High-Hardness, Water-Stable, and UV-Resistant Conductive Coatings Based on Waterborne PEDOT:PSS/Epoxy/(KH560/SiO2) Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
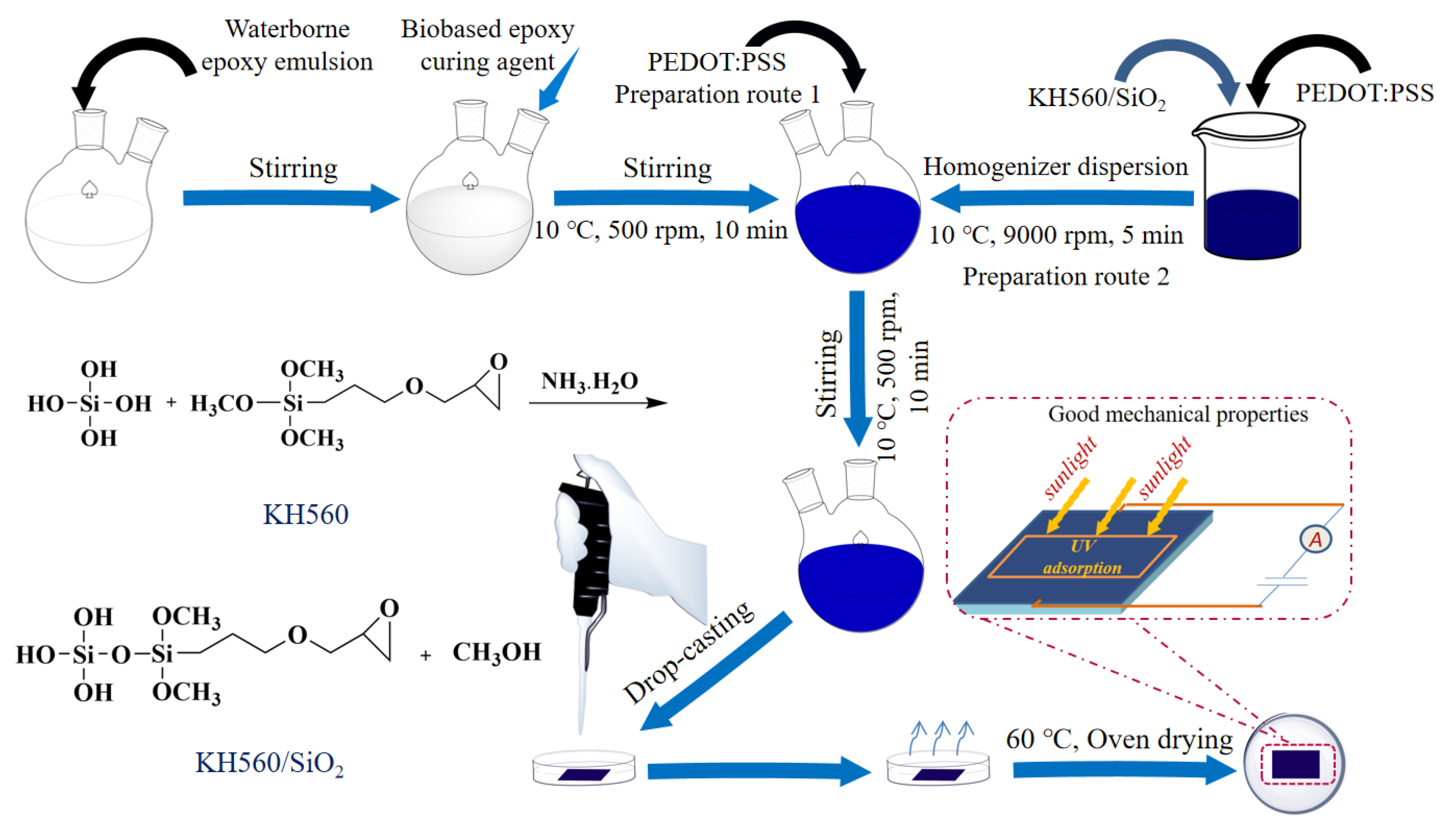
2.2. Preparation of KH560/SiO2
2.3. Preparation of PEDOT:PSS-Based Composite System
2.3.1. Preparation of PEDOT:PSS Aqueous Dispersion
2.3.2. Preparation of PEDOT:PSS/Epoxy and PEDOT:PSS/Epoxy(KH560/SiO2) Composite
2.4. Preparation of PEDOT:PSS/Epoxy and PEDOT:PSS/Epoxy/(KH560/SiO2) Composite Coatings
2.5. Characterizations
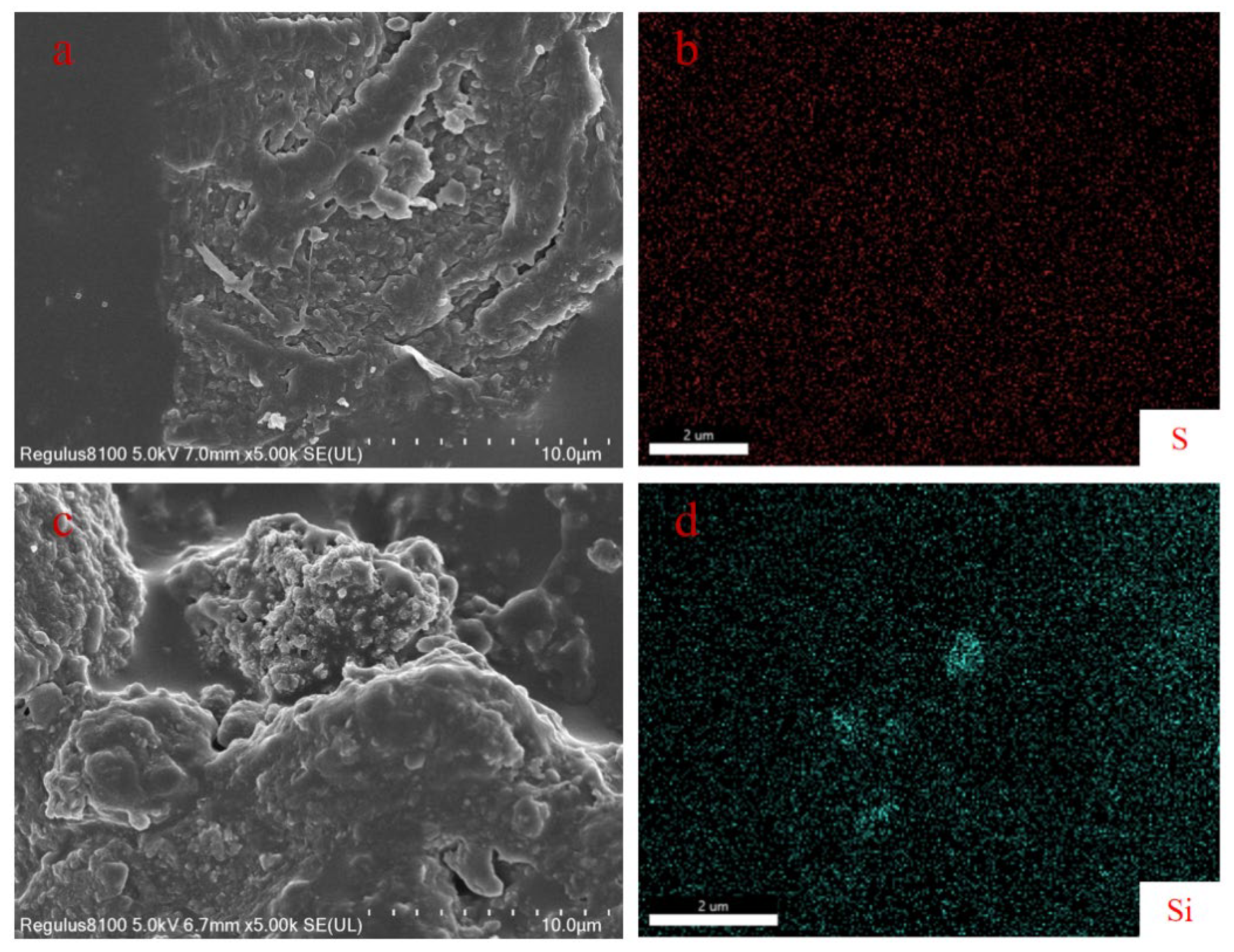
2.5.1. Microstructure and Morphology
2.5.2. Bulk Resistance
2.5.3. Surface Wettability and Water Resistance Stability
2.5.4. Pencil Hardness Property
2.5.5. UV Resistant Property
3. Results and Discussion
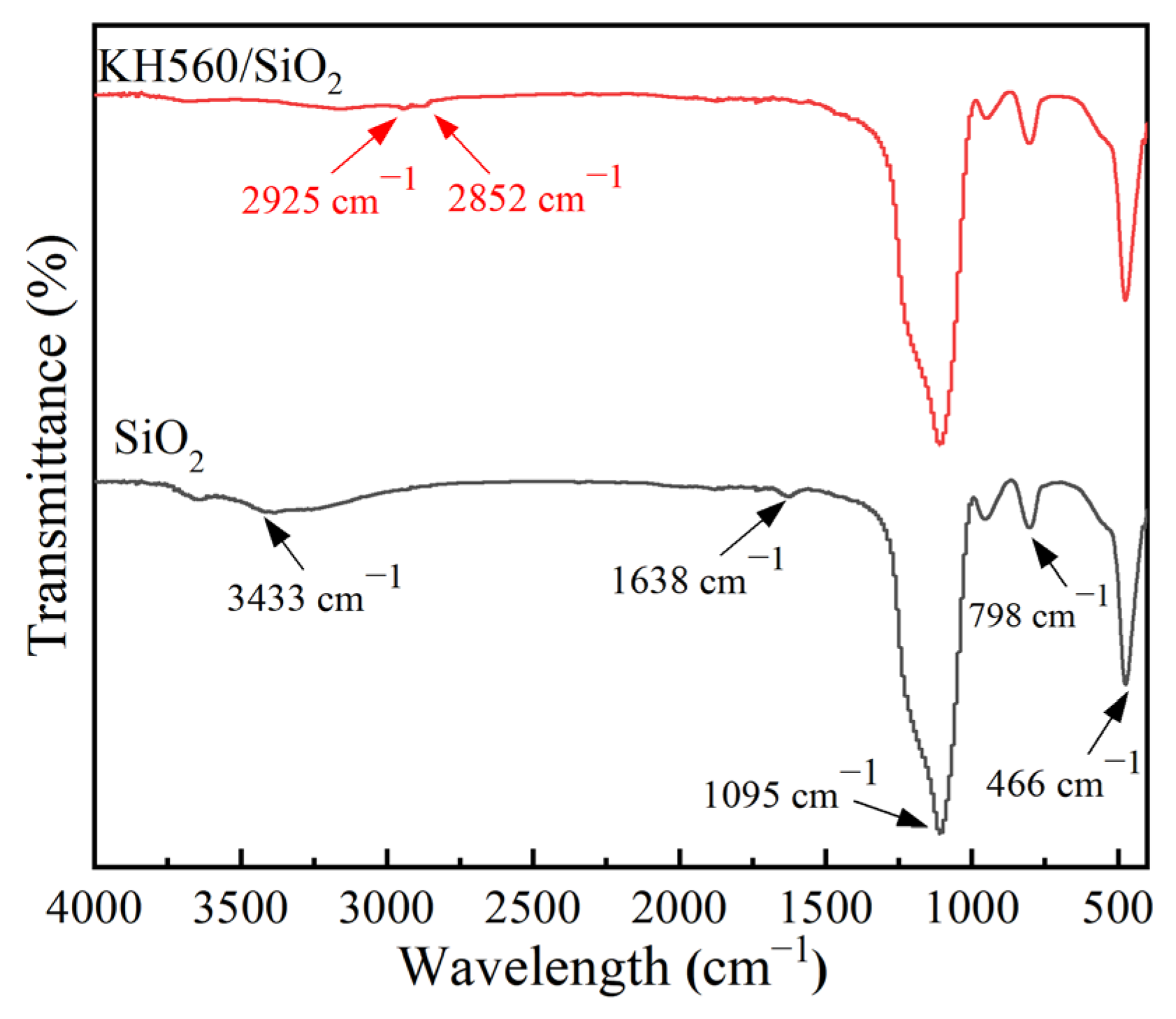
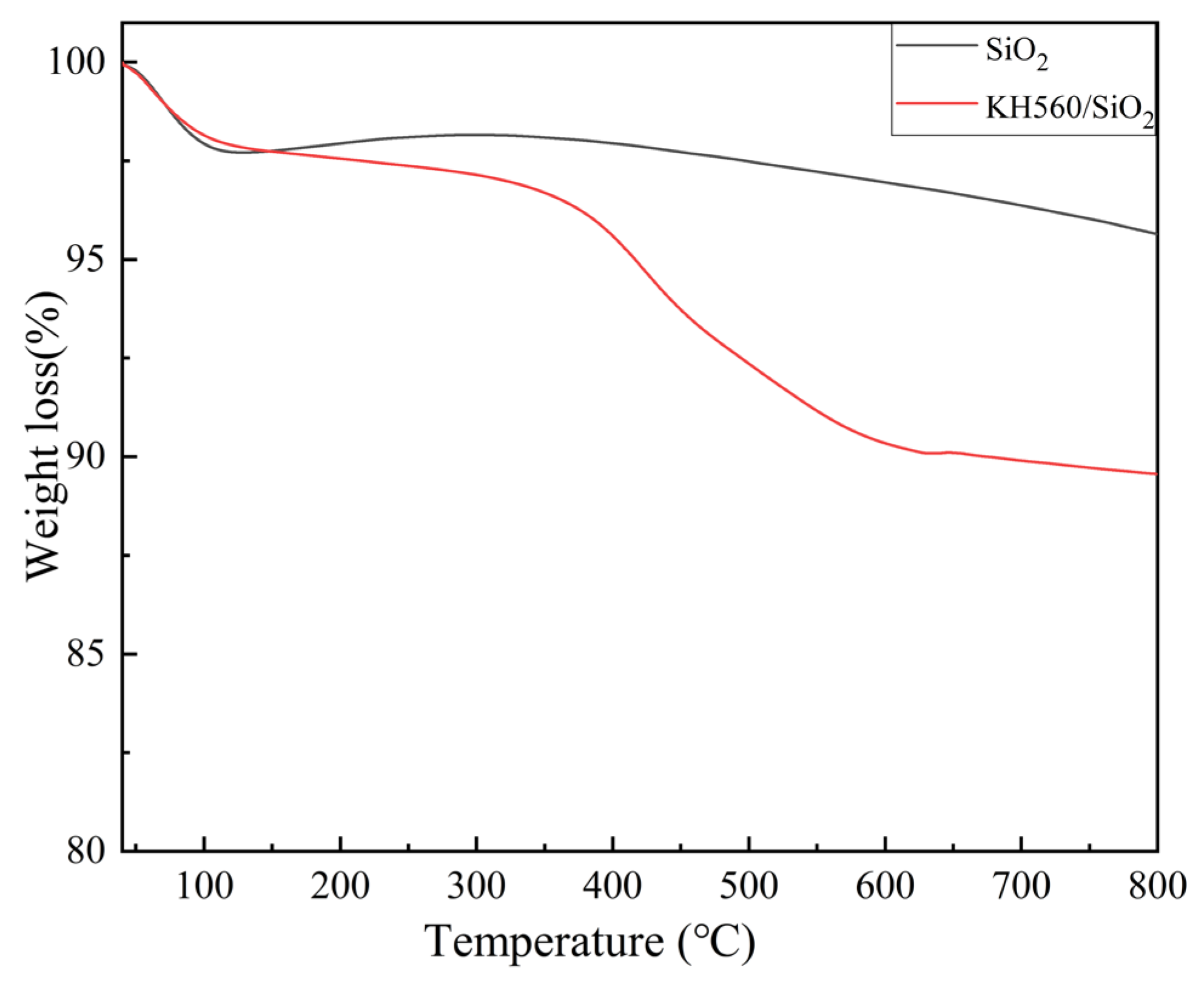
3.1. The Structural Characterization of SiO2 and KH560/SiO2
3.2. Effect of P:E and P:E:K Ratios on the Preparation and Properties of PEDOT:PSS/Epoxy and PEDOT:PSS/Epoxy/(KH560/SiO2)
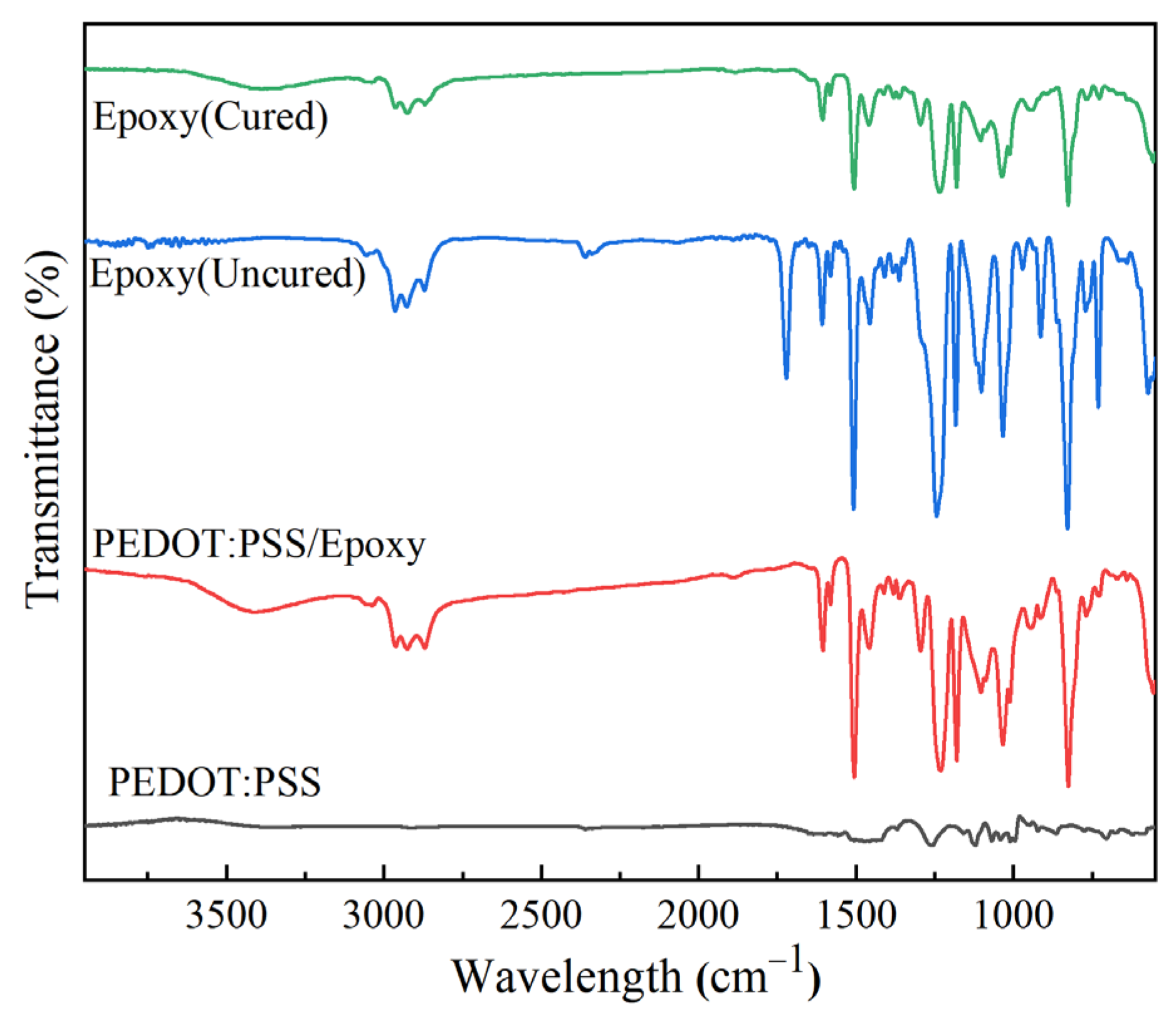
3.2.1. Preparation and Properties of PEDOT:PSS/Epoxy and PEDOT:PSS/Epoxy/(KH560/SiO2) Composite Aqueous Dispersions
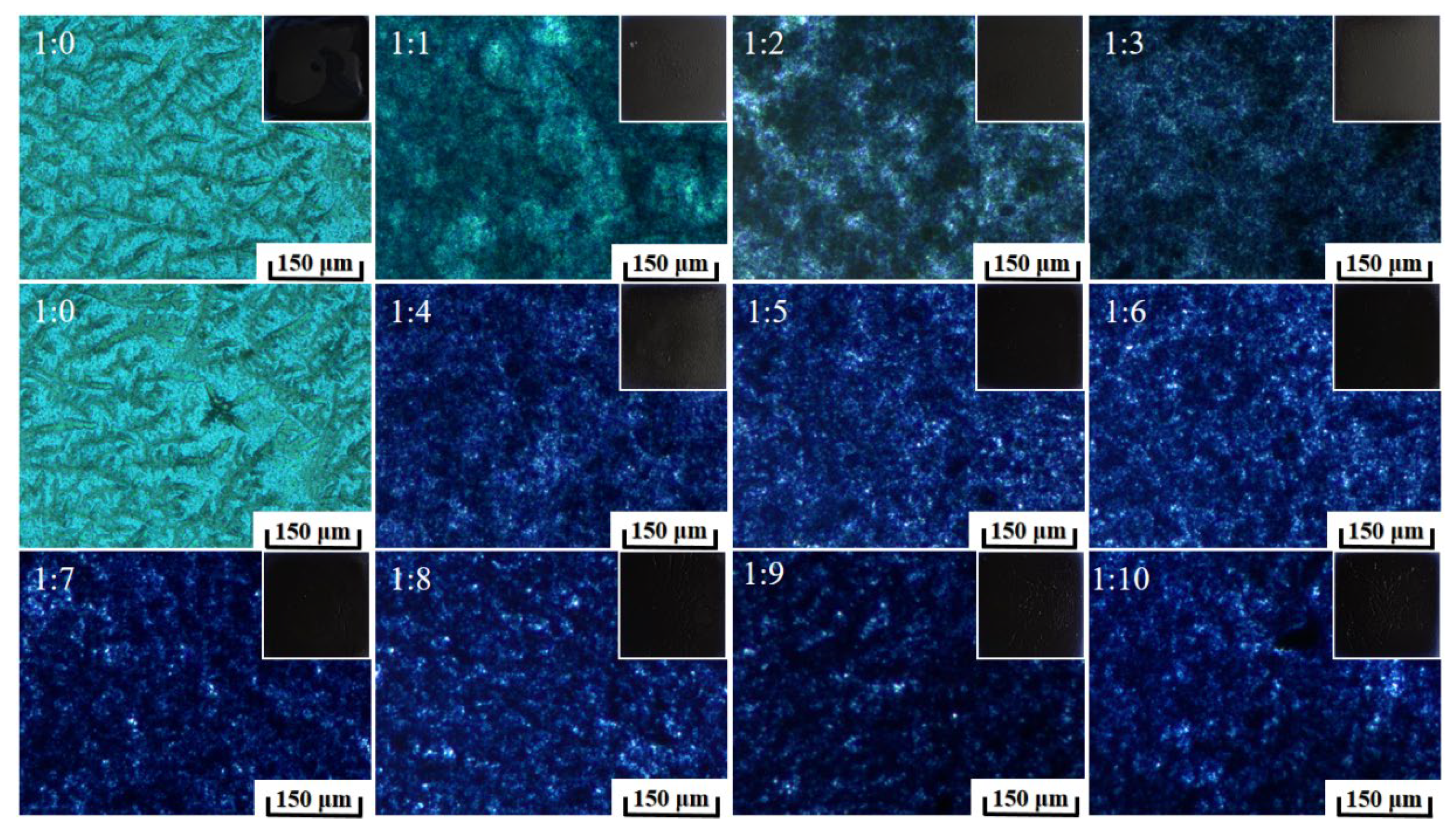
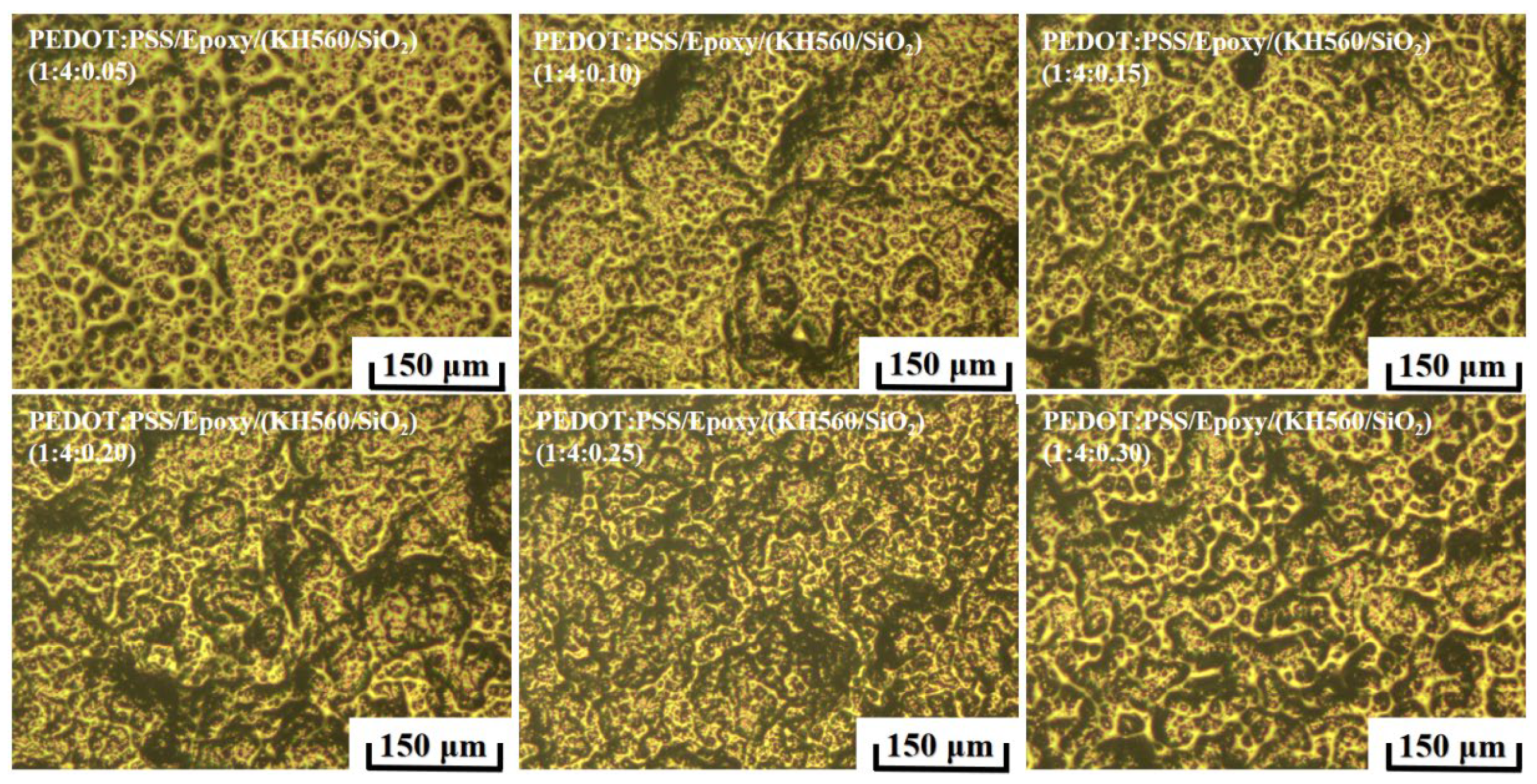
3.2.2. Preparation and Morphology Properties of PEDOT:PSS/Epoxy and KH560/SiO2 Composite Coatings
