The Role of Physical Activity and Nutrition in the Sarcopenia of Cirrhosis
Abstract
:1. Introduction
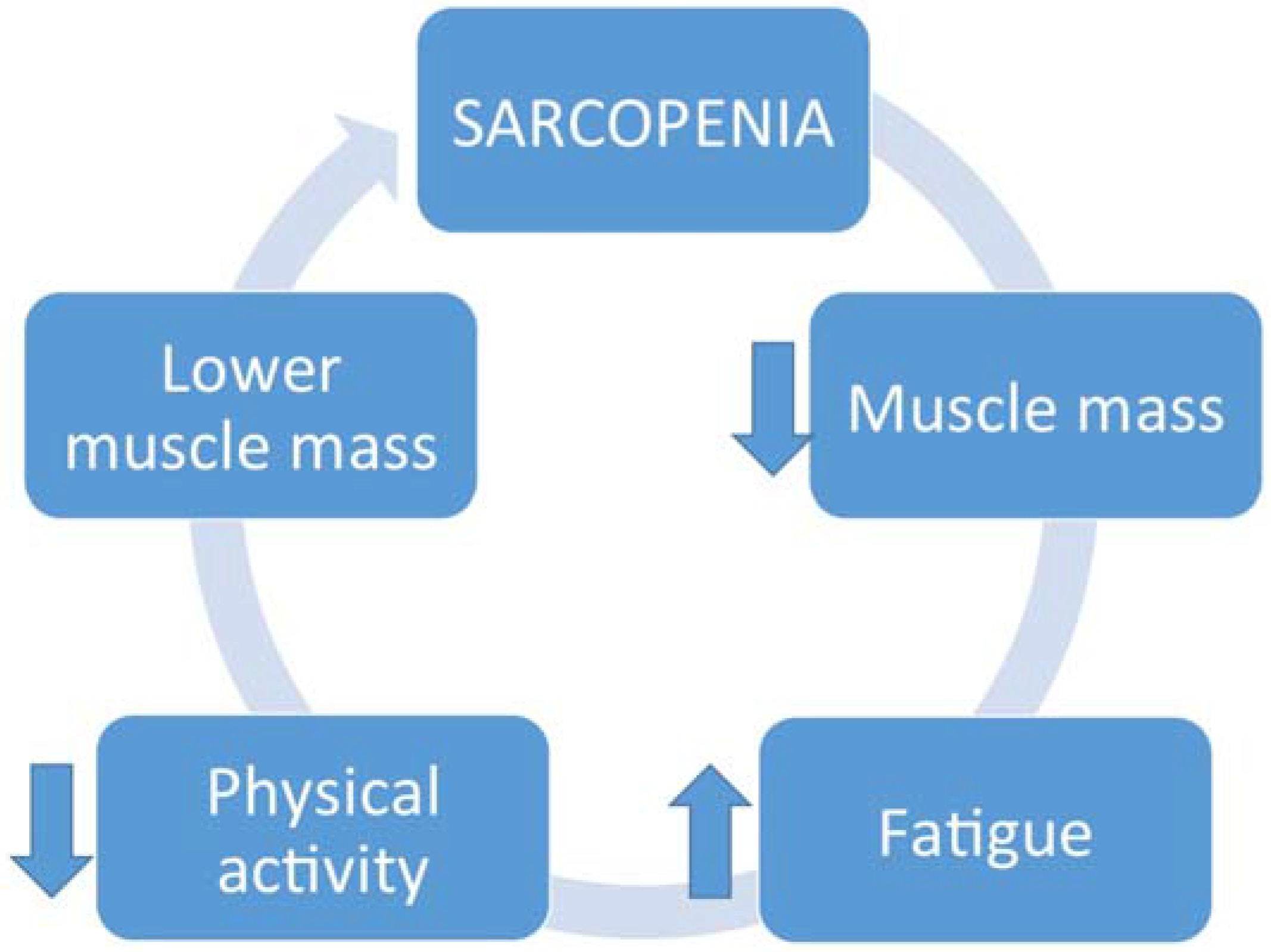
2. Sarcopenia
3. Physical Activity
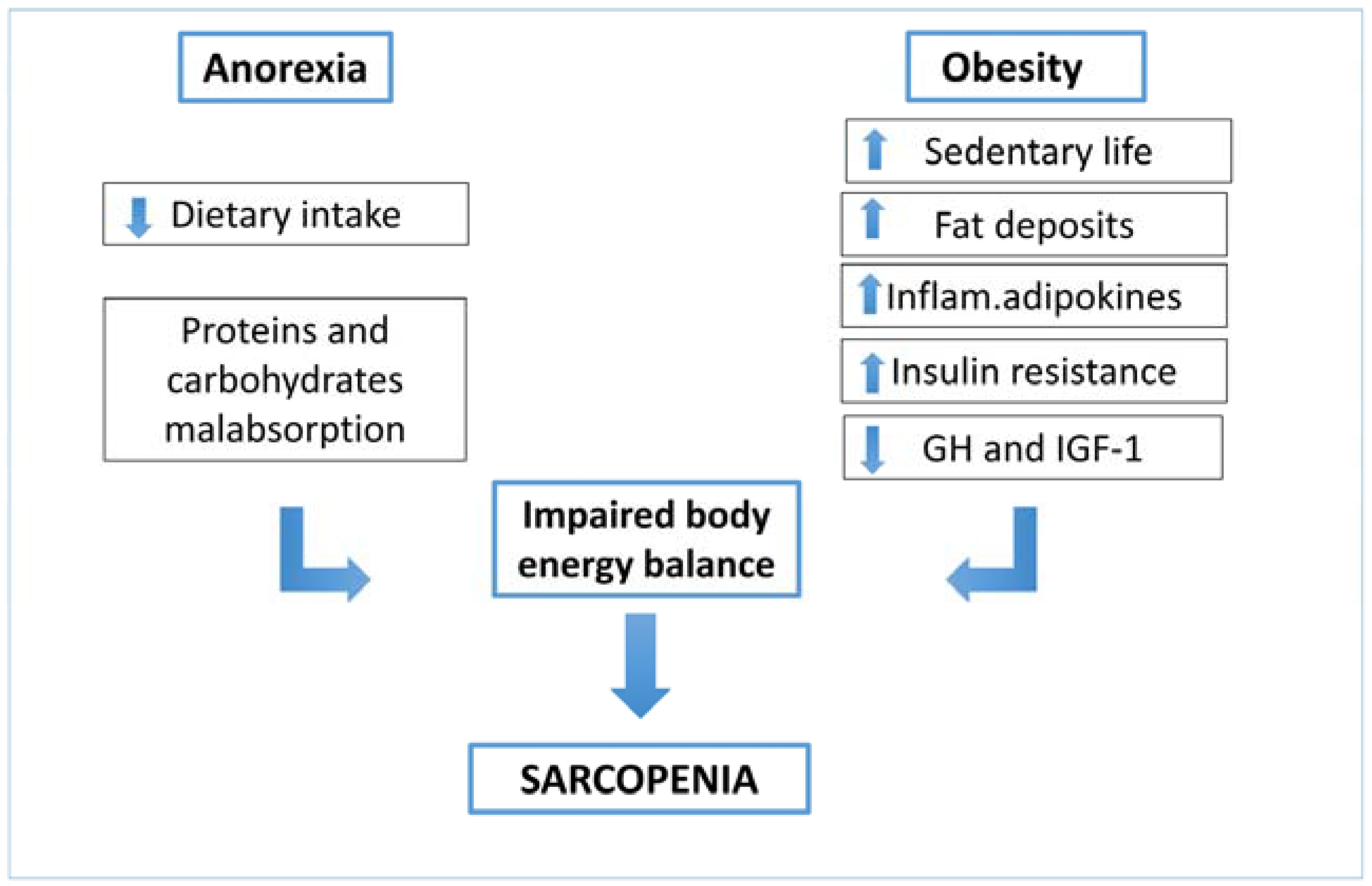
4. Nutrition
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbrevation
| BCAA | Branched Chain Amino Acids |
| EB | Energy Balance |
| MELD | Mayo End stage Liver Disease |
| NAFLD | Non-alcoholic Fatty Liver Disease |
References
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Ozgoren, A.A.; Abdalla, S.; Abd-Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Ferreira, L.G.; Martins, A.I.F.; Cunha, C.E.; Anastácio, L.R.; Lima, A.S.; Correia, M.I. Negative energy balance secondary to inadequate dietary intake of patients on the waiting list for liver transplantation. Nutrition 2013, 29, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Bémeur, C.; Butterworth, R.F. Nutrition in the management of cirrhosis and its neurological complications. J. Clin. Exp. Hepatol. 2014, 4, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty liver index associates with relative sarcopenia and GH/IGF-1 status in obese subjects. PLoS ONE 2016, 11, e0145811. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 4, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E.; Björnsson, E. Hepatic encephalopathy in patients with liver cirrhosis: Is there a role of malnutrition? World J. Gastroenterol. 2008, 14, 3438–3439. [Google Scholar]
- Madden, A.M.; Bradbury, W.; Morgan, M.Y. Taste perception in cirrhosis: Its relationship to circulating micronutrients and food preferences. Hepatology 1997, 26, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Gastrointestinal dysfunction in liver disease and portal hypertension. Gut-liver interactions revisited. Dig. Dis. Sci. 1996, 41, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Caregaro, L.; Pattenò, E.; Marcon, M.; Del Piccolo, F.; Gatta, A. Vegetarian diets in hepatic encephalopathy: Facts or fantasies? Dig. Liver Dis. 2001, 33, 492–500. [Google Scholar] [CrossRef]
