Variability of Upper Cervical Anatomy: A Reflection on Its Clinical Relevance
Abstract
:1. Introduction
2. Experimental Section
3. Results
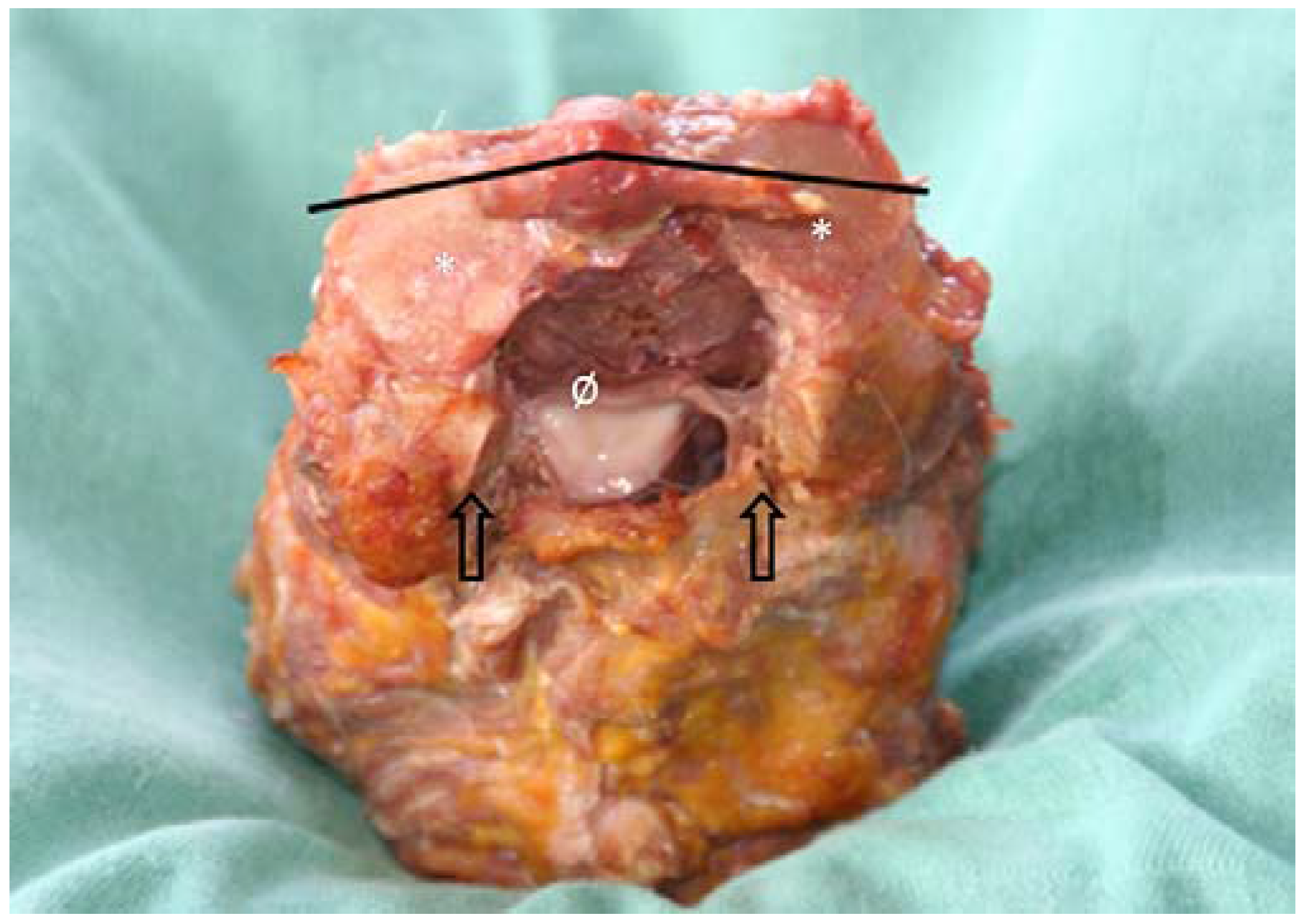
3.1. Morphological Features of the Transverse Ligament of the Atlas and the Alar Ligaments
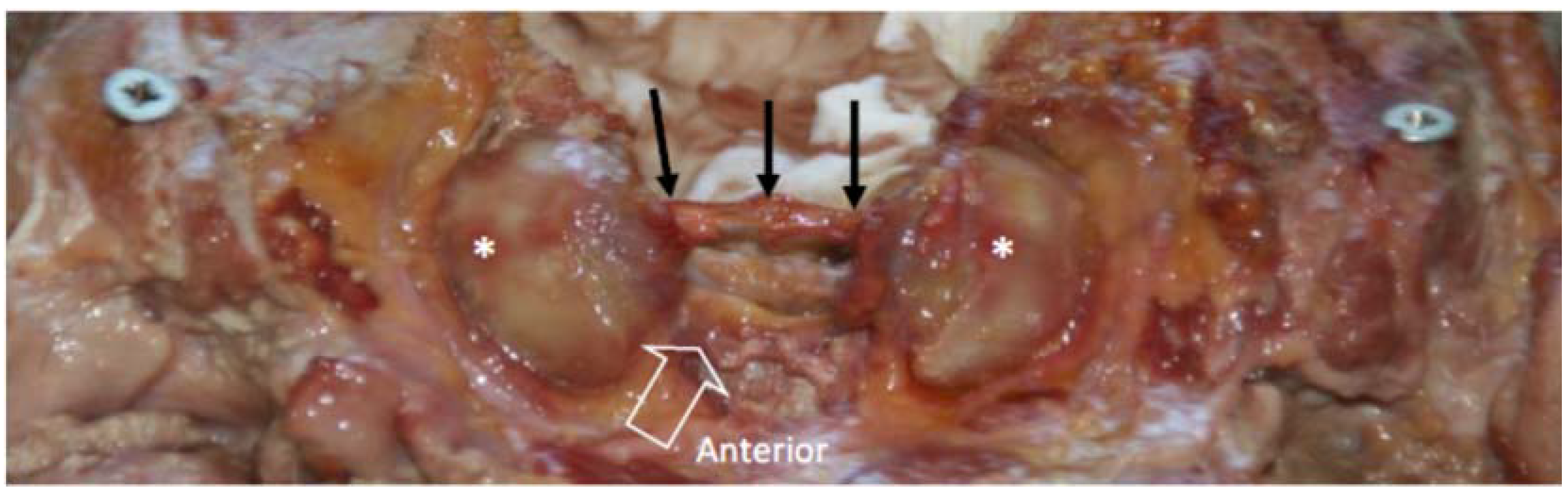
3.2. Morphological Features of the Lateral Atlanto-Axial and Atlanto-Occipital Joints
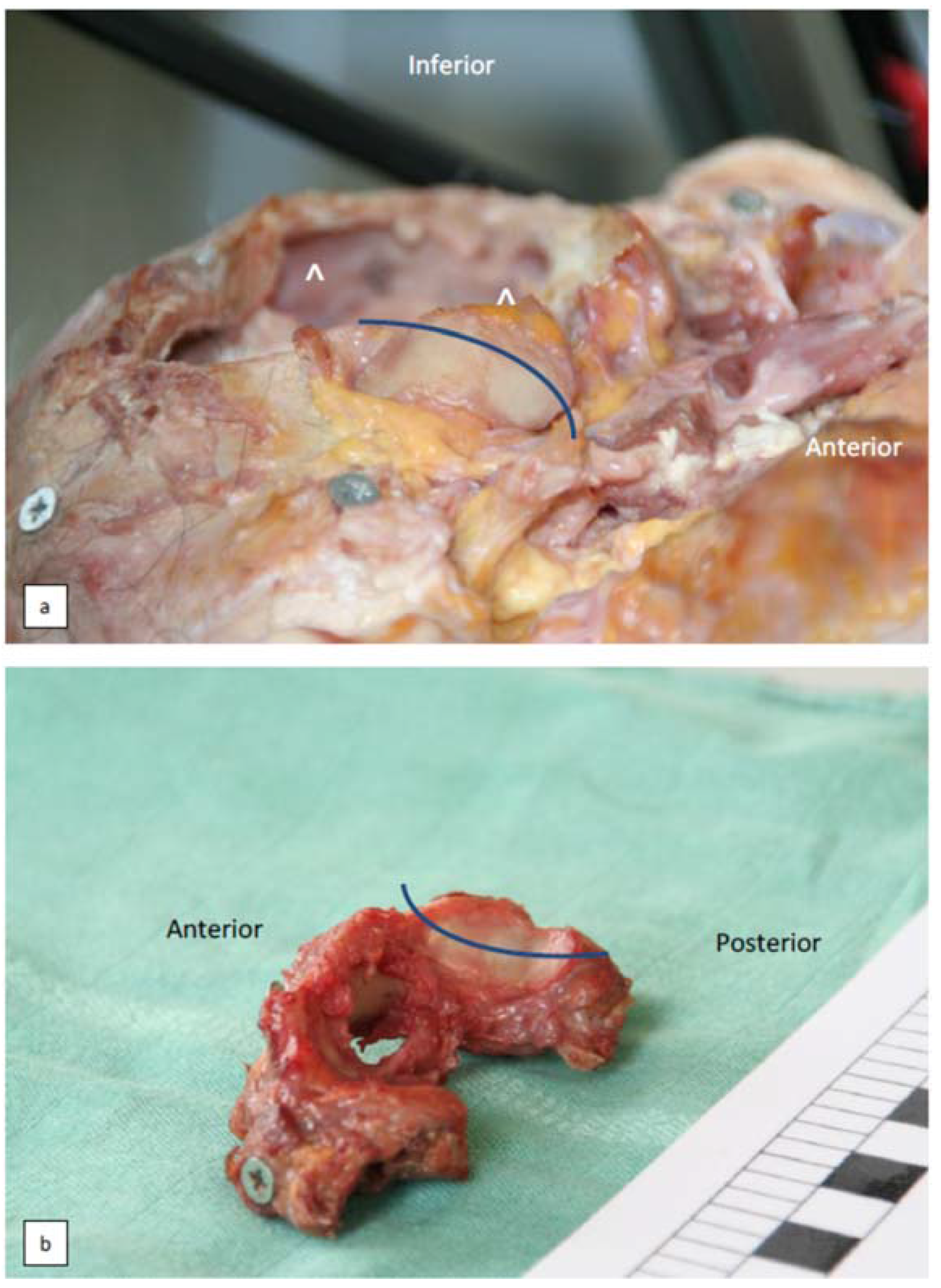
3.3. Additional Joint Configurations of the Atlanto-Occipital Junction
3.4. Muscular Attachments to the Joint Capsule of the Lateral Atlanto-Occipital Joint
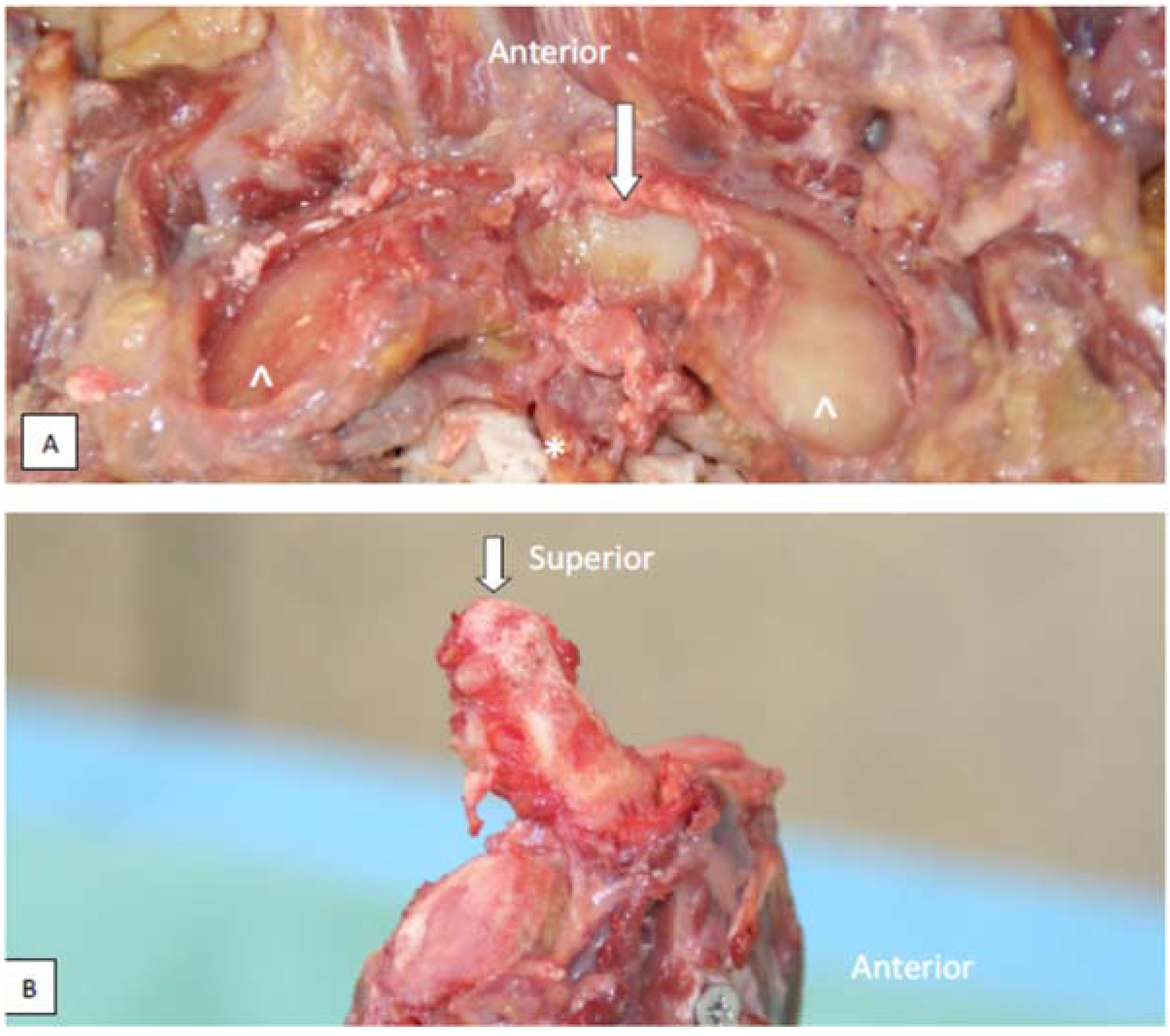
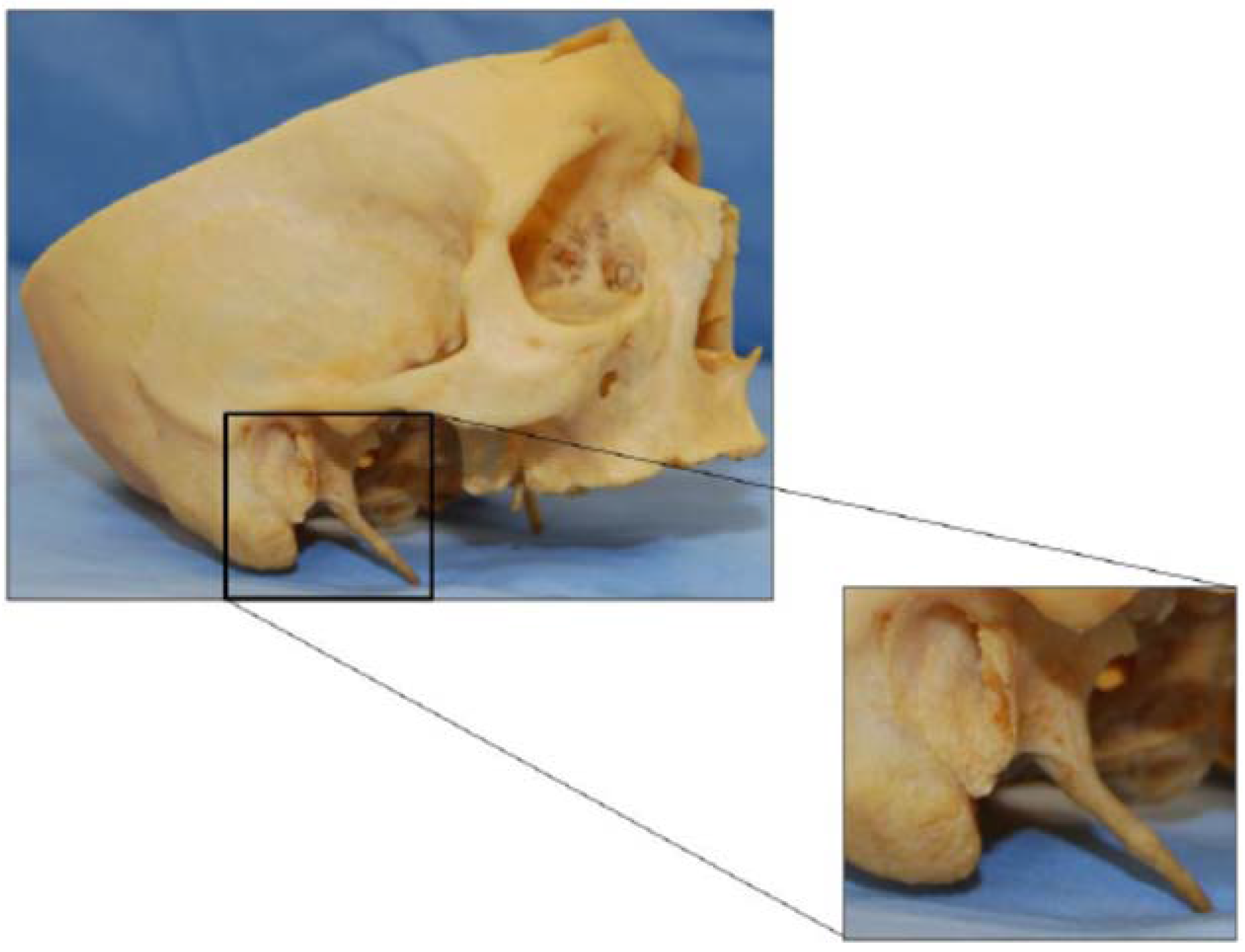
3.5. The processus Styloideus and Its Relationship with Movements of the Upper Cervical Joints
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reeves, N.P.; Narendra, K.S.; Cholewicki, J. Spine stability: The six blind man and the elephant. Clin. Biomech. 2007, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The stabilizing system of the spine .1. Function, dysfunction, adaptation, and enhancement. J. Spinal Disord. 1992, 5, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Testut, L. Les Anomalies Musculaires chez l’Homme, Expliquées par Lanatomie Comparée; G. Masson: Paris, France, 1884. [Google Scholar]
- Le Double, A.F. Traité des Variations Musculaires et Leur Significations au Point de Vue de l’Anthropologie Zoologique; Schleicher-Frères: Paris, France, 1897. [Google Scholar]
- Krause, W. Anatomische Varietäten; Hahn’sche Buchhandlung: Hannover, Germany, 1880. [Google Scholar]
- Bergman, R.A.; Afifi, A.K. Illustrated Encyclopedia of Human Anatomic Variation; The University of Iowa: Iowa City, IA, USA, 1996. [Google Scholar]