3.2.3. Bulk Resistance Property of PEDOT:PSS/Epoxy and PEDOT:PSS/Epoxy(KH560/SiO2) Coatings
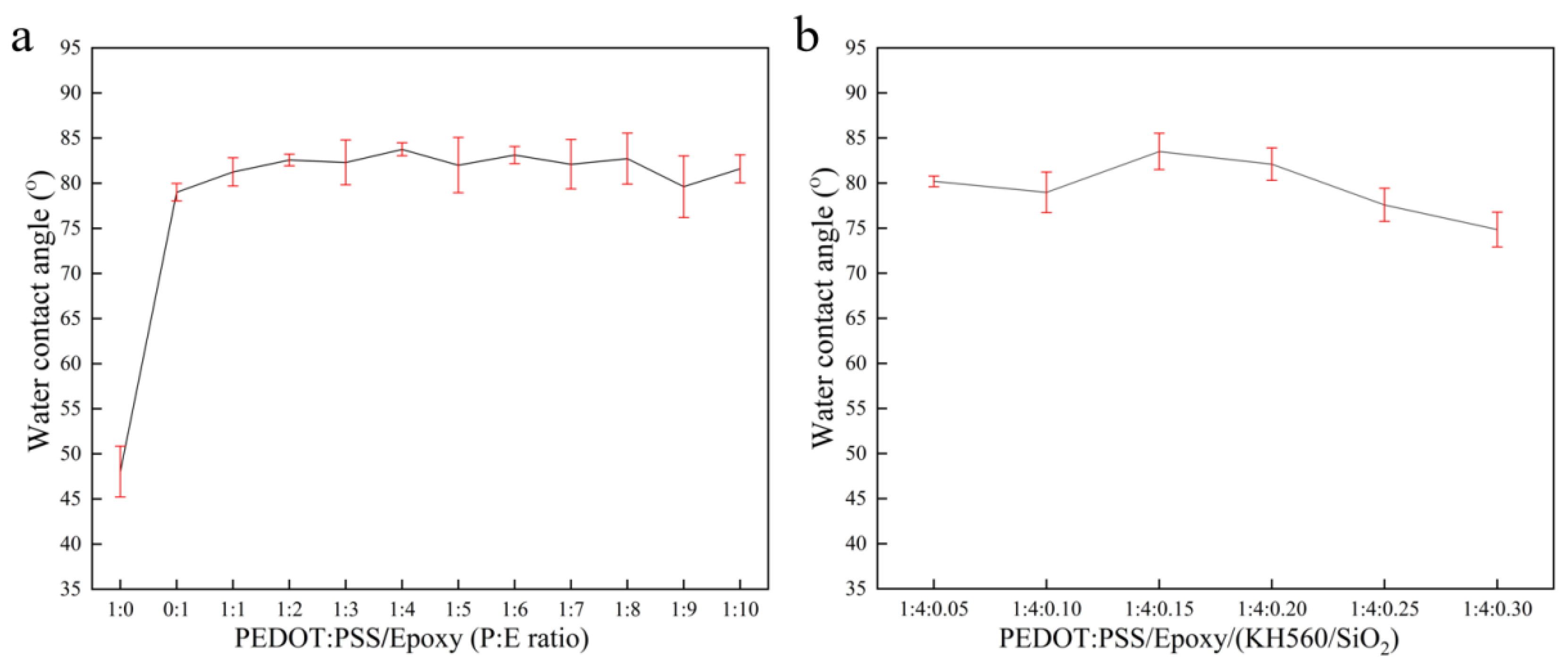
3.2.4. Surface Wettability and Water Resistance of PEDOT:PSS/Epoxy and PEDOT:PSS/Epoxy/(KH560/SiO2) Coatings
3.2.5. Pencil Hardness Property of PEDOT:PSS/Epoxy and KH560/SiO2 Composite Coatings
3.2.6. UV Resistance Properties of PEDOT:PSS/Epoxy and KH560/SiO2 Composite Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, X.; Nie, W.Y.; Tsai, S.H.; Wang, N.X.; Huang, H.H.; Cheng, Y.J.; Wen, R.J.; Ma, L.J.; Yan, F.; Xia, Y.G. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.J.; Si, H.W.; Zhang, X.D.; Lin, S.W. Functional Sensing Interfaces of PEDOT:PSS Organic Electrochemical Transistors for Chemical and Biological Sensors: A Mini Review. Sensors 2019, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yu, J.R.; Tian, Q.Y.; Shi, J.F.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and Its Composites in Electrochemical and Electronic Chemosensors. Chemosensors 2021, 9, 79. [Google Scholar] [CrossRef]
- Xia, Y.J.; Yan, G.W.; Lin, J. Review on Tailoring PEDOT:PSS Layer for Improved Device Stability of Perovskite Solar Cells. Nanomaterials 2021, 11, 3119. [Google Scholar] [CrossRef]
