Distribution, Spread, and Habitat Predictability of a Small, Invasive, Piscivorous Fish in an Important Estuarine Fish Nursery
Abstract
:1. Introduction
2. Materials and Methods
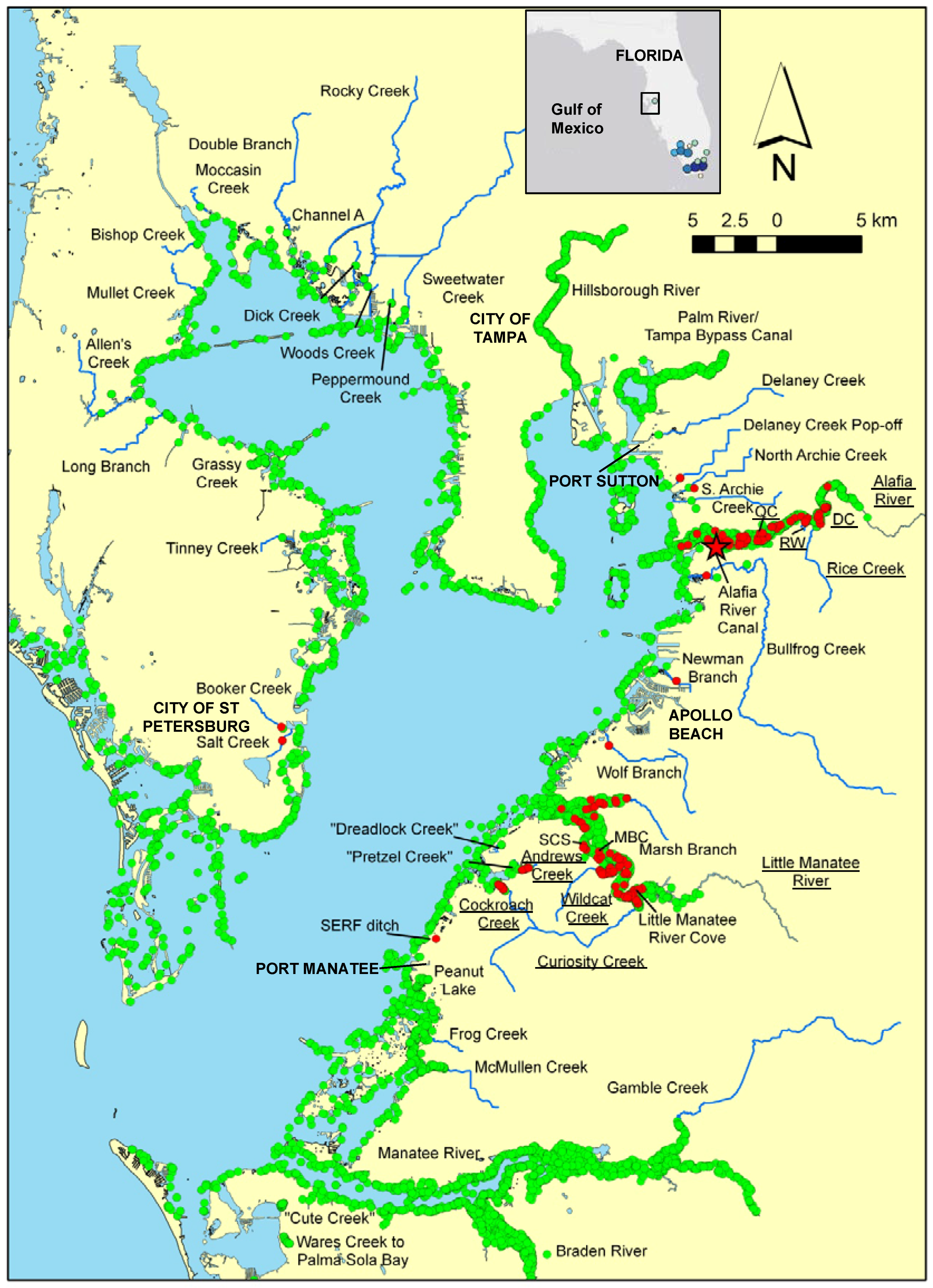
2.1. Study Area and Sampling Techniques
2.2. Data Analysis
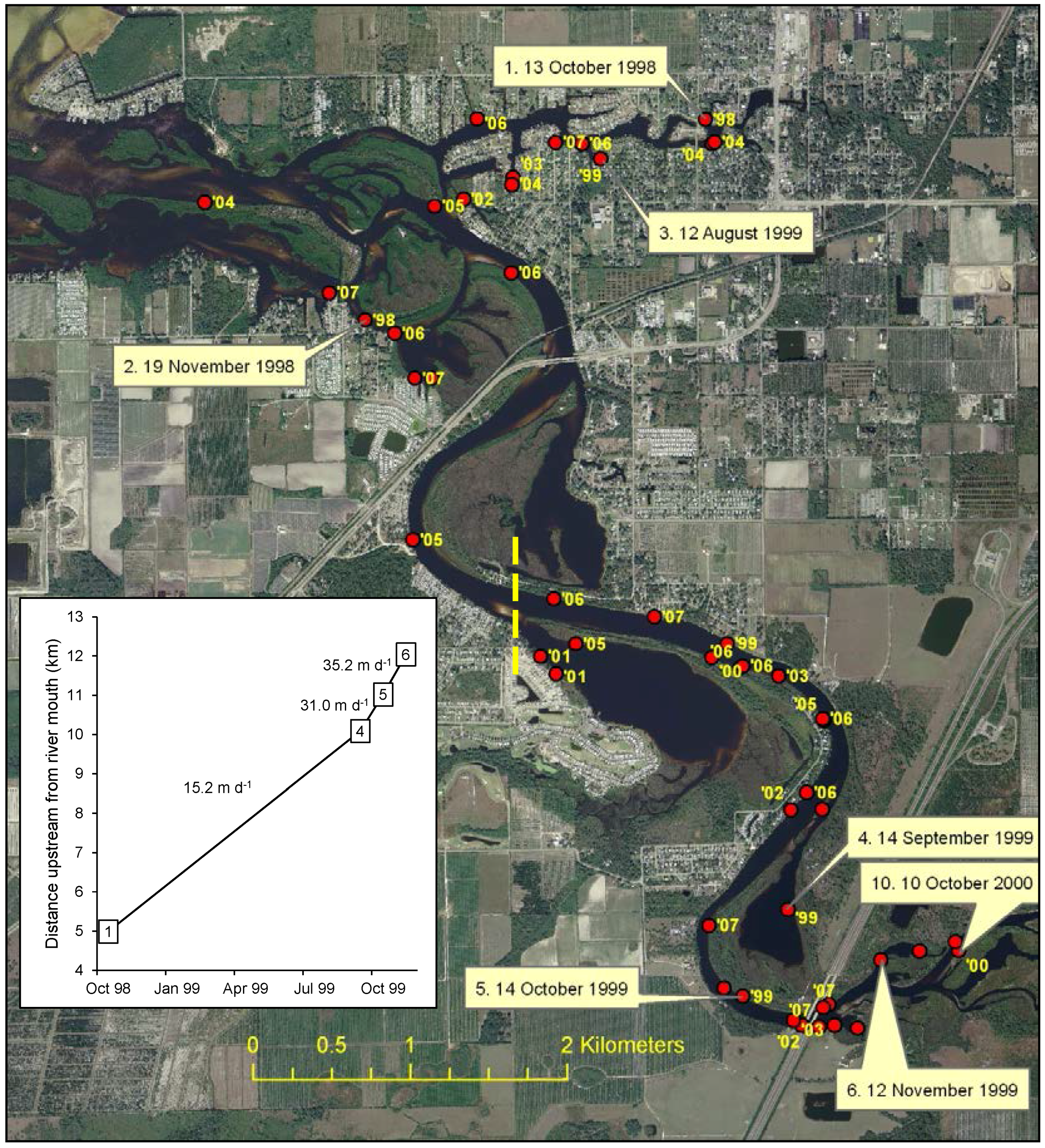
2.2.1. Distribution and Spread Rate
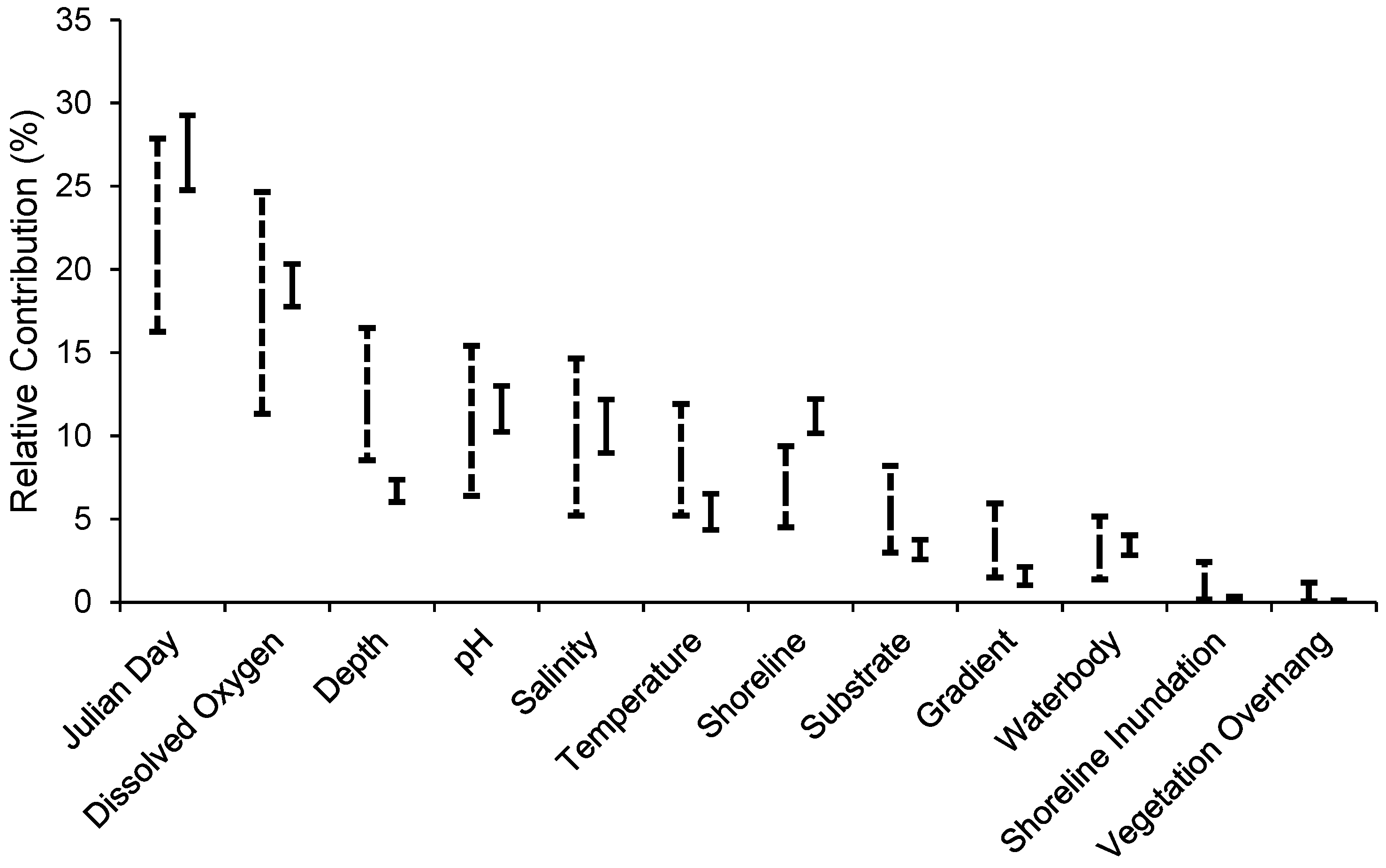