- Bergman, R.A.; Thompson, S.A.; Afifi, A.K.; Saadeh, F.A. Compendium of Human Anatomic Variation; Urban and Schwarzenberg: Baltimore, MD, USA, 1988. [Google Scholar]
- Cattrysse, E.; Barbero, M.; Gagey, O.; Kool, P.; Clarys, J.P.; Van Roy, P. 3D morphometry of the transverse and alar ligaments in the occipito-atlanto-axial spine: An in vitro analysis. Clin. Anat. 2007, 20, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Cattrysse, E.; Gagey, O.; Clarys, J.P.; Van Roy, P. In vitro 3-dimensional morphometry of the lateral atlanto-axial articular surfaces. Spine 2008, 33, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Wellons, J.C., 3rd; Banks, J.; Blount, J.P.; Oakes, W.J. Quantitative anatomy of the transverse ligament tubercles. J. Neurosurg. 2002, 97, 343–345. [Google Scholar] [PubMed]
- Burguet, J.L.; Sick, H.; Dirheimer, Y.; Wackenheim, A. CT of the main ligaments of the cervico-occipital hinge. Neuroradiology 1985, 27, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Krakenes, J.; Kaale, B.R.; Rorvik, J.; Gilhus, N.E. Mri assessment of normal ligamentous structures in the craniovertebral junction. Neuroradiology 2001, 43, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Saldinger, P.; Dvorak, J.; Rahn, B.A.; Perren, S.M. Histology of the alar and transverse ligaments. Spine 1990, 15, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Mayer, T.E.; Drescher, R. Delineation of alar ligament morphology: Comparison of magnetic resonance imaging at 1.5 and 3 tesla. Orthopedics 2012, 35, E1635–E1639. [Google Scholar] [CrossRef] [PubMed]
- Lummel, N.; Zeif, C.; Kloetzer, A.; Linn, J.; Bruckmann, H.; Bitterling, H. Variability of morphology and signal intensity of alar ligaments in healthy volunteers using mr imaging. Am. J. Neuroradiol. 