- Zhu, D.L.; Miao, M.; Du, X.J.; Peng, Y.Y.; Wang, Z.; Liu, S.J.; Xing, J.F. Long/Short chain Crosslinkers-optimized and PEDOT:PSS-enhanced covalent double network hydrogels rapidly prepared under green LED irradiation as flexible strain sensor. Eur. Polym. J. 2022, 174, 111327. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, G.J.; Cheng, X.; Deng, H.; Fu, Q. Stretchable and Healable Conductive Elastomer Based on PEDOT:PSS/Natural Rubber for Self-Powered Temperature and Strain Sensing. ACS Appl. Mater. Inter. 2021, 13, 14599–14611. [Google Scholar] [CrossRef]
- Tan, Z.T.; Li, H.W.; Huang, Y.N.; Gong, X.; Qi, J.N.; Li, J.; Chen, X.S.; Ji, D.Y.; Lv, W.B.; Li, L.Q.; et al. Breathing-effect assisted transferring large-area PEDOT:PSS to PDMS substrate with robust adhesion for stable flexible pressure sensor. Compos. Part. A Appl. Sci. Manuf. 2021, 143, 106299. [Google Scholar] [CrossRef]
- Huang, J.M.; Ren, Z.W.; Zhang, Y.K.; Liu, K.; Zhang, H.K.; Tang, H.; Yan, C.Q.; Zheng, Z.J.; Li, G. Stretchable ITO-Free Organic Solar Cells with Intrinsic Anti-Reflection Substrate for High-Efficiency Outdoor and Indoor Energy Harvesting. Adv. Funct. Mater. 2021, 31, 2010172. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.L.; Wang, Z.; Wang, J.N.; Huang, T.; Dong, J.; Zhang, Q.H. Flexible PEDOT:PSS/polyimide aerogels with linearly responsive and stable properties for piezoresistive sensor applications. Chem. Eng. J. 2020, 395, 125115. [Google Scholar] [CrossRef]