2.2.2. Habitat Predictability
3. Results
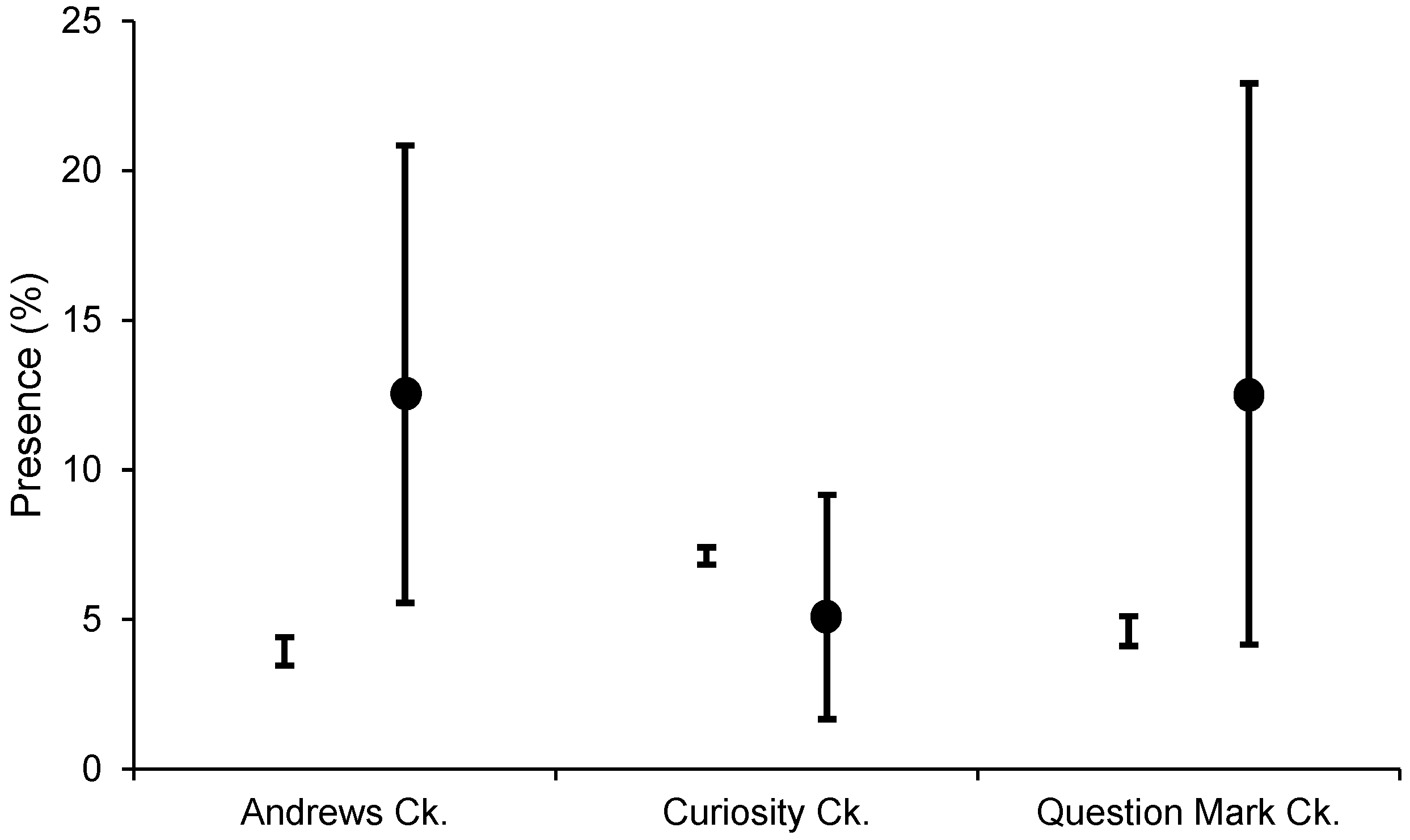
3.1. Distribution and Spread Rate
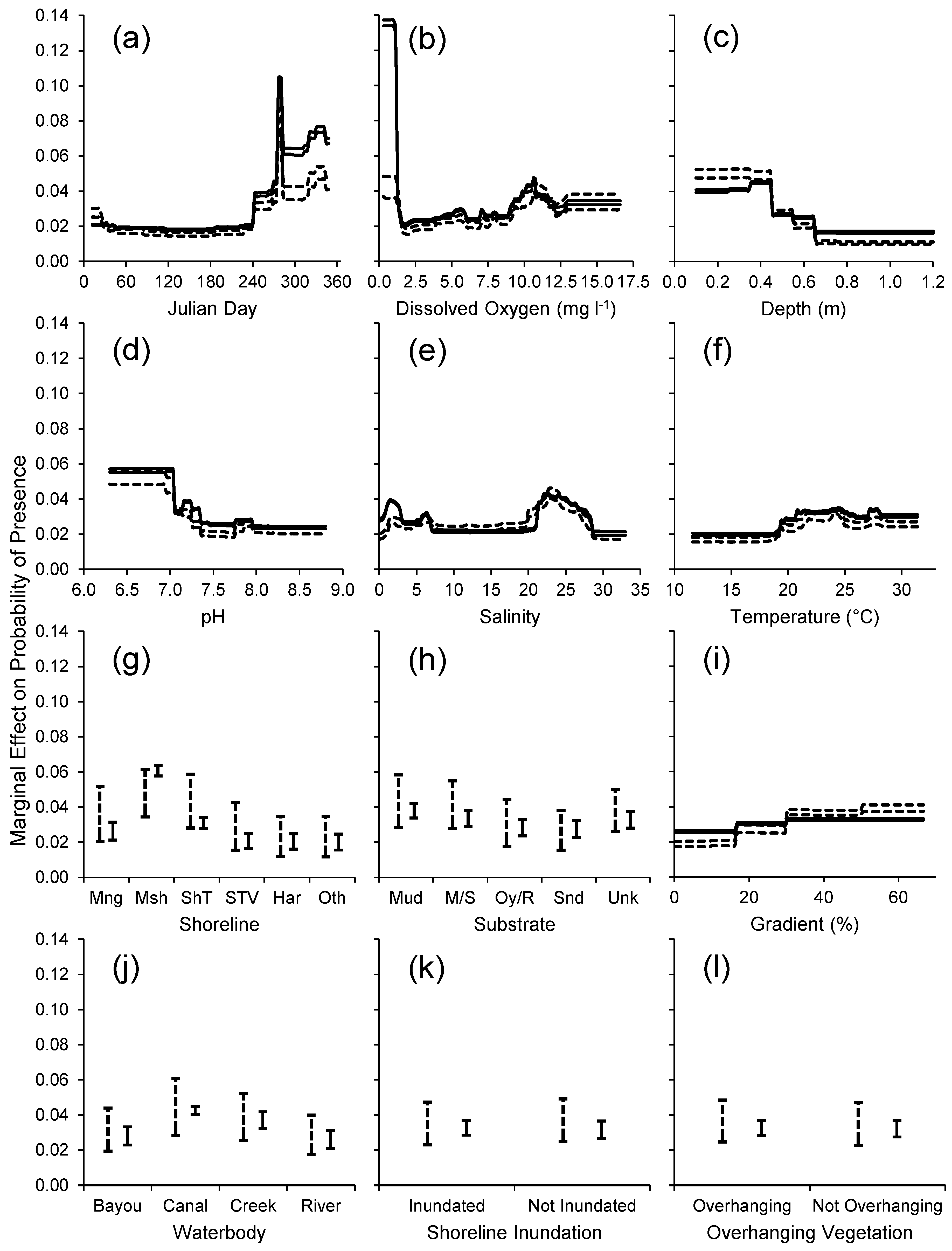
3.2. Habitat Predictability
4. Discussion
4.1. Distribution and Spread Rate
4.2. Habitat Predictability
4.3. Management Implications
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying Threats to Imperiled Species in the United States. Bioscience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Obama, B. Executive Order—Safeguarding the Nation from the Impacts of Invasive Species; The White House, Office of the Press Secretary: Washington, DC, USA, 2016. Available online: https://www.whitehouse.gov/the-press-office/2016/12/05/executive-order-safeguarding-nation-impacts-invasive-species (accessed on 11 January 2017).
