2.1. All Sharks
The result of the hierarchical, agglomerative classification (CLUSTER) of trypanorhynch assemblages of 50 species of Selachii revealed five major clusters of shark species at an arbitrary dissimilarity level of 78% (
Figure 1). The two-dimensional ordination plots resulting from nMDS of this agglomerative classification, with sharks coded for taxonomic order, diet, habitat, and depth distribution are shown in
Figure 2. The analysis of similarities (ANOSIM) revealed significant differences in trypanorhynch assemblage composition between sharks that primarily consume invertebrates versus those that primarily consume teleosts (global
R = 0.279;
p < 0.01), between those from benthic and pelagic habitats (global
R = 0.275;
p < 0.01), and between sharks occurring in deep versus shallow water (global
R = 0.327;
p < 0.01), thus supporting the significance of the major clusters resulting from the classification and ordination methods. With respect to taxonomy, the ANOSIM showed significant differences among shark orders (global
R = 0.381;
p < 0.01), suggesting a relationship between trypanorhynch genera and shark orders. In ANOSIM pairwise comparison, the trypanorhych assemblages of the Carcharhiniformes were significantly different from those of the Squaliformes (global
R = 0.546;
p < 0.01), the Orectolobiformes (global
R = 0.466;
p < 0.1), the Lamniformes (global
R = 0.312;
p < 0.01), and the Hexanchiformes (global
R = 0.275;
p < 0.05). In addition, the trypanorhynch assemblages of the Lamniformes were significantly different from those of the Orectolobiformes (global
R = 0.432;
p < 0.01) and Squaliformes (global
R = 0.355;
p < 0.05), and the assemblages of the Orectolobiforms were significantly different from those of the Squaliformes (global
R = 0.620;
p < 0.01). No significant differences were found between the trypanorhynch assemblages of any of the other 16 pairs of shark orders.
The following similarity percentage (SIMPER) analysis indicated which trypanorhynch genera contributed most to the differences between the studied shark species (
Table S2). The average similarity was 22.29% for the pelagic sharks, with the genera
Nybelinia Poche, 1926,
Otobothrium Linton, 1891, and
Dasyrhynchus Pintner, 1928, accounting for a cumulative contribution of 54.30%. Including
Heteronybelinia Palm, 1999,
Callitetrarhynchus Pintner, 1931,
Tentacularia Bosc, 1797,
Grillotia Guiart, 1927, and
Floriceps Cuvier 1817, the trypanorhynch genera reached a cumulative contribution of 91.51%. The benthic sharks which had an average similarity of 15.64% were characterised by five genera,
Dollfusiella Campbell and Beveridge, 1994,
Nybelinia,
Grillotia,
Lacistorhynchus Pintner, 1913, and
Gilquinia Guiart 1927, which accounted for a cumulative contribution of 92.84%. The genera
Nybelinia (12.48%),
Otobothrium (8.26%),
Dasyrhynchus (6.85%),
Heteronybelinia (6.44%), and
Callitetrarhynchus (5.07%) for the pelagic sharks and
Dollfusiella (7.81%) and
Grillotia (7.93%) for the benthic sharks contributed over 50% to the total dissimilarity. Other characteristic trypanorhynch genera of the benthic sharks were
Gilquinia,
Prochristianella Dollfus, 1946,
Diesingium Pintner, 1929,
Deanicola Beveridge, 1990 and
Pseudolacistorhynchus Palm, 1995. The parasite genera whose species numbers significantly differed between pelagic and benthic sharks were
Tentacularia (
U = 171.0,
p = 0.003),
Nybelinia (
U = 177.5,
p = 0.025),
Heteronybelinia (
U = 193.0,
p = 0.029),
Pseudogrillotia Dollfus, 1969 (
U = 216.0,
p = 0.033),
Dasyrhynchus (
U = 144.0,
p = 0.000),
Lacistorhynchus (
U = 195.0,
p = 0.013),
Pseudolacistorhynchus (
U = 232.0,
p = 0.034),
Callitetrarhynchus (
U = 180.0,
p = 0.013),
Floriceps (
U = 180.0,
p = 0.005),
Poecilancistrum Dollfus, 1929 (
U = 207.0,
p = 0.020),
Otobothrium (
U = 155.0,
p = 0.002),
Tetrarhynchobothrium Diesing, 1854 (
U = 217.5,
p = 0.012) and
Dollfusiella (
U = 156.5,
p = 0.001).