- Cho, H.S.; Jang, E.; Liu, H.; Cho, G. Applicability of poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate) impregnated polyurethane nanoweb as a transmission line for smart textiles. Text. Res. J. 2021, 91, 1253–1262. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Li, J.; Zhao, H.; Wang, Y.; Zhou, Y. Mesoporous cellulose nanofibers-interlaced PEDOT:PSS hybrids for chemiresistive ammonia detection. Microchim. Acta 2022, 189, 308. [Google Scholar] [CrossRef]
- Si, P.X.; Trinidad, J.; Chen, L.; Lee, B.; Chen, A.; Persic, J.; Lyn, R.; Leonenko, Z.; Zhao, B.X. PEDOT:PSS nano-gels for highly electrically conductive silver/epoxy composite adhesives. J. Mater. Sci. Mater. Electron. 2018, 29, 1837–1846. [Google Scholar] [CrossRef]
- Zeng, F.J.; Zhao, X.; Luo, M.Y.; Wang, W.; Qing, X.; Lu, Y.; Zhong, W.B.; Liu, Q.Z.; Luo, J.; Li, M.F.; et al. A transparent PEDOT:PSS/PVA-co-PE/epoxy thermoelectric composite device with excellent flexibility and environmental stability. Compos. Sci. Technol. 2022, 218, 109153. [Google Scholar] [CrossRef]
- Wu, Y.Q.; He, Y.; Zhou, T.G.; Chen, C.L.; Zhong, F.; Xia, Y.Q.; Xie, P.; Zhang, C. Synergistic functionalization of h-BN by mechanical exfoliation and PEI chemical modification for enhancing the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 142, 105541. [Google Scholar] [CrossRef]