- Matlock, G.C. Temporal Trends in Non-Native Fishes Established in the Continental United States. Manag. Biol. Invasion 2014, 5, 349–355. [Google Scholar] [CrossRef]
- Mills, M.D.; Rader, R.B.; Belk, M.C. Complex Interactions between Native and Invasive Fish: The Simultaneous Effects of Multiple Negative Interactions. Oecologia 2004, 141, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Moyle, P.B.; Marchetti, M.P. Predicting Invasion Success: Freshwater Fishes in California as a Model. Bioscience 2006, 56, 515–524. [Google Scholar] [CrossRef]
- Huang, J.; Frimpong, E.A. Limited Transferability of Stream-Fish Distribution Models among River Catchments: Reasons and Implications. Freshw. Biol. 2016, 61, 729–744. [Google Scholar] [CrossRef]
- Wenger, S.J.; Olden, J.D. Assessing Transferability of Ecological Models: An Underappreciated Aspect of Statistical Validation. Methods Ecol. Evol. 2012, 3, 260–267. [Google Scholar] [CrossRef]
- Houlahan, J.E.; McKinney, S.T.; Anderson, T.M.; McGill, B.J. The Priority of Prediction in Ecological Understanding. Oikos 2016, 126, 1–7. [Google Scholar] [CrossRef]
- Shafland, P.L.; Gestring, K.B.; Stanford, M.S. Florida’s Exotic Freshwater Fishes—2007. Fla. Sci. 2008, 71, 220–245. [Google Scholar]
- Tuckett, Q.M.; Ritch, J.L.; Lawson, K.M.; Hill, J.E. Landscape-Scale Survey of Non-Native Fishes near Ornamental Aquaculture Facilities in Florida, USA. Biol. Invasions 2017, 19, 223–237. [Google Scholar] [CrossRef]
- Schofield, P.J.; Loftus, W.F. Non-Native Fishes in Florida Freshwaters: A Literature Review and Synthesis. Rev. Fish Biol. Fish 2015, 25, 117–145. [Google Scholar] [CrossRef]
- Schofield, P.J.; Morris, J.A.; Akins, L. Field Guide to Nonindigenous Marine Fishes of Florida; NOAA Technical Memorandum NOS NCCOS 92; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2009. Available online: https://repository.library.noaa.gov/view/noaa/12675 (accessed on 13 February 2017).
- Belshe, J.F. Observations of an Introduced Tropical Fish (Belonesox belizanus) in Southern Florida. Master’s Thesis, University of Miami, Coral Gables, FL, USA, 1961. [Google Scholar]
- Schofield, P.J.; Nico, L.; Neilson, M. Belonesox belizanus; USGS Nonindigenous Aquatic Species Database: Gainesville, FL, USA, 2013. Available online: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=843 (accessed on 19 January 2017).
- Obregon-Barboza, H.; Contreras-Balderas, S.; Lozano-Vilano, M.L. The Fishes of Northern and Central Veracruz, Mexico. Hydrobiologia 1994, 286, 79–95. [Google Scholar] [CrossRef]
- Miller, R.R.; Minckley, W.L.; Norris, S.M. Freshwater Fishes of México; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Ferry, L.A.; Paig-Tran, E.M.; Gibb, A.C. Suction, Ram, and Biting: Deviations and Limitations to the Capture of Aquatic Prey. Integr. Comp. Biol. 2015, 55, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Miley, W.W. Ecological Impact of the Pike Killifish, Belonesox belizanus Kner, (Poeciliidae) in Southern Florida. Master’s Thesis, Florida Atlantic University, Boca Raton, FL, USA, 1978. [Google Scholar]
- Turner, J.S.; Snelson, F.F.J. Population Structure, Reproduction and Laboratory Behavior of the Introduced Belonesox belizanus (Poeciliidae) in Florida. Environ. Biol. Fishes 1984, 10, 89–100. [Google Scholar] [CrossRef]
- Greenwood, M.F.D.; Malkin, E.; Peebles, E.B.; Stahl, S.D.; Courtney, F.X. Assessment of the Value of Small Tidal Streams, Creeks, and Backwaters as Critical Habitat for Nekton in the Tampa Bay Watershed. Report to the Florida State Wildlife Grants Program, Project SWG05-15; Florida Fish and Wildlife Conservation Commission Fish and Wildlife Research Institute and University of South Florida College of Marine Science: St Petersburg, FL, USA, 2008; Available online: https://www.researchgate.net/publication/313398996_Assessment_of_the_value_of_small_tidal_streams_creeks_and_backwaters_as_critical_habitat_for_nekton_in_the_Tampa_Bay_watershed_Report_to_the_Florida_State_Wildlife_Grants_Program_Project_SWG05–15 (accessed on 13 February 2017).
- Harms, C.A.; Turingan, R.G. Dietary Flexibility Despite Behavioral Stereotypy Contributes to Successful Invasion of the Pike Killifish, Belonesox belizanus in Florida, USA. Aquat. Invasions 2012, 7, 547–553. [Google Scholar] [CrossRef]
- Lawson, L.L., Jr.; Hill, J.E.; Hardin, S.; Vilizzi, L.; Copp, G.H. Evaluation of the Fish Invasiveness Screening Kit (Fisk V2) for Peninsular Florida. Manag. Biol. Invasion 2015, 6, 413–422. [Google Scholar] [CrossRef]
- Greenwood, M.F.D. Assessing Effects of the Nonindigenous Pike Killifish on Indigenous Fishes in Tampa Bay, Florida, Using a Weighted-Evidence Approach. Trans. Am. Fish Soc. 2012, 141, 84–99. [Google Scholar] [CrossRef]
- Fulford, R.; Yoskowitz, D.; Russell, M.; Dantin, D.; Rogers, J. Habitat and Recreational Fishing Opportunity in Tampa Bay: Linking Ecological and Ecosystem Services to Human Beneficiaries. Ecosyst. Serv. 2016, 17, 64–74. [Google Scholar] [CrossRef]
- Stevens, P.W.; Blewett, D.A.; Boucek, R.E.; Rehage, J.S.; Winner, B.L.; Young, J.M.; Whittington, J.A.; Paperno, R. Resilience of a Tropical Sport Fish Population to a Severe Cold Event Varies across Five Estuaries in Southern Florida. Ecosphere 2016, 7, e01400. [Google Scholar] [CrossRef]
- MacDonald, T.C.; Matheson, R.E.; Peebles, E.B.; McMichael, R.H.; Greenwood, M.F.D. Tidal Creeks as Nekton Habitat in the Tampa Bay Estuary. In Proceedings of the Tampa Bay Area Scientific Information Symposium (BASIS 5), St Petersburg, FL, USA, 20–23 October 2009; Cooper, S.T., Ed.; Tampa Bay Estuary Program and Tampa Bay Regional Planning Council: Tampa Bay, FL, USA, 2010; pp. 109–122. [Google Scholar]
- Krebs, J.M.; Bell, S.S. Risk of Predation Reflects Variation in the Reproductive Strategy of a Dominant Forage Fish in Mangrove Tidal Tributaries. Oecologia 2012, 168, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.M.; McIvor, C.C.; Bell, S.S. Nekton Community Structure Varies in Response to Coastal Urbanization near Mangrove Tidal Tributaries. Estuar. Coasts 2014, 37, 815–831. [Google Scholar] [CrossRef]
