Pressing and Sintering of Titanium Aluminide Powder after Ball Milling in Silane-Doped Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
3. Results
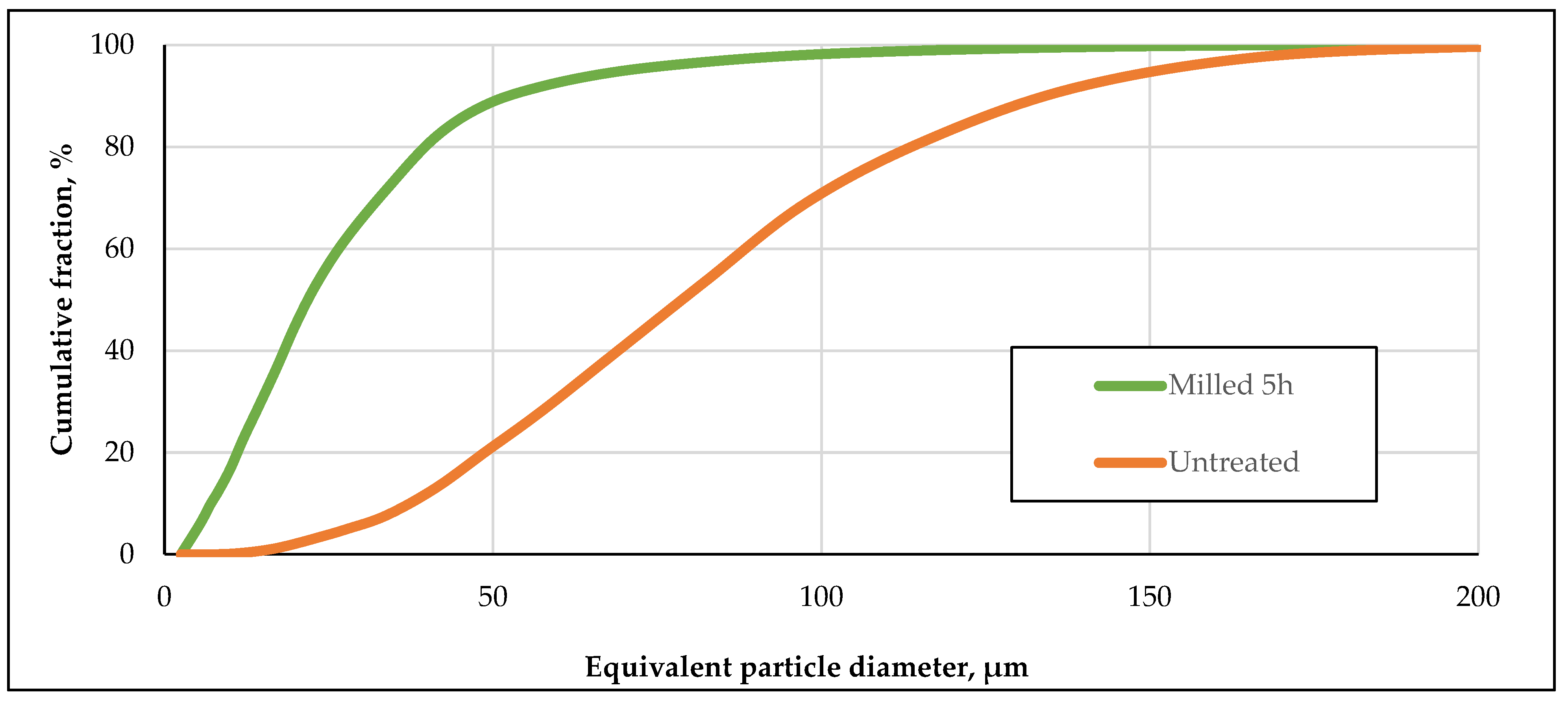
3.1. Particle Size Distribution
3.2. X-ray Photoelectron Spectroscopy
3.3. Pressability
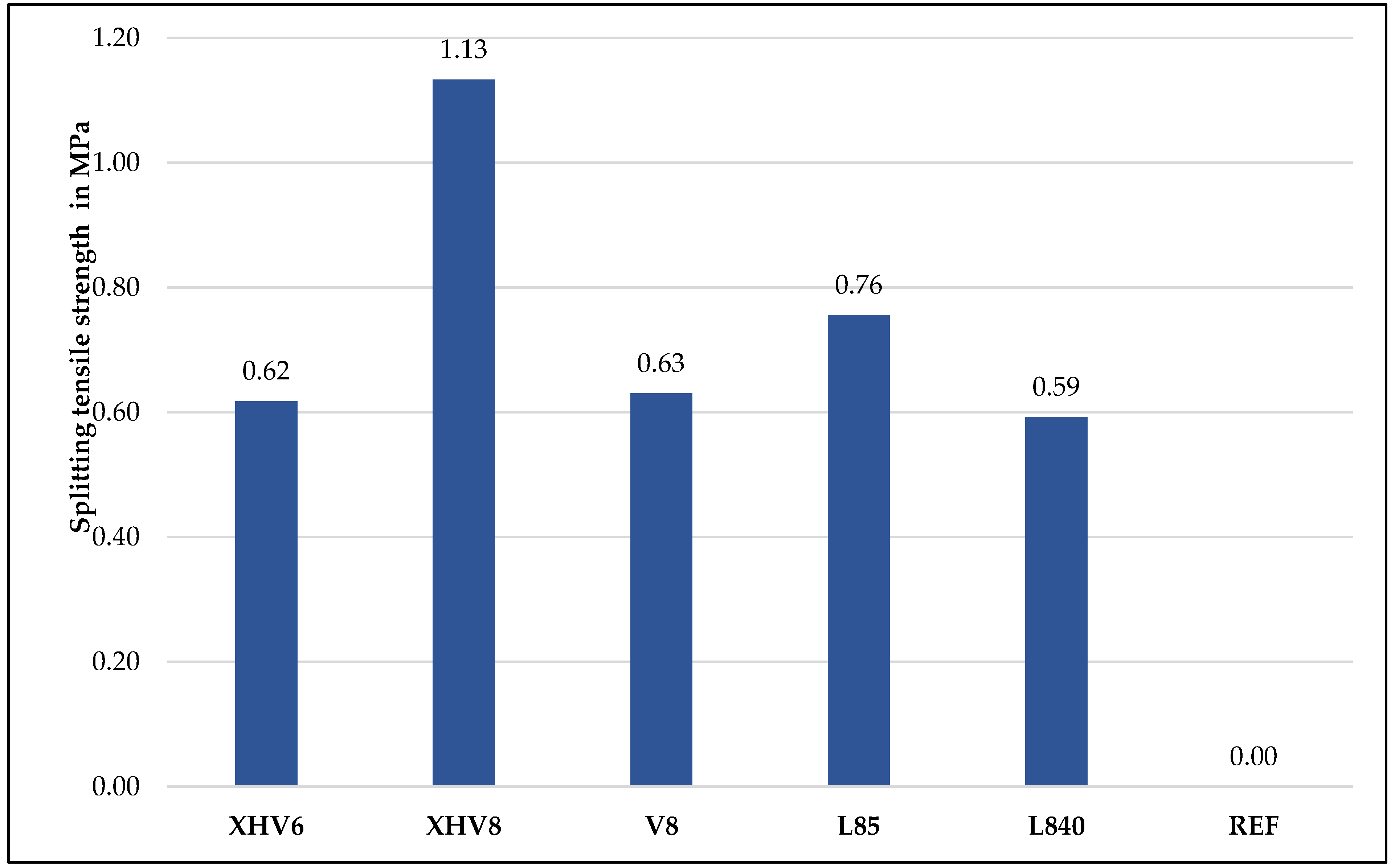
3.4. Splitting Tensile Strength
3.5. Density
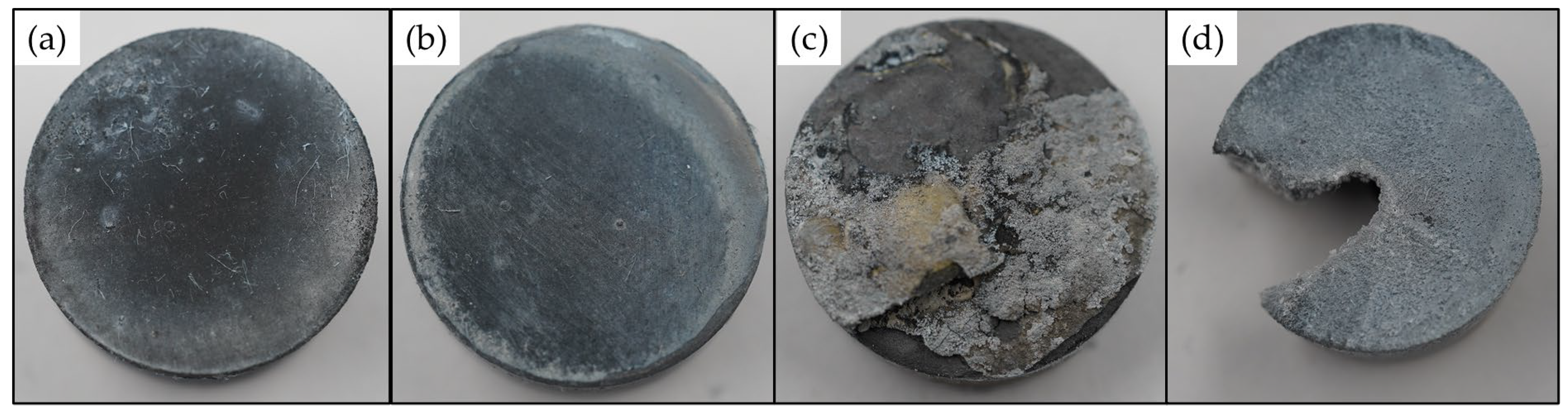
3.6. Microstructure
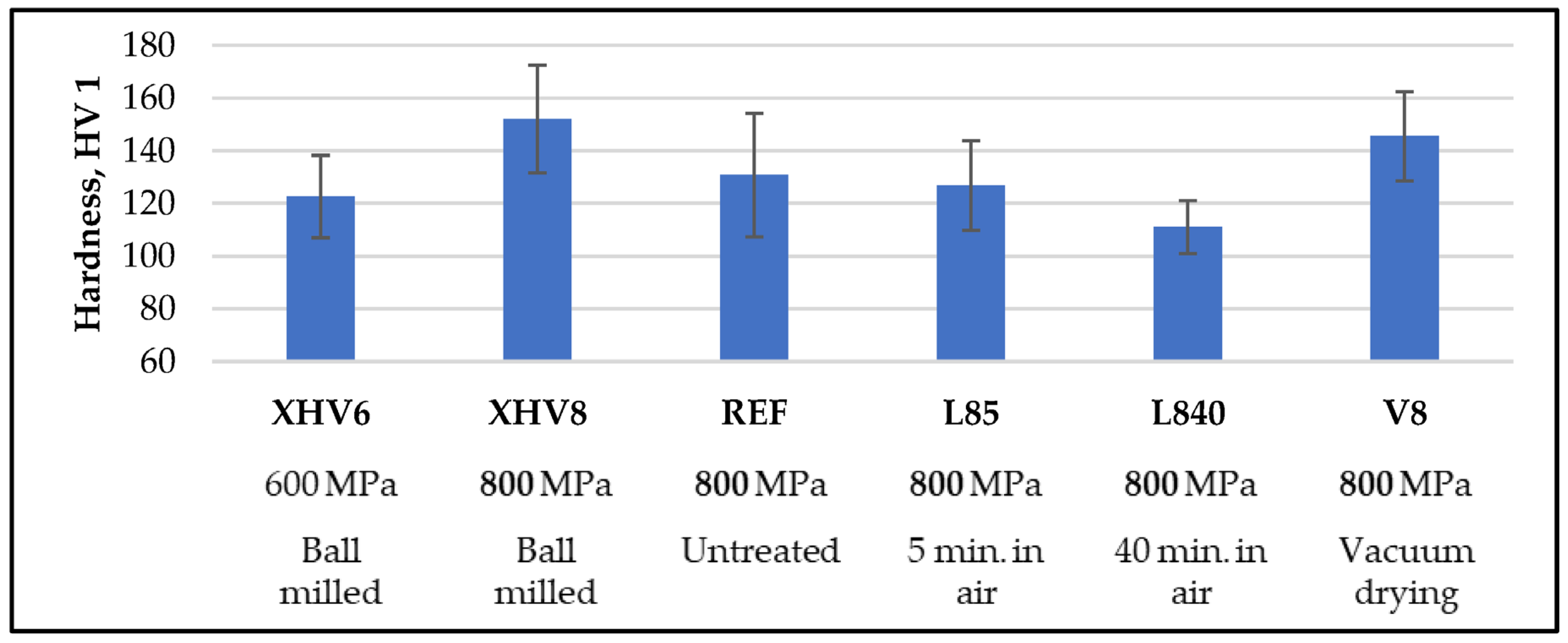
3.7. Hardness
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Liu, Y.; Zhang, W.; Huang, J.S. Hot deformation behavior of TiAl alloys prepared by blended elemental powders. Intermetallics 2011, 19, 154–159. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S.; Heilmaier, M. Pulvermetallurgische Herstellung von innovativen Hochtemperaturwerkstoffen. BHM Berg-Und Hüttenmännische Monatshefte 2021, 166, 1–7. [Google Scholar] [CrossRef]
- Voisin, T.; Monchoux, J.-P.; Durand, L.; Karnatak, N.; Thomas, M.; Couret, A. An Innovative Way to Produce γ-TiAl Blades: Spark Plasma Sintering. Adv. Eng. Mater. 2015, 17, 1408–1413. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Mogale, N.; Matizamhuka, W. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Beiss, P. Pulvermetallurgische Fertigungstechnik; Springer Vieweg: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Kariya, S.; Issariyapat, A.; Bahador, A.; Umeda, J.; Shen, J.; Kondoh, K. Ductility improvement of high-strength Ti–O material upon heteromicrostructure formation. Mater. Sci. Eng. A 2022, 842, 143041. [Google Scholar] [CrossRef]