- Li, W.; Xu, G.L.; Xu, B.Q.; Wang, Y.; Yang, J.; Hu, J. Preparation of waterborne P-N containing epoxy resin curing and its performances. Pigm. Resin. Technol. 2016, 45, 308–312. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.P.; Wang, L.; Liu, H.; Ban, X.Y.; Ye, J.J. Investigation on the epoxy/polyurethane modified asphalt binder cured with bio-based curing agent: Properties and optimization. Constr. Build. Mater. 2022, 320, 12. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Manalo, A.; Aravinthan, T.; Turner, J.; Ferdous, W.; Hota, G. Effects of ultraviolet solar radiation on the properties of particulate-filled epoxy based polymer coating. Polym. Degrad. Stabil. 2020, 181, 109352. [Google Scholar] [CrossRef]
- Noe, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89. [Google Scholar] [CrossRef]
- Wang, X.Q.; Fan, H.N.; Li, W.R.; Zhang, Y.Y.; Shang, R.Q.; Yin, F.H.; Wang, L.M. Effect of Ultraviolet-A Radiation on Alicyclic Epoxy Resin and Silicone Rubber Used for Insulators. Polymers 2022, 14, 4889. [Google Scholar] [CrossRef]
- Zaghloul, M.Y.; Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of stress level and fibre volume fraction on fatigue performance of glass fibre-reinforced polyester composites. Polymers 2022, 14, 2662. [Google Scholar] [CrossRef]
- Vu, C.M.; Bach, Q.V.; Duong, L.X.; Thai, N.V.; Thao, V.D.; Duc, P.T.; Nguyen, D.D.; Hoang, T.; Van, T.N. Silane coupling agent with amine group grafted nano/micro-glass fiber as novel toughener for epoxy resin: Fabrication and mechanical properties. Compos. Interfaces 2020, 27, 1085–1100. [Google Scholar]