- Miller, N. The Fishes of the Motagua River, Guatemala. Bull. Am. Mus. Nat. Hist. 1905, 23, 95–123. [Google Scholar]
- Greenfield, D.W.; Thomerson, J.E. Fishes of the Continental Waters of Belize; University Press of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Taylor, D.S.; Reyier, E.A.; McIvor, C.C.; Davis, W.P. An Assessment of Ichthyofaunal Assemblages within the Mangal of the Belize Offshore Cays. Bull. Mar. Sci. 2007, 80, 721–737. [Google Scholar]
- Lopez-Lopez, E.; Sedeno-Diaz, J.E.; Romero, F.L.; Trujillo-Jimenez, P. Spatial and Seasonal Distribution Patterns of Fish Assemblages in the Rio Champoton, Southeastern Mexico. Rev. Fish Biol. Fish 2008, 19, 127–142. [Google Scholar] [CrossRef]
- Esselman, P.; Allan, J. Relative Influences of Catchment-and Reach-Scale Abiotic Factors on Freshwater Fish Communities in Rivers of Northeastern Mesoamerica. Ecol. Freshw. Fish 2010, 19, 439–454. [Google Scholar] [CrossRef]
- Vega-Cendejas, M.E.; Santillana, M.H.d.; Norris, S. Habitat Characteristics and Environmental Parameters Influencing Fish Assemblages of Karstic Pools in Southern Mexico. Neotrop. Ichthyol. 2013, 11, 859–870. [Google Scholar] [CrossRef]
- Jourdan, J.; Krause, S.T.; Lazar, V.M.; Zimmer, C.; Sommer-Trembo, C.; Arias-Rodriguez, L.; Klaus, S.; Riesch, R.; Plath, M. Shared and Unique Patterns of Phenotypic Diversification Along a Stream Gradient in Two Congeneric Species. Sci. Rep. 2016, 6, 38971. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, J.R., Jr.; Lorenz, J.J.; Turingan, R.G. Environmental Correlates of the Abundance and Distribution of Belonesox belizanus in a Novel Environment. Environ. Biol. Fishes 2011, 92, 125–139. [Google Scholar] [CrossRef]
- Rahel, F.J.; Olden, J.D. Assessing the Effects of Climate Change on Aquatic Invasive Species. Conserv. Biol. 2008, 22, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.R.; Estevez, E.D. The Ecology of Tampa Bay, Florida: An Estuarine Profile; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1988.
- Raabe, E.A.; Roy, L.C.; McIvor, C.C. Tampa Bay Coastal Wetlands: Nineteenth to Twentieth Century Tidal Marsh-to-Mangrove Conversion. Estuar. Coasts 2012, 35, 1145–1162. [Google Scholar] [CrossRef]
- Sherwood, E.T.; Greening, H.S. Potential Impacts and Management Implications of Climate Change on Tampa Bay Estuary Critical Coastal Habitats. Environ. Manag. 2014, 53, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.R., III; Robison, D. Setting Priorities for Tampa Bay Habitat Protection and Restoration: Restoring the Balance; Tampa Bay National Estuary Program: St. Petersburg, FL, USA, 1996. [Google Scholar]
- Greenwood, M.F.D.; Matheson, R.E., Jr.; McMichael, R.H., Jr.; MacDonald, T.C. Community Structure of Shoreline Nekton in the Estuarine Portion of the Alafia River, Florida: Differences Along a Salinity Gradient and Inflow-Related Changes. Estuar. Coast. Shelf Sci. 2007, 74, 223–238. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Tomasini, J.A.; Guilhaumon, F.; Romdhane, M.S.; Do Chi, T.; Mouillot, D. Ecological Correlates of Dispersal Success of Lessepsian Fishes. Mar. Ecol. Prog. Ser. 2008, 363, 273–286. [Google Scholar] [CrossRef]
- Tampa Bay Tidal Tributary Research Team. Tampa Bay Tidal Tributary Habitat Initiative Project: Final Report and Management Recommendations. Tampa Bay Estuary Program Technical Report #02-08; Report to the Pinellas County Environmental Fund; Sherwood, E.T., Ed.; Tampa Bay Estuary Program: St. Petersburg, FL USA, 2008; Available online: https://www.tbeptech.org/TBEP_TECH_PUBS/2008/TBEP_02_08_Tidal_Tributary_Habitat_Initiative_Integrated_Summary_Report.pdf (accessed on 13 February 2017).
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Rozas, L.P.; Minello, T.J. Estimating Densities of Small Fishes and Decapod Crustaceans in Shallow Estuarine Habitats: A Review of Sampling Design with Focus on Gear Selection. Estuaries 1997, 20, 199–213. [Google Scholar] [CrossRef]
- Cocheret de la Moriniere, E.; Nagelkerken, I.; van der Melj, H.; van derVelde, G. What Attracts Juvenile Coral Reef Fish to Mangroves: Habitat Complexity or Shade? Mar. Biol. 2004, 144, 139–145. [Google Scholar] [CrossRef]
- Ellis, W.L.; Bell, S.S. Conditional Use of Mangrove Habitats by Fishes: Depth as a Cue to Avoid Predators. Estuaries 2004, 27, 966–976. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.1-1. 2016. Available online: https://CRAN.R-project.org/package=dismo (accessed on 22 October 2016).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 22 October 2016).
- Broennimann, O.; Guisan, A. Predicting Current and Future Biological Invasions: Both Native and Invaded Ranges Matter. Biol. Lett. 2008, 4, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Allen, M.S.; Beard, T.D. Data Management and Statistical Techniques. In Fisheries Techniques, 3rd ed.; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 15–77. [Google Scholar]
- Friedman, J.H.; Meulman, J.J. Multiple Additive Regression Trees with Application in Epidemiology. Stat. Med. 2003, 22, 1365–1381. [Google Scholar] [CrossRef] [PubMed]
- Ridgeway, G. with Contributions from others. gbm: Generalized Boosted Regression Models. R Package Version 2.1.1. 2015. Available online: https://CRAN.R-project.org/package=gbm (accessed on 12 October 2016).
- Lawson, K.M.; Tuckett, Q.M.; Ritch, J.L.; Nico, L.G.; Fuller, P.L.; Matheson, R.E.; Gestring, K.; Hill, J.E. Distribution and Status of Five Non-native Fish Species in the Tampa Bay Drainage (USA), a Hot Spot for Fish Introductions. Bioinvasions Rec. (under review).
- MacDonald, T. Personal Communication, Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute: St. Petersburg, FL, USA, 2016.