2011, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Osmotherly, P.G.; Rawson, O.A.; Rowe, L.J. The relationship between dens height and alar ligament orientation: A radiologic study. J. Manip. Physiol. Ther. 2011, 34, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Panjabi, M.M. Functional-anatomy of the alar ligaments. Spine 1987, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Penning, L.; Hayek, J.; Panjabi, M.M.; Grob, D.; Zehnder, R. Functional diagnostics of the cervical spine using computer-tomography. Neuroradiology 1988, 30, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Osmotherly, P.G.; Rivett, D.A.; Mercer, S.R. Revisiting the clinical anatomy of the alar ligaments. Eur. Spine J. 2013, 22, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Van Roy, P.; Barbaix, E.; Clarys, J.P. Functional anatomy of the cervical spine. In The Degenerative Cervical Spine; Spalski, M., Gunzburg, R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 3–27. [Google Scholar]
- Okazaki, K. Anatomical study of the ligaments in the occipito-atlantoaxial complex. Nippon Seikeigeka Gakkai Zasshi 1995, 69, 1259–1267. [Google Scholar] [PubMed]
- Tubbs, R.S.; Mortazavi, M.M.; Louis, R.G.; Loukas, M.; Shoja, M.M.; Chern, J.J.; Benninger, B.; Cohen-Gadol, A.A. The anterior atlantodental ligament: Its anatomy and potential functional significance. World Neurosurg. 2012, 77, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Moore, G.D.; Panchani, P.N.; DiLandro, A.C.; Battaglia, F.; Tubbs, R.S.; Shoja, M.M.; Loukas, M.; Kozlowski, P.B.; D’Antoni, A.V. The transverse occipital ligament: An anatomic, histologic, and radiographic study. Spine J. 2012, 12, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Radcliff, K.E.; Ben-Galim, P.; Dreiangel, N.; Martin, S.B.; Reitman, C.A.; Lin, J.N.; Hipp, J.A. Comprehensive computed tomography assessment