- Wahyuningsih, K.; Yuliani, S.; Hoerudin. Characteristics of Silica Nanoparticles from Rice Husk as Influenced by Surface Modification with Used Solvent Containing Silane. J. Eng. Technol. Sci. 2021, 53, 11. [Google Scholar] [CrossRef]
- Gao, Z.L.; Gao, T.; Geng, Q.; Lin, G.L.; Li, Y.F.; Chen, L.; Li, M.C. Improving light absorption of active layer by adjusting PEDOT:PSS film for high efficiency Si-based hybrid solar cells. Sol. Energy 2021, 228, 299–307. [Google Scholar] [CrossRef]
- Hu, L.Q.; Pu, Z.J.; Tian, Y.H.; Zheng, X.Y.; Cheng, J.; Zhong, J.C. Preparation and properties of fluorinated silicon two-component polyurethane hydrophobic coatings. Polym. Int. 2020, 69, 448–456. [Google Scholar] [CrossRef]
- Chen, S.; Lu, B.Y.; Xu, J.K.; Qin, L.Q.; Wang, Z.P.; Duan, X.M. Preparation and characterization of aqueous dispersions of poly(3,4-ethylenedithiathiophene-co-3,4-ethylenedioxythiophene)/ poly(styrene sulfonate) and their conducting films. J. Appl. Polym. Sci. 2013, 129, 1717–1725. [Google Scholar] [CrossRef]
- Xin, X.; Yu, J.R.; Gao, N.; Xie, X.W.; Chen, S.; Zhong, J.; Xu, J.K. Effects of POSS composition on PEDOT:PSS conductive film. Synth. Met. 2021, 282, 116947. [Google Scholar] [CrossRef]