- Rehage, J.S.; Sih, A. Dispersal Behavior, Boldness, and the Link to Invasiveness: A Comparison of Four Gambusia Species. Biol. Invasions 2004, 6, 379–391. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Bestgen, K.R. Life-History Strategies Predict Fish Invasions and Extirpations in the Colorado River Basin. Ecol. Monogr. 2006, 76, 25–40. [Google Scholar] [CrossRef]
- Córdova-Tapia, F.; Zambrano, L. Fish Functional Groups in a Tropical Wetland of the Yucatan Peninsula, Mexico. Neotrop. Ichthyol. 2016, 14. [Google Scholar] [CrossRef]
- Armstrong, R.C. Dispersal Rates of Mosquitofish through Cattails in Drainage Ditches. Proc. Calif. Mos. Control Assoc. 1977, 45, 103–104. [Google Scholar]
- Alemadi, S.D.; Jenkins, D.G. Behavioral Constraints for the Spread of the Eastern Mosquitofish, Gambusia Holbrooki (Poeciliidae). Biol. Invasions 2008, 10, 59–66. [Google Scholar] [CrossRef]
- Deacon, A.E.; Ramnarine, I.W.; Magurran, A.E. How Reproductive Ecology Contributes to the Spread of a Globally Invasive Fish. PLoS ONE 2011, 6, e24416. [Google Scholar] [CrossRef] [PubMed]
- Bronnenhuber, J.E.; Dufour, B.A.; Higgs, D.M.; Heath, D.D. Dispersal Strategies, Secondary Range Expansion and Invasion Genetics of the Nonindigenous Round Goby, Neogobius melanostomus, in Great Lakes Tributaries. Mol. Ecol. 2011, 20, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Kornis, M.; Mercado-Silva, N.; Vander Zanden, M. Twenty Years of Invasion: A Review of Round Goby Neogobius melanostomus Biology, Spread and Ecological Implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.; Cerwenka, A.F.; Schliewen, U.K.; Geist, J. Bigger Is Better: Characteristics of Round Gobies Forming an Invasion Front in the Danube River. PLoS ONE 2013, 8, e73036. [Google Scholar] [CrossRef] [PubMed]
- Ligtvoet, W.; Mkumbo, O.C. Synopsis of ecological and fishery research on Nile perch (Lates niloticus) in Lake Victoria, conducted by HEST/TAFIRI. In Proceedings of the CIFA. Report of the 5th session of the Sub-Committee for the Development and Management of the Fisheries in Lake Victoria, Mwanza, Tanzania, 12–24 September 1989; FAO Fisheries Report 430. Food and Agriculture Organization of the United Nations: Rome, Italy, 1990; pp. 35–74. [Google Scholar]
- Peters, L.M.; Pegg, M.A.; Reinhardt, U.G. Movements of Adult Radio-Tagged Bighead Carp in the Illinois River. Trans. Am. Fish Soc. 2006, 135, 1205–1212. [Google Scholar] [CrossRef]
- Lapointe, N.W.R.; Odenkirk, J.S.; Angermeier, P.L. Seasonal Movement, Dispersal, and Home Range of Northern Snakehead Channa argus (Actinopterygii, Perciformes) in the Potomac River Catchment. Hydrobiologia 2013, 709, 73–87. [Google Scholar] [CrossRef]
- Nico, L.G.; Beamish, W.H.; Musikasinthorn, P. Discovery of the Invasive Mayan Cichlid Fish “Cichlasoma” urophthalmus (Günther 1862) in Thailand, with Comments on Other Introductions and Potential Impacts. Aquat. Invasions 2007, 2, 197–214. [Google Scholar] [CrossRef]
- Harrison, E.; Lorenz, J.J.; Trexler, J.C. Per Capita Effects of Non-Native Mayan Cichlids (Cichlasoma urophthalmus; Gunther) on Native Fish in the Estuarine Southern Everglades. Copeia 2013, 2013, 80–96. [Google Scholar] [CrossRef]
- Paperno, R.; Ruiz-Carus, R.; Krebs, J.M.; McIvor, C.C. Expansion of the Mayan Cichlid, Cichlasoma urophthalmus (Pisces, Cichlidae), above the 28° N Latitude in Florida. Fla. Sci. 2008, 71, 293–304. [Google Scholar]
- Bisazza, A.; Novarini, N.; Pilastro, A. Male Body Size and Male-Male Competition: Interspecific Variation in Poeciliid Fishes. Ital. J. Zool. 1996, 63, 365–369. [Google Scholar] [CrossRef]
- Padilla, D.K.; Williams, S.L. Beyond Ballast Water: Aquarium and Ornamental Trades as Sources of Invasive Species in Aquatic Ecosystems. Front. Ecol. Environ. 2004, 2, 131–138. [Google Scholar] [CrossRef]
- Rahel, F.J. Biogeographic Barriers, Connectivity and Homogenization of Freshwater Faunas: It’s a Small World after All. Freshw. Biol. 2007, 52, 696–710. [Google Scholar] [CrossRef]
- Frederick, P.C.; Spalding, M.G.; Sepulveda, M.S.; Williams, G.E.; Nico, L.; Robins, R. Exposure of Great Egret (Ardea albus) Nestlings to Mercury through Diet in the Everglades Ecosystem. Environ. Toxicol. Chem. 1999, 18, 1940–1947. [Google Scholar] [CrossRef]
- Gilliam, J.F.; Fraser, D.F. Movement in Corridors: Enhancement by Predation Threat, Disturbance, and Habitat Structure. Ecology 2001, 82, 258–273. [Google Scholar] [CrossRef]
- Bond, N.R.; Lake, P.S. Local Habitat Restoration in Streams: Constraints on the Effectiveness of Restoration for Stream Biota. Ecol. Manag. Restor. 2003, 4, 193–198. [Google Scholar] [CrossRef]
- McIvor, C.C.; Odum, W.E. Food, Predation Risk, and Microhabitat Selection in a Marsh Fish Assemblage. Ecology 1988, 69, 1341–1351. [Google Scholar] [CrossRef]
- Idelberger, C.F.; Stafford, C.J.; Erickson, S.E. Distribution and Abundance of Introduced Fishes in Florida’s Charlotte Harbor Estuary. Gulf. Caribb. Res. 2011, 23, 13–22. [Google Scholar] [CrossRef]
- Idelberger, C.F.; Stevens, P.W.; Weather, E. Fishes of the Lemon Bay Estuary and a Comparison of Fish Community Structure to Nearby Estuaries Along Florida’s Gulf Coast. Fla. Sci. 2016, 79, 159–177. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming Limitations of Modelling Rare Species by Using Ensembles of Small Models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Hegel, T.M.; Cushman, S.A.; Evans, J.; Huettmann, F. Current State of the Art for Statistical Modelling of Species Distributions. In Spatial Complexity, Informatics, and Wildlife Conservation; Cushman, S.A., Huettmann, F., Eds.; Springer: New York, NY, USA, 2010; pp. 273–311. [Google Scholar]