of the upper cervical anatomy: What is normal? Spine J. 2010, 10, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rauschning, W. Detailed sectional anatomy of the spine. In Multiplanar CT of the Spine; Rothman, S., Glenn, W.V., Eds.; University Park Press: Baltimore, MD, USA, 1985; pp. 33–35. [Google Scholar]
- Rauschning, W. Anatomy and pathology of the cervical spine. In The Adult Spine: Principles and Practice; Frymoyer, J.W., Ed.; Raven Press: New York, NY, USA, 1991; pp. 907–928. [Google Scholar]
- Rauschning, W.; Bergstrom, K.; Pech, P. Correlative craniospinal anatomy by computed-tomography and cryomicrotomy. J. Comput. Assist. Tomogr. 1983, 7, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rauschning, W.; Glenn, W.V. Atlas of Sectional Anatomy: Head, Neck and Thrunk. N. Engl. J. Med. 1984, 311, 267–268. [Google Scholar] [CrossRef]
- Rothman, S.; Glenn, W.V. Multiplanar CT of the Spine; University Park Press: Baltimore, MD, USA, 1985. [Google Scholar]
- Konig, S.A.; Goldammer, A.; Vitzthum, H.E. Anatomical data on the craniocervical junction and their correlation with degenerative changes in 30 cadaveric specimens. J. Neurosurg. Spine 2005, 3, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, R.C.; Cattrysse, E.; Zrull, J.M. In vitro characterization of the anterior to posterior curvature of the superior articular facets of the atlas as a function of age. Spine J. 2011, 11, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lofrese, G.; De Iure, F.; Cappuccio, M.; Amendola, L. Occipital condyles congenital dislocation and condylus tertius an unstable association revealing a new abnormality of the craniocervical junction. Spine 2015, 40, E992–E995. [Google Scholar] [CrossRef] [PubMed]