- Groza, J.R.; Zavaliangos, A. Sintering activation by external electrical field. Mater. Sci. Eng. A 2000, 287, 171–177. [Google Scholar] [CrossRef]
- Jiao, L.; Feng, Q.; He, S.; Duan, B.; Dou, Z.; Li, C.; Lu, X. Direct oxygen removal from titanium aluminide scraps by yttrium reduction. Nonferrous Met. Soc. China 2021, 32, 2428–2437. [Google Scholar] [CrossRef]
- Behrens, B.-A.; Bonhage, M.; Bohr, D. Neuartige Verfahrenskombination zur Verarbeitung von Werkstoffen auf Titanaluminid-Basis unter sauerstofffreier Atmosphäre 2020, 23. Umformtechnisches Kolloquium Hannover, Aktuelle Entwicklungen im Bereich der Umformtechnik, ISBN: 978-3-95900-423-7. Available online: https://www.tewiss-verlag.de/katalog/details/?isbn=978-3-95900-423-7 (accessed on 6 July 2023).
- Bakulin, A.V.; Hocker, S.; Kulkova, S.E. Role of Intermediate Metal and Oxide Layers in Change of Adhesion Properties of TiAl/Al2O3 Interface. Phys. Mesomech. 2021, 24, 523–532. [Google Scholar] [CrossRef]
- Attia, M. Cold-isostatic pressing of metal powders: A review of the technology and recent developments. Solid State Mater. Sci. 2021, 46, 587–610. [Google Scholar] [CrossRef]