- Trexler, J.C.; Loftus, W.F.; Jordan, F.; Lorenz, J.J.; Chick, J.H.; Kobza, R.M. Empirical Assessment of Fish Introductions in a Subtropical Wetland: An Evaluation of Contrasting Views. Biol. Invasions 2000, 2, 265–277. [Google Scholar] [CrossRef]
- Nordlie, F.G. Physicochemical Environments and Tolerances of Cyprinodontoid Fishes Found in Estuaries and Salt Marshes of Eastern North America. Rev. Fish Biol. Fish 2006, 16, 51–106. [Google Scholar] [CrossRef]
- Stevens, P.W.; Blewett, D.A.; Casey, J.P. Short-Term Effects of a Low Dissolved Oxygen Event on Estuarine Fish Assemblages Following the Passage of Hurricane Charley. Estuar. Coasts 2006, 29, 997–1003. [Google Scholar] [CrossRef]
- Domenici, P.; Lefrancois, C.; Shingles, A. Hypoxia and the Antipredator Behaviours of Fishes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 2105–2121. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.J.; Loftus, W.F.; Brown, M.E. Hypoxia Tolerance of Two Centrarchid Sunfishes and an Introduced Cichlid from Karstic Everglades Wetlands of Southern Florida, U.S.A. J. Fish Biol. 2007, 71, 87–99. [Google Scholar] [CrossRef]
- Iacarella, J.C.; Ricciardi, A. Dissolved Ions Mediate Body Mass Gain and Predatory Response of an Invasive Fish. Biol. Invasions 2015, 17, 3237–3246. [Google Scholar] [CrossRef]
- Duarte, C.M.; Hendriks, I.E.; Moore, T.S.; Olsen, Y.S.; Steckbauer, A.; Ramajo, L.; Carstensen, J.; Trotter, J.A.; McCulloch, M. Is Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater Ph. Estuar. Coasts 2013, 36, 221–236. [Google Scholar] [CrossRef]
- Shafland, P.L.; Pestrak, J.M. Lower Lethal Temperatures for Fourteen Non-Native Fishes in Florida. Environ. Biol. Fishes 1982, 7, 149–156. [Google Scholar] [CrossRef]
- Allanson, B.R.; Bok, A.; van Wyk, N.I. The Influence of Exposure to Low Temperature on Tilapia Mossambica Peters (Cichlidae). II. Changes in Serum Osmolarity, Sodium and Chlorida Ion Concentrations. J. Fish Biol. 1971, 3, 181–185. [Google Scholar] [CrossRef]
- Stauffer, J.R.J.; Boltz, S.E. Effect of Salinity on the Temperature Preference and Tolerance of Age-0 Mayan Cichlids. Trans. Am. Fish Soc. 1993, 123, 101–107. [Google Scholar] [CrossRef]
- Peterson, M.S.; Ross, S.T. Dynamics of Littoral Fishes and Decapods Along a Coastal River-Estuarine Gradient. Estuar. Coast. Shelf Sci. 1991, 33, 467–483. [Google Scholar] [CrossRef]
- Gilmore, R.G., Jr.; Bullock, L.H.; Berry, F.H. Hypothermal Mortality in Marine Fishes of South-Central Florida, January, 1977. Northeast Gulf. Sci. 1978, 2, 77–97. [Google Scholar]
- Kerfoot, J. Thermal Tolerance of the Invasive Belonesox Belizanus, Pike Killifish, Throughout Ontogeny. J. Exp. Zool. A Ecol. Genet. Physiol. 2012, 317, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Boucek, R.E.; Rehage, J.S. Examining the Effectiveness of Consumer Diet Sampling as a Nonnative Detection Tool in a Subtropical Estuary. Trans. Am. Fish Soc. 2014, 143, 489–494. [Google Scholar] [CrossRef]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the Long-Term Effects of Species Invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Everman, E.; Klawinski, P. Human-Facilitated Jump Dispersal of a Non-Native Frog Species on Hawai’i Island. J. Biogeogr. 2013, 40, 1961–1970. [Google Scholar] [CrossRef]
- Gertzen, E.; Familiar, O.; Leung, B. Quantifying Invasion Pathways: Fish Introductions from the Aquarium Trade. Can. J. Fish Aquat. Sci. 2008, 65, 1265–1273. [Google Scholar] [CrossRef]
- Stevens, P.W. Sampling Fish Communities in Saltmarsh Impoundments in the Northern Indian River Lagoon, Florida: Cast Net and Culvert Trap Gear Testing. Fla. Sci. 2006, 69, 135–147. [Google Scholar]
- Winner, B.L.; Blewett, D.A.; McMichael, R.H.; Guenther, C.B. Relative Abundance and Distribution of Common Snook Along Shoreline Habitats of Florida Estuaries. Trans. Am. Fish Soc. 2010, 139, 62–79. [Google Scholar] [CrossRef]
- Kerfoot, J.R., Jr.; Turingan, R.G. Similarity and Disparity in Prey-Capture Kinematics between the Invasive Pike Killifish (Belonesox Belizanus) and the Native Florida Largemouth Bass (Micropterus Floridanus). Fla. Sci. 2011, 74, 137. [Google Scholar]
- Henningsen, B.F.; Norton, N.T.; Young, B.E. The Rock Ponds Ecosystem Restoration Project—A True Mosaic of Coastal Habitats for Tampa Bay. Proceedingso of the Tampa Bay Area Scientific Information Symposium (BASIS 6), St. Petersburg, FL, USA, 28–30 September 2015; Burke, M., Ed.; Tampa Bay Estuary Program and Tampa Bay Regional Planning Council: St. Petersburg, FL, USA, 2015; p. 60. Available online: https://www.tbeptech.org/BASIS/BASIS6/BASIS6_Proceedings_FINAL.pdf#page=67 (accessed on 12 February 2017).
- Motta, P.J. Fisheries Evaluation of Restoration of Peanut Lake, Tampa Bay, Florida; Department of Biology, University of South Florida: Tampa, FL, USA, 1995. [Google Scholar]
- Kurz, R.C.; Fenwick, R.W.; Davis, K.A. A Comparison of Fish Communities in Restored and Natural Salt Marshes in Tampa Bay, Florida; Southwest Florida Water Management District: Tampa, FL, USA, 1998. Available online: http://images.library.wisc.edu/EcoNatRes/EFacs/Wetlands/Wetlands24/reference/econatres.wetlands24.rkurz.pdf (accessed on 13 February 2017).
- Bahk, B.M. Succession of Ichthyofauna in a Constructed Tidal Creek Salt Marsh in Cockroach Bay, Florida; Resource Management Department, Southwest Florida Water Management District: Brooksville, FL, USA, 1999.