The average similarity and genus composition of trypanorhynchs for fish vs. invertebrate feeding sharks is compiled in
Table S3. Fish-feeding sharks had a 19.38% average similarity, with the trypanorhynch genera
Nybelinia (30.43%),
Grillotia (14.98%),
Heteronybelinia (10.53%),
Otobothrium (9.89%),
Dasyrhynchus (9.72%),
Callitetrarhynchus (6.96%),
Tentacularia (4.57%) and
Floriceps (3.29%) having a contribution of 90.37%. Invertebrate-feeding sharks had an average similarity of 18.76%, and were characterized by the genera
Dollfusiella (40.53%),
Nybelinia (30.13%),
Lacistorhynchus (8.27%),
Prochristianella (4.29%),
Diesingium,
Trigonolobium Dollfus, 1929 and
Tetrarhynchobothrium (together 9.66%). The decreasing contribution to the total similarity, up to 100%, is given in
Table S3. The parasite genera whose species numbers significantly differed between fish and invertebrate-feeding sharks were
Tentacularia (
U = 126.5,
p = 0.025),
Heteronybelinia (
U = 124.0,
p = 0.036),
Kotorella Euzet and Radujkovic, 1989 (
U = 160.0,
p = 0.044),
Dasyrhynchus (
U = 110.0,
p = 0.009),
Grillotia (
U = 129.0,
p = 0.048),
Paragrillotia Dollfus, 1969 (
U = 160.0,
p = 0.044),
Lacistorhynchus (
U = 123.0,
p = 0.007),
Pseudolacistorhynchus (
U = 144.0,
p = 0.007),
Diesingium (
U = 128.0,
p = 0.001),
Floriceps (
U = 132.0,
p = 0.035),
Otobothrium (
U = 123.0,
p = 0.034),
Tetrarhynchobothrium (
U = 128.0,
p = 0.001),
Zygorhynchus Beveridge & Campbell, 1988 (
U = 160.0,
p = 0.044),
Eutetrarhynchus Pintner, 1913 (
U = 149.5,
p = 0.047),
Dollfusiella (
U = 74.5,
p = 0.000),
Trigonolobium (
U = 144.0,
p = 0.007),
Trimacracanthus Beveridge and Campbell, 1987 (
U = 160,
p = 0.044) and
Prochristianella (
U = 133.0,
p = 0.008).
The average similarity and genus composition of trypanorhynchs for fish vs. invertebrate feeding sharks is compiled in
Table S3. Fish-feeding sharks had a 19.38% average similarity, with the trypanorhynch genera
Nybelinia (30.43%),
Grillotia (14.98%),
Heteronybelinia (10.53%),
Otobothrium (9.89%),
Dasyrhynchus (9.72%),
Callitetrarhynchus (6.96%),
Tentacularia (4.57%) and
Floriceps (3.29%) having a contribution of 90.37%. Invertebrate-feeding sharks had an average similarity of 18.76%, and were characterized by the genera
Dollfusiella (40.53%),
Nybelinia (30.13%),
Lacistorhynchus (8.27%),
Prochristianella (4.29%),
Diesingium,
Trigonolobium Dollfus, 1929 and
Tetrarhynchobothrium (together 9.66%). The decreasing contribution to the total similarity, up to 100%, is given in
Table S3. The parasite genera whose species numbers significantly differed between fish and invertebrate-feeding sharks were
Tentacularia (
U = 126.5,
p = 0.025),
Heteronybelinia (
U = 124.0,
p = 0.036),
Kotorella Euzet and Radujkovic, 1989 (
U = 160.0,
p = 0.044),
Dasyrhynchus (
U = 110.0,
p = 0.009),
Grillotia (
U = 129.0,
p = 0.048),
Paragrillotia Dollfus, 1969 (
U = 160.0,
p = 0.044),
Lacistorhynchus (
U = 123.0,
p = 0.007),
Pseudolacistorhynchus (
U = 144.0,
p = 0.007),
Diesingium (
U = 128.0,
p = 0.001),
Floriceps (
U = 132.0,
p = 0.035),
Otobothrium (
U = 123.0,
p = 0.034),
Tetrarhynchobothrium (
U = 128.0,
p = 0.001),
Zygorhynchus Beveridge & Campbell, 1988 (
U = 160.0,
p = 0.044),
Eutetrarhynchus Pintner, 1913 (
U = 149.5,
p = 0.047),
Dollfusiella (
U = 74.5,
p = 0.000),
Trigonolobium (
U = 144.0,
p = 0.007),
Trimacracanthus Beveridge and Campbell, 1987 (
U = 160,
p = 0.044) and
Prochristianella (
U = 133.0,
p = 0.008).