- Bay, N. Mechanisms Producing Metallic Bonds in Cold Welding. Weld. J. 1983, 62, 137. [Google Scholar]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of g-TiAl-based alloys e A review. Intermetallics 2014, 56, 1–14. [Google Scholar] [CrossRef]
- Gökce, A.; Findik, F. Mechanical and physical properties of sintered aluminum powders. J. Achievments Mater. Manuf. Eng. 2008, 30, 157–164. [Google Scholar]
- Mphahlele, M.R.; Olubambi, P.A.; Olevsky, E.A. Advances in Sintering of Titanium Aluminide: A Review. JOM 2023, 75, 2877–2896. [Google Scholar] [CrossRef]
- Gerling, R.; Clemens, H.; Schimansky, F.P. Powder Metallurgical Processing of Intermetallic Gamma Titanium Aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Meier, G.H.; Pettit, F.S.; Hu, S. Oxidation behavior of titanium aluminides. J. Phys. 1993, 3, 395–402. [Google Scholar] [CrossRef]
- Diaz, M.R.; Szafarska, M.; Gustus, R.; Möhwald, K.; Maier, H.J. Oxide Free Wire Arc Sprayed Coatings—An Avenue to Enhanced Adhesive Tensile Strength. Metals 2022, 12, 684. [Google Scholar] [CrossRef]
- Holländer, U.; Wulff, D.; Langohr, A.; Möhwald, K.; Maier, H.J. Brazing inSiH 4-Doped Inert Gases: A New Approach to an Environment Friendly Production Process. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 7, 1059–1071. [Google Scholar] [CrossRef]