- Udare, A.S.; Bansal, D.; Patel, B.; Mondel, P.K.; Aiyer, S. Condylus tertius with atlanto-axial rotatory fixation: An unreported association. Skelet. Radiol. 2014, 43, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Bodon, G.; Glasz, T.; Olerud, C. Anatomical changes in occipitalization: Is there an increased risk during the standard posterior approach? Eur. Spine J. 2013, 22, S512–S516. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.; Hart, J.; Navis, M.; Clayton, S.; Boone, R. Surface area congruence of atlas superior articulating facets and occipital condyles. J. Chiropr. Med. 2008, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N.; Mercer, S. Biomechanics of the cervical spine. I: Normal kinematics. Clin. Biomech. 2000, 15, 633–648. [Google Scholar] [CrossRef]
- Ishii, T.; Mukai, Y.; Hosono, N.; Sakaura, H.; Fujii, R.; Nakajima, Y.; Tamura, S.; Iwasaki, M.; Yoshikawa, H.; Sugamoto, K. Kinematics of the cervical spine in lateral bending in vivo three-dimensional analysis. Spine 2006, 31, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mukai, Y.; Hosono, N.; Sakaura, H.; Nakajima, Y.; Sato, Y.; Sugamoto, K.; Yoshikawa, H. Kinematics of the upper cervical spine in rotation—In vivo three-dimensional analysis. Spine 2004, 29, E139–E144. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.A.; Binkert, C.A.; Zanetti, M.; Boos, N.; Hodler, J. Functional MR imaging of the craniocervical junction. Correlation with alar ligaments and occipito-atlantoaxial joint morphology: A study in 50 asymptomatic subjects. Schweiz. Med. Wochenschr. 2000, 130, 645–651. [Google Scholar] [PubMed]