| Waterbody Name | Waterbody Type | Year Sampled | Number of 9.1-m Seine Samples | Temperature (°C) | Salinity (Practical Salinity Scale) | Dissolved Oxygen (mg L−1) | pH | Depth (m) | Gradient (%) |
|---|---|---|---|---|---|---|---|---|---|
| Alafia River | River | 2006 | 167 | 22.9 (13.9–30.3) | 3.5 (0.1–20.1) | 7.3 (3.4–16.5) | 7.7 (6.8–8.8) | 0.5 (0.1–1.0) | 20.6 (0.0–66.7) |
| Andrews Creek | Creek | 2007 | 72 | 25.1 (17.3–32.3) | 22.4 (6.3–32.8) | 5.5 (2.9–9.9) | 7.8 (7.5–8.1) | 0.5 (0.2–1.0) | 21.4 (0.0–66.7) |
| Cockroach Creek | Creek | 2007 | 120 | 23.8 (16.1–29.7) | 19.3 (0.4–33.0) | 3.8 (0.3–12.2) | 7.3 (6.8–8.1) | 0.5 (0.2–1.2) | 21.1 (0.0–60.0) |
| Curiosity Creek | Creek | 2006 | 120 | 22.5 (11.9–30.0) | 3.3 (0.2–17.2) | 6.5 (2.7–10.6) | 7.3 (6.4–7.9) | 0.7 (0.1–1.2) | 26.8 (0.0–66.7) |
| Dogleg Creek | Canal | 2006 | 48 | 22.6 (15.1–28.3) | 1.3 (0.2–4.0) | 6.9 (4.5–9.8) | 7.7 (7.3–8.3) | 0.7 (0.3–1.0) | 29.0 (6.7–53.3) |
| Hayes Bayou | Bayou | 2006 | 48 | 23.7 (12.8–30.5) | 11.6 (0.6–23.6) | 7.0 (3.6–10.3) | 7.6 (7.0–8.3) | 0.4 (0.1–1.0) | 14.3 (0.0–40.0) |
| Little Manatee River | River | 2006 | 96 | 23.8 (11.8–31.4) | 8.5 (0.1–27.0) | 6.7 (3.6–12.9) | 7.4 (6.3–7.9) | 0.5 (0.1–1.0) | 18.3 (0.0–53.3) |
| Question Mark Creek | Canal | 2006 | 48 | 23.4 (13.4–30.1) | 8.0 (1.0–16.4) | 6.5 (2.6–13.7) | 7.6 (6.9–8.4) | 0.5 (0.2–1.0) | 23.1 (0.0–46.7) |
| Rice Creek | Creek | 2006 | 48 | 21.7 (15.4–29.2) | 1.3 (0.1–8.1) | 8.1 (2.0–13.5) | 7.6 (7.1–8.8) | 0.5 (0.1–1.0) | 12.6 (0.0–66.7) |
| Riverview West Creek | Canal | 2006 | 48 | 23.3 (15.1–30.6) | 2.5 (0.2–8.9) | 7.8 (2.7–16.6) | 7.7 (6.9–8.7) | 0.5 (0.1–1.0) | 15.7 (0.0–40.0) |
| Wildcat Creek | Creek | 2006 | 120 | 22.7 (11.6–28.9) | 7.3 (0.2–23.4) | 6.5 (2.7–15.2) | 7.4 (7.0–8.1) | 0.5 (0.1–1.2) | 21.0 (0.0–66.7) |
| Overall | 935 | 23.2 (11.6–32.3) | 8.3 (0.1–33.0) | 6.4 (0.3–16.6) | 7.5 (6.3–8.8) | 0.5 (0.1–1.2) | 20.9 (0.0–66.7) |
| Waterbody Name | Shoreline Type | Substrate Type | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mangrove | Marsh | Shrubs/Trees | Small Terrestrial Veg. | Hardened | Mud | Mud-Sand | Sand | Oyster-Rock | |
| Alafia River | 0.0% | 14.4% | 21.6% | 13.8% | 49.1% | 7.2% | 26.3% | 49.7% | 16.8% |
| Andrews Creek | 94.4% | 0.0% | 1.4% | 0.0% | 2.8% | 72.2% | 18.1% | 1.4% | 6.9% |
| Cockroach Creek | 99.2% | 0.0% | 0.0% | 0.0% | 0.0% | 69.2% | 20.8% | 1.7% | 8.3% |
| Curiosity Creek | 0.0% | 50.8% | 27.5% | 20.0% | 0.0% | 19.2% | 35.8% | 45.0% | 0.0% |
| Dogleg Creek | 0.0% | 0.0% | 39.6% | 29.2% | 4.2% | 33.3% | 41.7% | 20.8% | 4.2% |
| Hayes Bayou | 0.0% | 100.0% | 0.0% | 0.0% | 0.0% | 33.3% | 25.0% | 41.7% | 0.0% |
| Little Manatee River | 21.9% | 56.3% | 3.1% | 2.1% | 12.5% | 3.1% | 22.9% | 55.2% | 18.8% |
| Question Mark Creek | 2.1% | 10.4% | 66.7% | 4.2% | 14.6% | 22.9% | 41.7% | 33.3% | 2.1% |
| Rice Creek | 0.0% | 8.3% | 18.8% | 33.3% | 27.1% | 10.4% | 31.3% | 54.2% | 4.2% |
| Riverview West Creek | 0.0% | 2.1% | 0.0% | 25.0% | 72.9% | 68.8% | 18.8% | 6.3% | 6.3% |
| Wildcat Creek | 4.2% | 86.7% | 4.2% | 2.5% | 0.0% | 50.8% | 40.0% | 9.2% | 0.0% |
| Waterbody Name | Number of 9.1-m Seine Samples | Number of Fish | % Presence |
|---|---|---|---|
| Alafia River | 167 | 2 | 1.2% |
| Andrews Creek | 72 | 14 | 12.5% |
| Cockroach Creek | 120 | 26 | 11.7% |
| Curiosity Creek | 120 | 10 | 5.0% |
| Dogleg Creek | 48 | 2 | 4.2% |
| Hayes Bayou | 48 | 3 | 6.3% |
| Little Manatee River | 96 | 6 | 5.2% |
| Question Mark Creek | 48 | 8 | 12.5% |
| Rice Creek | 48 | 0 | 0.0% |
| Riverview West Creek | 48 | 4 | 6.3% |
| Wildcat Creek | 120 | 22 | 14.2% |
| Overall | 935 | 97 | 7.2% |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greenwood, M.F.D. Distribution, Spread, and Habitat Predictability of a Small, Invasive, Piscivorous Fish in an Important Estuarine Fish Nursery. Fishes 2017, 2, 6. https://doi.org/10.3390/fishes2020006
Greenwood MFD. Distribution, Spread, and Habitat Predictability of a Small, Invasive, Piscivorous Fish in an Important Estuarine Fish Nursery. Fishes. 2017; 2(2):6. https://doi.org/10.3390/fishes2020006
Chicago/Turabian StyleGreenwood, M. F. D. 2017. "Distribution, Spread, and Habitat Predictability of a Small, Invasive, Piscivorous Fish in an Important Estuarine Fish Nursery" Fishes 2, no. 2: 6. https://doi.org/10.3390/fishes2020006
APA StyleGreenwood, M. F. D. (2017). Distribution, Spread, and Habitat Predictability of a Small, Invasive, Piscivorous Fish in an Important Estuarine Fish Nursery. Fishes, 2(2), 6. https://doi.org/10.3390/fishes2020006