- Murty, B.S.; Rao, M.M.; Ranganthan, S. Milling maps and amorphization during mechanical alloying. Acta Metall. Mater. 1994, 43, 2443–2450. [Google Scholar] [CrossRef]
- Qu, S.; Li, X.; Li, Y.; Hu, L.; Erde, W. Manufacturing a TiAl alloy by high-energy ball milling and subsequent reactive sintering. Rare Met. 2006, 25, 21–26. [Google Scholar] [CrossRef]
- Behrens, B.A.; Brunotte, K.; Peddinghaus, J.; Heymann, A. Influence of dwell time and pressure on SPS process with tita-2 nium aluminides. Metals 2021, 12, 83. [Google Scholar] [CrossRef]
- Gustus, R.; Szafarska, M.; Maus-Friedrichs, W. Oxygen-free transport of samples in silane-doped inert gas atmospheres for surface analysis. J. Vac. Sci. Technol. B 2021, 39, 54204. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1975, 8, 129–137. [Google Scholar] [CrossRef]
- Reilman, R.F.; Msezane, A.; Manson, S.T. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 389–394. [Google Scholar] [CrossRef]
- Heymann, A.; Peddinghaus, J.; Brunotte, K.; Behrens, B.-A. Investigations on the Consolidation of TNM Powder by Admixing Different Elemental Powders. In Metal 2022, 31st International Conference on Metallurgy and Materials: Conference Proceedings; Tanger: Ostrava, Czech Republic, 2022; pp. 639–644. [Google Scholar]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- DIN EN ISO 6507; Metallische Werkstoffe—Härteprüfung nach Vickers—Teil 1: Prüfverfahren. Beuth Verlag: Berlin, Germany, 2018.