Only three trypanorhynch genera, mainly
Grillotia with a 94.25% total similarity contribution,
Gilquinia (4.24%), and
Nybelinia (1.51%) characterised the deep water sharks (
Table S4). The other trypanorhynch genera were characteristic of shallow water shark species occurring above 200 m depth.
Nybelinia (39.30%),
Otobothrium (10.10%) and
Dollfusiella (9.60%) contributed most, followed by
Dasyrhynchus (6.23%),
Callitetrarhynchus (6.15%),
Heteronybelinia (5.07%),
Grillotia (3.99%), and
Tentacularia,
Mixonybelinia Palm, 1999, and
Lacistorhynchus (together 10.20%) (
Table S4). The parasite genera whose species numbers significantly differed between shallow and deep water sharks were
Nybelinia (
U = 85.0,
p = 0.016),
Chimaerarhynchus Beveridge and Campbell, 1989 (
U = 136.5,
p = 0.014),
Dasyrhynchus (
U = 108.0,
p = 0.038),
Deanicola (
U = 136.5,
p = 0.014),
Grillotia (
U = 34.5,
p = 0.000),
Callitetrarhynchus (
U = 104.0,
p = 0.030),
Dollfusiella (
U = 108.0,
p = 0.037), and
Otobothrium (
U = 100.0,
p = 0.025).
Similarity percentage analysis further revealed the importance of the different trypanorhynch genera for the characterisation of the shark orders (
Table S5). The Carcharhiniformes were characterized by
Nybelinia (33.03%),
Otobothrium (13.43%),
Dasyrhynchus (10.14%),
Callitetrarhynchus (9.98%),
Heteronybelinia (8.83%),
Dollfusiella (4.90%),
Tentacularia (4.67%),
Floriceps (4.39%), and
Lacistorhynchus (2.88%). The genera
Grillotia and
Nybelinia contributed 73.33% and 26.67% to the similarity of the Hexanchiformes, while the Lamniforms were characterised by
Nybelinia (35.68%),
Sphyriocephalus Pintner, 1913 (20.43%),
Mixonybelinia (19.68%),
Gymnorhynchus Rudolphi, 1819 (11.09%), and
Molicola Dollfus 1935 (9.24%). The genera
Dollfusiella (83.56%) and
Mixonybelinia (9.86%) contributed most to the similarity of the Orectolobiformes, and
Grillotia (86.11%) and
Gilquinia (13.89%) to that of the Squaliformes (
Table S5).