- Cheung, K.; Lee, S.S.; Raman, M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin. Gastroenterol. Hepatol. 2012, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Barre, L.K.; Mackenzie, T.A.; Pratt, S.I.; Lopez-Jimenez, F.; Bartels, S.J. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: Dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J. Am. Geriatr. Soc. 2013, 61, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Román, E.; Torrades, M.T.; Nadal, M.J.; Cárdenas, G.; Nieto, J.C.; Vidal, S.; Bascuñana, H.; Juárez, C.; Guarner, C.; Córdoba, J.; et al. Randomized pilot study: Effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig. Dis. Sci. 2014, 59, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin. Transl. Gastroenterol. 2015, 6, e102. [Google Scholar] [CrossRef] [PubMed]
- Montero-Fernandez, N.; Serra-Rexach, J.A. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil. Med. 2013, 49, 131–143. [Google Scholar] [PubMed]
- Jones, J.C.; Coombes, J.S.; Macdonald, G.A. Exercise capacity and muscle strength in patients. with cirrhosis. Liver Transpl. 2012, 18, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Ritland, S.; Petlund, C.F.; Knudsen, T.; Skrede, S. Improvement of physical capacity after long-term training in patients with chronic active hepatitis. Scand. J. Gastroenterol. 1983, 18, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Saran, U.; Dufour, J.F. Physical activity and liver diseases. Hepatology 2015. [Google Scholar] [CrossRef]
- Hayashi, F.; Matsumoto, Y.; Momoki, C.; Yuikawa, M.; Okada, G.; Hamakawa, E.; Kawamura, E.; Hagihara, A.; Toyama, M.; Fujii, H.; et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol. Res. 2013, 43, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Toshikuni, N.; Arisawa, T.; Tsutsumi, M. Nutrition and exercise in the management of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7286–7297. [Google Scholar] [CrossRef] [PubMed]
- Venu, M.; Saeian, K.; Gawrieh, S. High prevalence of vitamin A and D deficiency in patients evaluated for liver transplantation. Liver Transpl. 2013, 19, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Corey, R.; Whitaker, M.; Crowell, M.D.; Keddis, M.T.; Aqel, B.; Balan, V.; Byrne, T.; Carey, E.; Douglas, D.D.; Harrison, M.E.; et al. Vitamin D deficiency, parathyroid hormone levels, and bone disease among patients with end-stage liver disease and normal serum creatinine awaiting liver transplantation. Clin Transplant. 