Habets, R.; Langermans, H.; Overbeek, A.; Scheerder, J.; Thies, J.; Viets, N. Innovation at DSM: State of the Art Single Layer Anti-Reflective Coatings for Solar Cell Cover. Glass Performance Days, 17 June 2010; 505–507. [Google Scholar]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; Van Blaaderen, A. A general method to coat colloidal particles with silica. Langmuir 2003, 19, 6693–6700. [Google Scholar] [CrossRef]
- Castillo, S.I.R.; Ouhajji, S.; Fokker, S.; Erné, B.H.; Schneijdenberg, C.T.W.M.; Thies-Weesie, D.M.E.; Philipse, A.P. Silica cubes with tunable coating thickness and porosity: From hematite filled silica boxes to hollow silica bubbles. Microporous Mesoporous Mater. 2014, 195, 75–86. [Google Scholar] [CrossRef]
- Wang, Y.; Su, X.; Ding, P.; Lu, S.; Yu, H. Shape-controlled synthesis of hollow silica colloids. Langmuir 2013, 29, 11575–11581. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dekker, F.; Tuinier, R.; Philipse, A.P. Synthesis of Hollow Silica Nanocubes with Tuneable Size and Shape, Suitable for Light Scattering Studies. Colloids Interfaces 2018, 2, 44. https://doi.org/10.3390/colloids2040044
Dekker F, Tuinier R, Philipse AP. Synthesis of Hollow Silica Nanocubes with Tuneable Size and Shape, Suitable for Light Scattering Studies. Colloids and Interfaces. 2018; 2(4):44. https://doi.org/10.3390/colloids2040044
Chicago/Turabian StyleDekker, Frans, Remco Tuinier, and Albert P. Philipse. 2018. "Synthesis of Hollow Silica Nanocubes with Tuneable Size and Shape, Suitable for Light Scattering Studies" Colloids and Interfaces 2, no. 4: 44. https://doi.org/10.3390/colloids2040044