The genera Grillotia (15.18%), Nybelinia (11.69%), Gilquinia (9.24%), Otobothrium (7.92%), and Dasyrhynchus (6.19%) contributed over 50% to the total dissimilarity of Carcharhiniformes and Squaliformes, and Dollfusiella (12.66%), and Pseudolacistorhynchus (4.78%) were important to separate the Orectolobiformes from the Carcharhiniformes. The genera Nybelinia (13.36%), Sphyriocephalus (9.15%), Otobothrium (7.98%), Mixonybelinia (6.44%), Dasyrhynchus (6.32%), and Gymnorhynchus (5.45%) contributed over 50% to the dissimilarity of the Carcharhiniformes and the Lamniformes, while the Lamniformes differed from the Orectolobiformes by Dollfusiella (15.4%), Nybelinia (14.18%), Sphyriocephalus (10.78%), Mixonybelinia (8.21%), and Pseudolacistorhynchus (5.25%), and differed from the Squaliformes by Grillotia (18.81%), Nybelinia (14.46%), Sphyriocephalus (12.40%), and Gilquinia (11.00%). The genera Grillotia (25.87%), Nybelinia (12.26%), and Otobothrium (8.04%) contributed over 50% to the dissimilarity of the Carcharhiniformes and the Hexanchiformes. Other orectolobiform characteristic genera contributed less than 5% (Hornelliella Yamaguti, 1954, Molicola, Hepatoxylon Bosc, 1811). Only four genera, Grillotia (20.90%), Dollfusiella (16.76%), Gilquinia (11.88%) and Deanicola (6.73%) contributed 58.02% to the dissimilarity between the Orectolobiformes and the Squaliformes. The parasite genera where the species numbers significantly differed in seven orders of shark were Nybelinia (H = 11.0, p = 0.044), Mixonybelinia (H = 13.8, p = 0.016), Sphyriocephalus (H = 15.9, p = 0.007), Gilquinia (H = 13.2, p = 0.020), Molicola (H = 13.3, p = 0.019), Gymnorhynchus (H = 13.5, p = 0.017), Grillotia (H =19.7, p = 0.002), Pseudolacistorhynchus (H = 18.4, p = 0.002), Callitetrarhynchus (H = 11.2, p = 0.004), Otobothrium (H = 11.1, p = 0.043), and Dollfusiella (H = 13.5, p = 0.018).
2.2. Carcharhiniformes
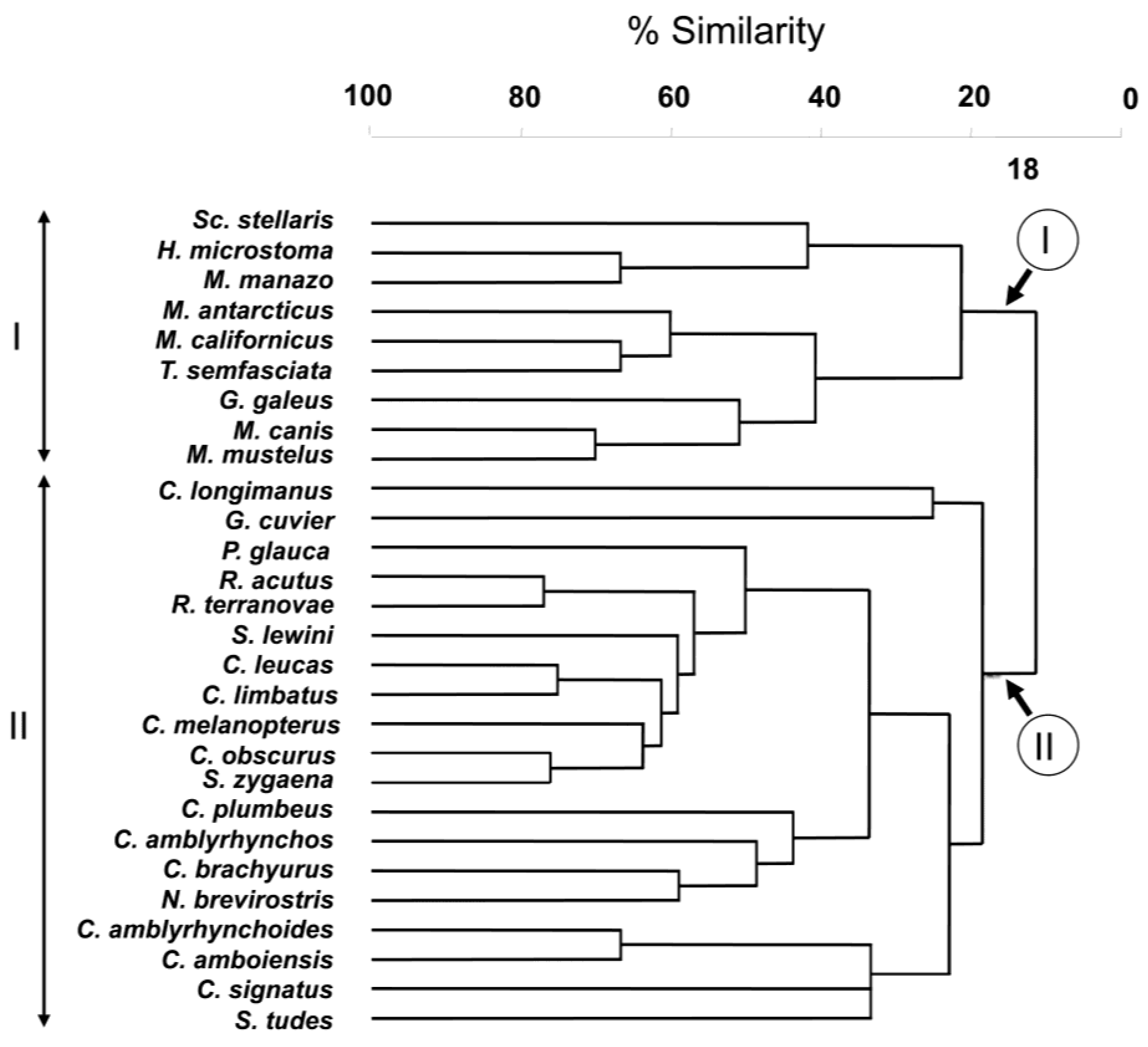
The result of the hierarchical, agglomerative classification (CLUSTER) of trypanorhynch assemblages of Carcharhiniformes, the order with the most comprehensive trypanorhynch dataset in Palm [
6], is shown in
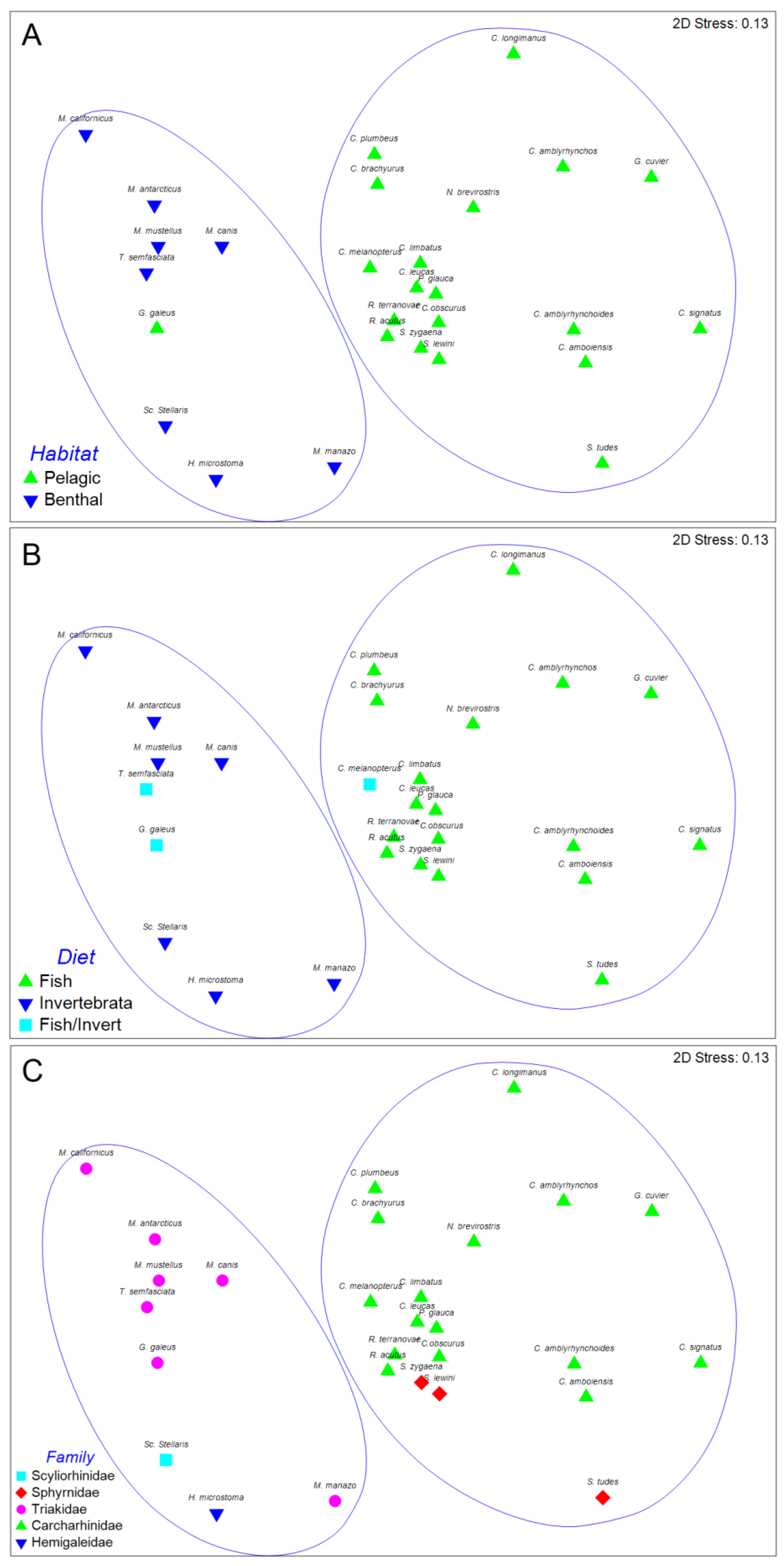
Figure 3. Two major clusters of sharks at an arbitrary dissimilarity level of 82% were recovered (I and II). The nMDS plots of the classification, with sharks coded for family, diet, and habitat are shown in
Figure 4. Analysis of similarities revealed significant differences in trypanorhynch assemblage composition between sharks that primarily consume invertebrates versus those that primarily consume teleosts (global
R = 0.683;
p < 0.01), and also between those from benthic and pelagic habitats (global
R = 0.590;
p < 0.01). Regarding taxonomy, however, ANOSIM pairwise comparisons, detected significant differences on trypanorhynch assemblages only between Carcharhinidae and Triakidae (global
R = 0.678;
p < 0.01) and between Sphyrnidae and Triakidae (global
R = 0.748;