2014, 28, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Kob, R.; Bollheimer, L.C.; Bertsch, T.; Fellner, C.; Djukic, M.; Sieber, C.C.; Fischer, B.E. Sarcopenic obesity: Molecular clues to a better understanding of its pathogenesis? Biogerontology 2015, 16, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Porter Starr, K.N.; McDonald, S.R.; Bales, C.W. Obesity and physical frailty in older adults: A scoping review of lifestyle intervention trials. J. Am. Med. Dir. Assoc. 2014, 15, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Bémeur, C.; Butterworth, R.F.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The nutritional management of hepatic encephalopathy in patients with cirrhosis: ISHEN consensus. Hepatology 2013. [Google Scholar] [CrossRef]
- Mendenhall, C.L.; Tosch, T.; Weesner, R.E.; Garcia-Pont, P.; Goldberg, S.J.; Kiernan, T.; Seeff, L.B.; Sorell, M.; Tamburro, C.; Zetterman, R.; et al. VA cooperative study on alcoholic hepatitis. Prognostic significance of protein-calorie malnutrition. Am. J. Clin. Nutr. 1986, 43, 213–218. [Google Scholar] [PubMed]
- Loguercio, C.; de Girolamo, V.; Federico, A.; Feng, S.L.; Cataldi, V.; del Vecchio Blanco, C.; Gialanella, G. Trace elements and chronic liver disease. J. Trace Elem. Med. Biol. 1997, 11, 158–161. [Google Scholar] [CrossRef]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.; Davuluri, G.; Singh, D.; Allawy, A.; Ten Have, G.A.; Thapaliya, S.; Schulze, J.M.; Barnes, D.; McCullough, A.J.; Engelen, M.P.; et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015, 61, 2018–2029. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trovato, F.M.; Aiello, F.C.; Larocca, L.; Taylor-Robinson, S.D. The Role of Physical Activity and Nutrition in the Sarcopenia of Cirrhosis. J. Funct. Morphol. Kinesiol. 2016, 1, 118-125. https://doi.org/10.3390/jfmk1010118
Trovato FM, Aiello FC, Larocca L, Taylor-Robinson SD. The Role of Physical Activity and Nutrition in the Sarcopenia of Cirrhosis. Journal of Functional Morphology and Kinesiology. 2016; 1(1):118-125. https://doi.org/10.3390/jfmk1010118
Chicago/Turabian StyleTrovato, Francesca Maria, Flavia Concetta Aiello, Licia Larocca, and Simon D. Taylor-Robinson. 2016. "The Role of Physical Activity and Nutrition in the Sarcopenia of Cirrhosis" Journal of Functional Morphology and Kinesiology 1, no. 1: 118-125. https://doi.org/10.3390/jfmk1010118