- Cattrysse, E.; Baeyens, J.P.; Clarys, J.P.; Van Roy, P. Three dimensional kinematics of manual upper cervical mobilization: Part 2: An in vitro analysis of manual axial rotation and lateral bending mobilization of the atlanto-axial joint. Man. Ther. 2007, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Niu, X.C.; Wu, C.Y.; Guo, L.F.; Liu, C.; Song, H.X.; Chhabra, A. Normative data on axial rotation of atlanto-occipital joint on 3 tesla MRI using a simple and reliable method of calculation. Acta Radiol. 2013, 54, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Oda, T.; Crisco, J.J.; Dvorak, J.; Grob, D. Posture affects motion coupling patterns of the upper cervical spine. J. Orthop. Res. 1993, 11, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Panjabi, M.; Gerber, M.; Wichmann, W. CT-functional diagnostics of the rotatory instability of upper cervical spine.1. An experimental-study on cadavers. Spine 1987, 12, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Miura, T.; Cripton, P.A.; Wang, J.L.; Nain, A.S.; DuBois, C. Development of a system for in vitro neck muscle force replication in whole cervical spine experiments. Spine 2001, 26, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Van Roy, P.; Caboor, D.; De Boelpaep, S.; Barbaix, E.; Clarys, J.P. Left-right asymmetries and other common anatomical variants of the first cervical vertebra. Man. Ther. 1997, 2, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lantz, C.A.; Klein, G.; Chen, J.; Mannion, A.; Solinger, A.B.; Dvorak, J. A reassessment of normal cervical range of motion. Spine 2003, 28, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Villas, C.; Arriagada, C.; Zubieta, J.L. Preliminary CT study of C1–C2 rotational mobility in normal subjects. Eur. Spine J. 1999, 8, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.Y.; Ye, F.; Kang, J.H. Three-dimensional CT study on normal anatomical features of atlanto-axial joints. Surg. Radiol. Anat. 2007, 29, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Buzzatti, L.; Provyn, S.; Van