- Gao, W.; Dang, Z.C.; Liu, F.S.; Wang, S.; Zhang, D.W.; Yan, M.X. Preparation of antistatic epoxy resin coatings based on double comb-like quaternary ammonium salt polymers. RSC Adv. 2020, 10, 43523–43532. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul MY, M.; Zaghloul MM, Y.; Zaghloul MM, Y. Developments in polyester composite materials–An in-depth review on natural fibres and nano fillers. Compo. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, G.C.; Wang, N.; Wang, Y.G.; Li, T.D.; Wang, F.K.; Ye, C. Enhancing the mechanical strength and toughness of epoxy resins with linear POSS nano-modifiers. Nanoscale Adv. 2022, 4, 1151–1157. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Tenjimbayashi, M.; Manabe, K.; Komine, M.; Navarrini, W.; Shiratori, S. Integrated Anti-Icing Property of Super-Repellency and Electrothermogenesis Exhibited by PEDOT:PSS/Cyanoacrylate Composite Nanoparticles. ACS Appl. Mater. Inter. 2016, 8, 24212–24220. [Google Scholar] [CrossRef]
- Parshina, M.S.; Tarasenkov, A.N.; Aysin, R.R.; Tebeneva, N.A.; Buzin, M.I.; Afanasyev, E.S.; Serenko, O.A.; Muzafarov, A.M. Monitoring the curing processes of epoxy oligomers with partially substituted polyethoxymetallosiloxanes by IR spectroscopy and thermomechanical analysis. J. Appl. Polym. Sci. 2021, 138, 50918. [Google Scholar] [CrossRef]