p < 0.01). No significant differences were found between the trypanorhynch assemblages of any of the other eight pairs of carcharhiniform families.
The results of the SIMPER analyses to explore the contributions of individual trypanorhynch genera to the degree of similarity observed among the conditions for the factors environment and diet are shown in
Tables S6 and S7, respectively. The average similarity was 31.16% for pelagic carcharhiniforms, with the genera
Nybelinia,
Otobothrium and
Dasyrhynchus having a cumulative contribution of 52.53%. Together with
Heteronybelinia,
Callitetrarhynchus,
Tentacularia, and
Floriceps, these trypanorhynch genera reached a cumulative contribution of 92.37%. Benthic carcharhiniforms that had an average similarity of 33.88% were characterized by four genera,
Dollfusiella,
Lacistorhynchus,
Nybelinia, and
Diesingium, having a cumulative contribution of 91.22%. Parasite genera whose species numbers significantly differed between pelagic and benthic carcharhiniforms were
Tentacularia (
U = 39.0,
p = 0.031 one-tailed),
Heteronybelinia (
U = 33.0,
p = 0.017),
Pintneriella Yamaguti 1934 (
U = 55.0,
p = 0.028),
Dasyrhynchus (
U = 30.0,
p = 0.012),
Lacistorhynchus (
U = 16.5,
p = 0.000),
Diesingium (
U = 35.5,
p = 0.002),
Floriceps (
U = 39.0,
p = 0.031),
Otobothrium (
U = 37.5,
p = 0.040),
Tetrarhynchobothrium (
U = 44.0,
p = 0.002),
Eutetrarhynchus (
U = 47.0,
p = 0.024),
Dollfusiella (
U = 19.0,
p = 0.000), and
Trimacracanthus (
U = 55.0,
p = 0.028).