Roy, P.; Cattrysse, E. Atlanto-axial facet displacement during rotational high-velocity low-amplitude thrust: An in vitro 3D kinematic analysis. Man. Ther. 2015, 20, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Mönckeberg, J.; Tome, C.; Matias, A.; Alonso, A.; Vasquez, J.; Zubieta, J. CT scan study of atlantoaxial rotatory mobility in asymptomatic adult subjects: A basis for better understanding C1–C2 rotatory fixation and subluxation. Spine 2009, 34, 1392–1295. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Willard, F.; Dixon, T.; Bogduk, N. Ventral innervation of the lateral C1–C2 joint: An anatomical study. Pain Med. 2008, 9, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Eagle, W.W. Elongated styloid process: Report of 2 cases. Arch. Otolaryngol. 1937, 25, 584–587. [Google Scholar] [CrossRef]
- Cattrysse, E.; Provyn, S.; Kool, P.; Clarys, J.P.; Van Roy, P. Morphology and kinematics of the atlanto-axial joints and their interaction during manual cervical rotation mobilization. Man. Ther. 2012, 16, 481–486. [Google Scholar] [CrossRef] [PubMed]



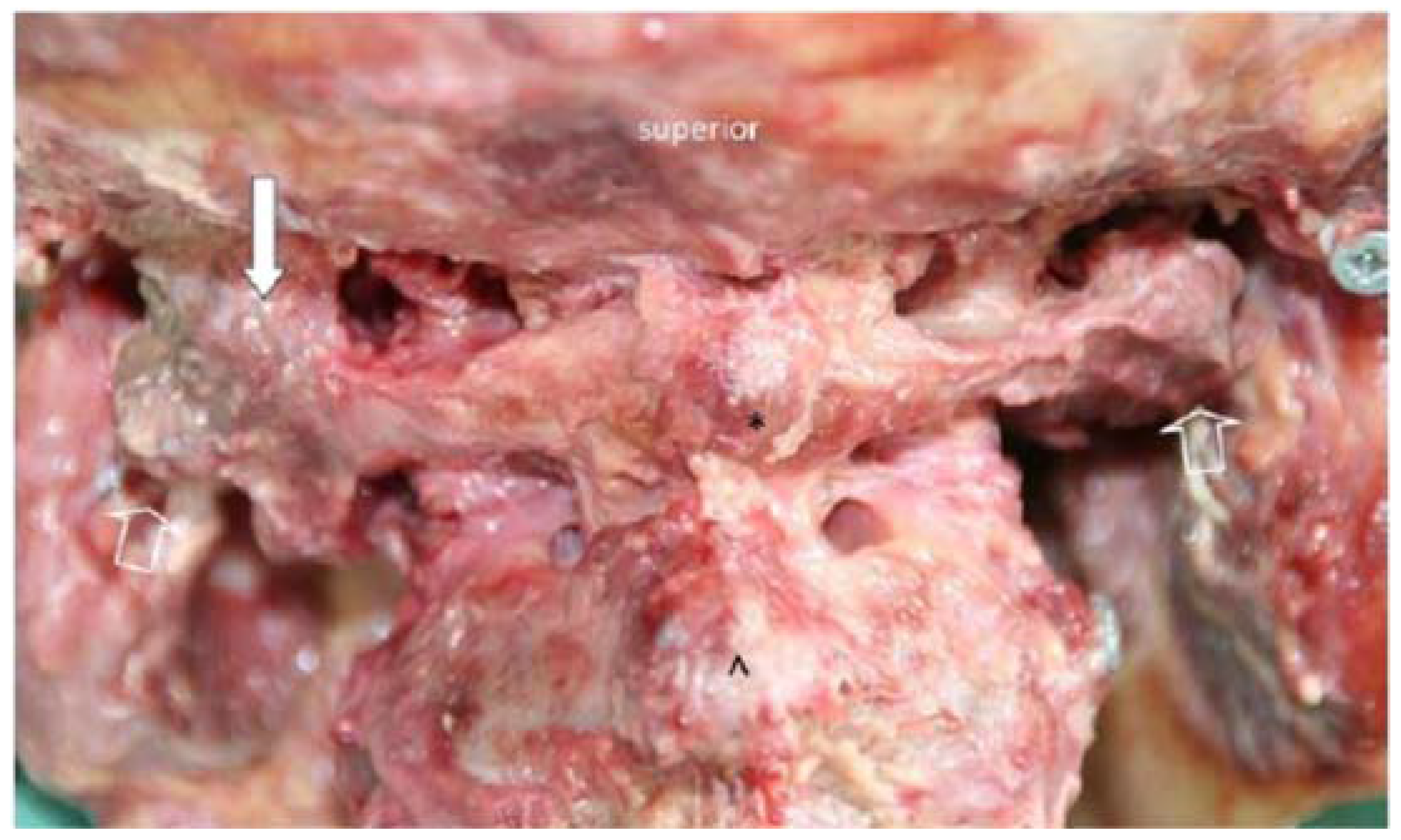


| Alar Ligaments | n | Mean | SD |
|---|---|---|---|
| Sup-inf width on occ: left | 20 | 7.2 | 1.7 |
| Ant-post depth on occ: left | 20 | 8.4 | 2.6 |
| Sup-inf width on dens: left | 20 | 8.4 | 1.6 |
| Ant-post depth on dens: left | 20 | 5.9 | 1.5 |
| Sup-inf width on occ: right | 20 | 6.5 | 1.8 |
| Ant-post depth on occ: right | 20 | 7.9 | 2.4 |
| Sup-inf width on dens: right | 20 | 7.3 | 1.6 |
| Ant-post depth on dens: right | 20 | 5.8 | 1.6 |
| Length left | 18 | 8.8 | 2.6 |
| Length right | 17 | 8.9 | 2.6 |
| Absolute angle between left and right ligament | 18 | 117.2 | 31.0 |
| Angle to sagittal plane | 20 | 6.4 | 4.3 |