- Szafarska, M. Gas Phase Reaction of Silane with Water at Different Temperatures and Supported by Plasma. ACS Omega 2023, 8, 8388–8396. [Google Scholar] [CrossRef]
- Maier, H.J.; Herbst, S.; Denkena, B.; Dittrich, M.A.; Schaper, F.; Worpenberg, S.; Gustus, R.; Maus-Friedrichs, W. Towards Dry Machining of Titanium-Based Alloys: A New Approach Using an Oxygen-Free Environment. Metals 2020, 10, 1161. [Google Scholar] [CrossRef]
- Barienti, K.; Werwein, S.; Herbst, S.; Maier, H.J.; Nürnberger, F. A novel way to reduce the critical deformation for cold roll bonding. Manuf. Lett. 2023, 36, 9–12. [Google Scholar] [CrossRef]
- Lee, P.A. Oxide formation on Fe and Ti thin films and on Fe thin films modified with ultrathin layers of Ti. Surf. Interface Anal. 1991, 17, 48–56. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Layered Machinable and Electrically Conductive Ti2AlC and Ti3AlC2 Ceramics: A Review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar] [CrossRef]
- Tallman, D.J.; Anasori, B.; Barsoum, M.W. A Critical Review of the Oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in Air. Mater. Res. Lett. 2013, 1, 115–125. [Google Scholar] [CrossRef]





| Designation | Pressure in MPa | Treatment |
|---|---|---|
| XHV6 | 600 | Ball-milled |
| XHV8 | 800 | Ball-milled |
| V8 | 800 | Vacuum drying |
| L85 | 800 | 5 min in air |
| L840 | 800 | 40 min in air |
| REF | 800 | Untreated |
| XHV6 | XHV8 | REF | L85 | L840 | V8 | |
|---|---|---|---|---|---|---|
| Density | 3.69 g/cm3 | 3.57 g/cm3 | 3.42 g/cm3 | 3.65 g/cm3 | 3.63 g/cm3 | 3.60 g/cm3 |
| Rel. density | 93% | 90% | 86% | 92% | 91% | 91% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behrens, B.-A.; Brunotte, K.; Peddinghaus, J.; Ursinus, J.; Döring, S.; Maus-Friedrichs, W.; Gustus, R.; Szafarska, M. Pressing and Sintering of Titanium Aluminide Powder after Ball Milling in Silane-Doped Atmosphere. J. Manuf. Mater. Process. 2023, 7, 171. https://doi.org/10.3390/jmmp7050171
Behrens B-A, Brunotte K, Peddinghaus J, Ursinus J, Döring S, Maus-Friedrichs W, Gustus R, Szafarska M. Pressing and Sintering of Titanium Aluminide Powder after Ball Milling in Silane-Doped Atmosphere. Journal of Manufacturing and Materials Processing. 2023; 7(5):171. https://doi.org/10.3390/jmmp7050171
Chicago/Turabian StyleBehrens, Bernd-Arno, Kai Brunotte, Julius Peddinghaus, Jonathan Ursinus, Sebastian Döring, Wolfgang Maus-Friedrichs, René Gustus, and Maik Szafarska. 2023. "Pressing and Sintering of Titanium Aluminide Powder after Ball Milling in Silane-Doped Atmosphere" Journal of Manufacturing and Materials Processing 7, no. 5: 171. https://doi.org/10.3390/jmmp7050171