Carcharhiniforms with different diet preferences, either fish or invertebrates, had an average similarity of 32.44 for fish and 29.47 for invertebrates. Similarly to the SIMPER analysis of benthic versus pelagic carcharhiniforms,
Nybelinia,
Otobothrium, and
Dasyrhynchus reached a cumulative contribution close to 50% (48.95%) for preliminary fish eating sharks (
Table S7). Together with
Heteronybelinia,
Callitetrarhynchus,
Tentacularia, and
Floriceps, the cumulative contribution was 91.43%. Invertebrate-feeding carcharhiniforms were characterized by the genus
Nybelinia, that had a contribution of 50.24%, and together with
Dollfusiella,
Lacistorhynchus,
Diesingium, and
Trigonolobium reached a cumulative contribution of 91.92%. The decreasing contribution to the total similarity, up to 100%, is given in
Table S7. The parasite genera whose species numbers were significantly different between fish and invertebrate feeders were
Tentacularia (
U = 35.0,
p = 0.018),
Heteronybelinia (
U = 24.5,
p = 0.004),
Gilquinia (
U = 45,
p = 0.010),
Dasyrhynchus (
U = 24.5,
p = 0.005),
Lacistorhynchus (
U = 27.0,
p = 0.000),
Diesingium (
U = 36.0,
p = 0.002),
Callitetrarhynchus (
U = 32.5,
p = 0.019),
Floriceps (
U = 35,
p = 0.018),
Poecilancistrum (
U = 42.0,
p = 0.043),
Otobothrium (
U = 30.5,
p = 0.016),
Tetrarhynchobothrium (
U = 45.0,
p = 0.010),
Eutetrarhynchus (
U = 45.0,
p = 0.010),
Dollfusiella (
U = 33.5,
p = 0.008), and
Trigonolobium (
U = 45.0,
p = 0.010).
The trypanorhynch composition within the shark family Carcharhinidae is characterised by the genera
Dasyrhynchus (20.05% contribution),
Nybelinia (14.52%),
Callitetrarhynchus (13.90%),
Otobothrium (12.61%),
Floriceps (9.87%),
Heteronybelinia (9.70%),
Tentacularia (8.19%), and
Poecilancistrum (3.96%) (
Table S8). The Triakidae differed significantly from the Carcharhinidae and Sphyrnidae, being characterized by the genera
Lacistorhynchus (31.15% contribution),
Nybelinia (31.15%),
Dollfusiella (23.20%), and
Diesingium (9.10%). The Sphyrnidae harbored the genera
Otobothrium (38.72%),
Heteronybelinia (31.20%), and
Nybelinia (22.56%). Only two genera,
Nybelinia and
Trigonolobium, were found in the Hemigaleidae, and
Nybelinia and
Gilquinia in the Scyliorhinidae. The parasite genera whose species numbers significantly differed in five families of carcharhiniforms were
Heteronybelinia (
H = 10.4,
p = 0.017),
Gilquinia (
H = 12.6,
p = 0.006),
Dasyrhynchus (
H = 10.5,
p = 0.016),
Pseudogrillotia (
H = 8.3,
p = 0.040),
Lacistorhynchus (
H = 21.9,
p = 0.000),
Diesingium (
H = 13.4,
p = 0.004),
Floriceps (
H = 9.6,
p = 0.024),
Otobothrium (
H = 8.5,
p = 0.037),
Dollfusiella (
H = 8.8,
p = 0.033), and
Trigonolobium (
H = 14.5,
p = 0.003).