| Angle to frontal plane | 20 | −10.4 | 51.6 |
| Transverse Ligaments | |||
| width | 19 | 9.2 | 2.2 |
| length | 19 | 21.5 | 3.0 |
| Absolute angle | 19 | 119.2 | 16.9 |
| n = 20 | Left | Right | ||
|---|---|---|---|---|
| Absolute dimensions | mean | SD | mean | SD |
| Axis superior mean diameter | 17.7 | 1.4 | 17.7 | 1.7 |
| Axis superior surface area | 234.5 | 38.6 | 235.2 | 41.0 |
| Atlas inferior mean diameter | 17.0 | 1.4 | 17.0 | 1.5 |
| Atlas inferior surface area | 213.0 | 33.8 | 210.5 | 37.0 |
| Axis height | 0.2 | 1.2 | 0.3 | 1.1 |
| Axis ratio | 0.01 | 0.07 | 0.02 | 0.07 |
| Atlas height | 0.0 | 0.4 | 0.1 | 0.3 |
| Atlas ratio | 0.00 | 0.02 | 0.00 | 0.04 |
| Relative angles | ||||
| Atlas | mean | SD | mean | SD |
| Angle to sagittal plane | 21.3 | 6.0 | −23.2 | 5.9 |
| Angle to frontal plane | −8.9 | 59.5 | 15.2 | 48.9 |
| Axis | mean | SD | mean | SD |
| Angle to sagittal plane | 23.3 | 5.1 | −24.7 | 5.7 |
| Angle to frontal plane | −9.6 | 52.2 | 0.3 | 57.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattrysse, E.; Buzzatti, L.; Provyn, S.; Barbero, M.; Van Roy, P. Variability of Upper Cervical Anatomy: A Reflection on Its Clinical Relevance. J. Funct. Morphol. Kinesiol. 2016, 1, 126-139. https://doi.org/10.3390/jfmk1010126
Cattrysse E, Buzzatti L, Provyn S, Barbero M, Van Roy P. Variability of Upper Cervical Anatomy: A Reflection on Its Clinical Relevance. Journal of Functional Morphology and Kinesiology. 2016; 1(1):126-139. https://doi.org/10.3390/jfmk1010126
Chicago/Turabian StyleCattrysse, Erik, Luca Buzzatti, Steven Provyn, Marco Barbero, and Peter Van Roy. 2016. "Variability of Upper Cervical Anatomy: A Reflection on Its Clinical Relevance" Journal of Functional Morphology and Kinesiology 1, no. 1: 126-139. https://doi.org/10.3390/jfmk1010126