- Aycan, D.; Dolapci, N.; Karaca, O.G.; Alemdar, N. Polysaccharide-based electroconductive films for controlled release of ciprofloxacin. J. Appl. Polym. Sci. 2022, 139, e52761. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]



| PEDOT:PSS/Epoxy | 1:0 | 1:1 | 1:2 | 1:3 | 1:4 | 1:5 | 1:6 | 1:7 | 1:8 | 1:9 | 1:10 | 0:1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness level | 3B | H | HB | H | F | F | F | F | H | H | H | F |
| PEDOT:PSS/Epoxy/(KH560/SiO2) | 1:4:0.05 | 1:4:0.10 | 1:4:0.15 | 1:4:0.20 | 1:4:0.25 | 1:4:0.30 |
|---|---|---|---|---|---|---|
| Hardness level | H | H | H | H | H | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhu, L.; Xie, X.; Zhou, M.; Fu, C.; Chen, S. High-Hardness, Water-Stable, and UV-Resistant Conductive Coatings Based on Waterborne PEDOT:PSS/Epoxy/(KH560/SiO2) Composite. J. Compos. Sci. 2023, 7, 51. https://doi.org/10.3390/jcs7020051
Li Z, Zhu L, Xie X, Zhou M, Fu C, Chen S. High-Hardness, Water-Stable, and UV-Resistant Conductive Coatings Based on Waterborne PEDOT:PSS/Epoxy/(KH560/SiO2) Composite. Journal of Composites Science. 2023; 7(2):51. https://doi.org/10.3390/jcs7020051
Chicago/Turabian StyleLi, Zhanqi, Ling Zhu, Xiaowen Xie, Meng Zhou, Changqing Fu, and Shuai Chen. 2023. "High-Hardness, Water-Stable, and UV-Resistant Conductive Coatings Based on Waterborne PEDOT:PSS/Epoxy/(KH560/SiO2) Composite" Journal of Composites Science 7, no. 2: 51. https://doi.org/10.3390/jcs7020051






