Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Development
2.1.1. Egg White Isolation and Heat Treatment
2.1.2. Alginate Preparation
2.1.3. Egg White-Alginate Hydrogel Preparation
2.1.4. Matrigel® Preparation
2.2. Physical Property Tests
2.2.1. Determining Optimal Alginate Percentage
2.2.2. Determining Degradation Rate
2.3. Biological Tests
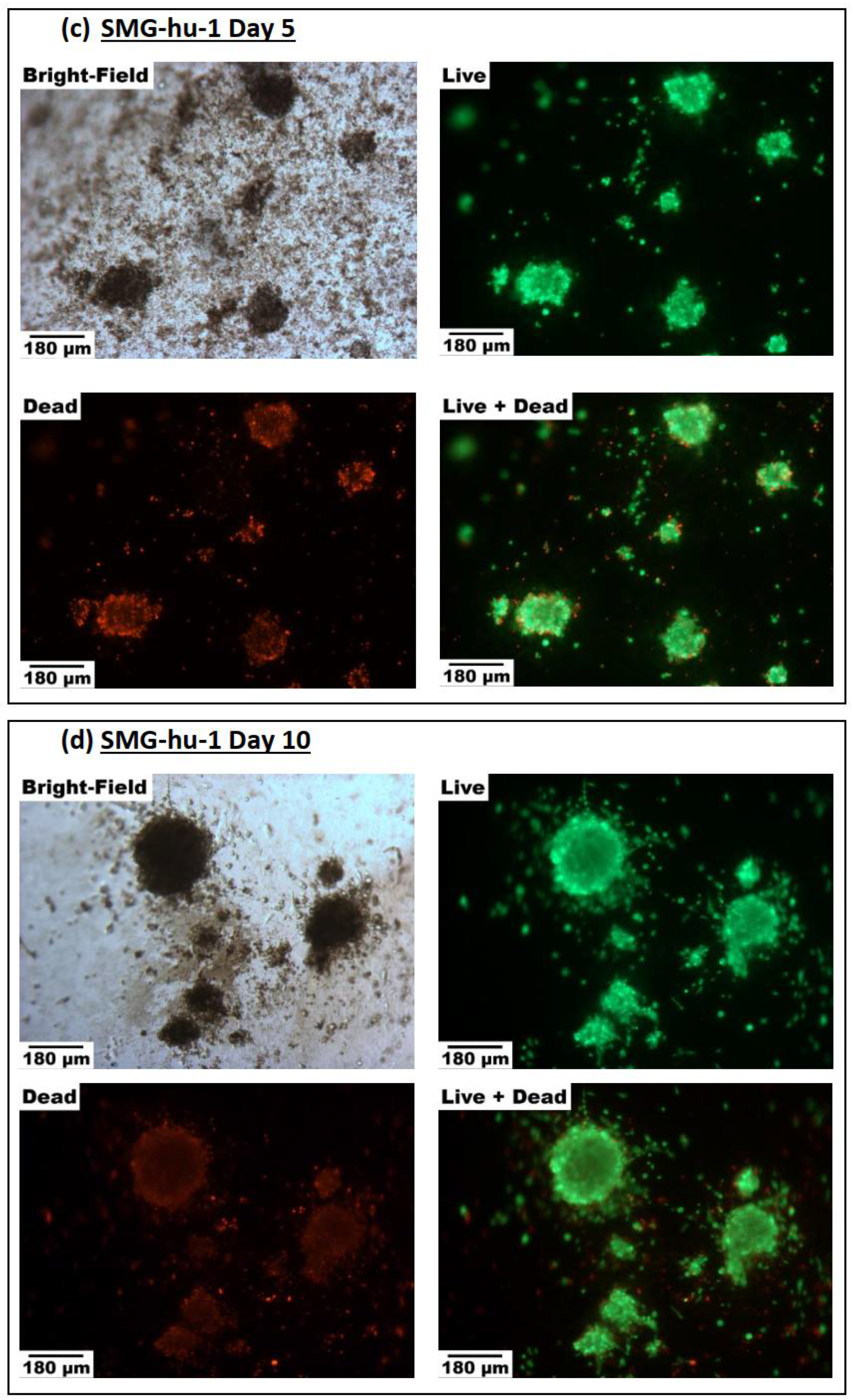
2.3.1. Cell Viability
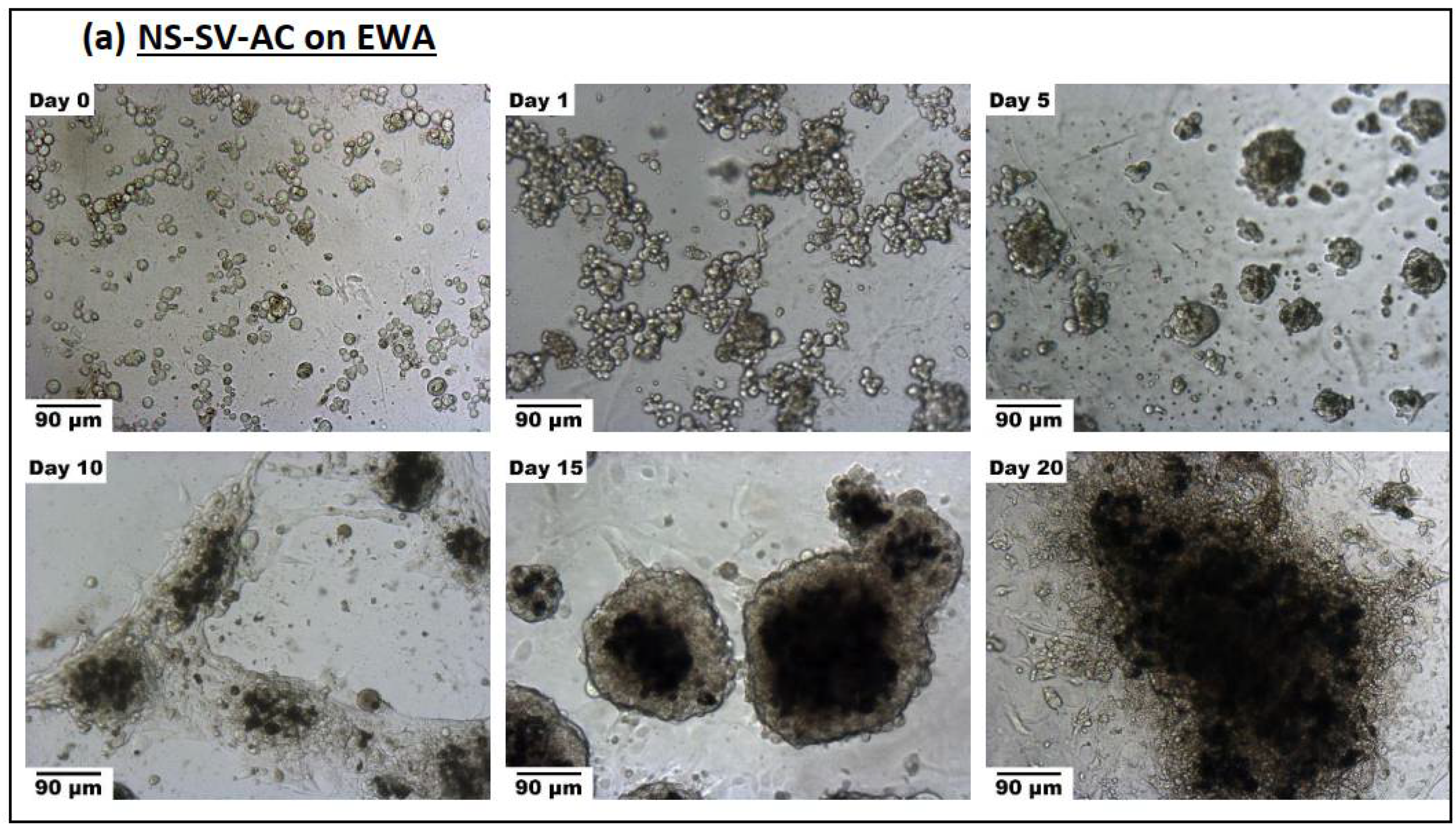
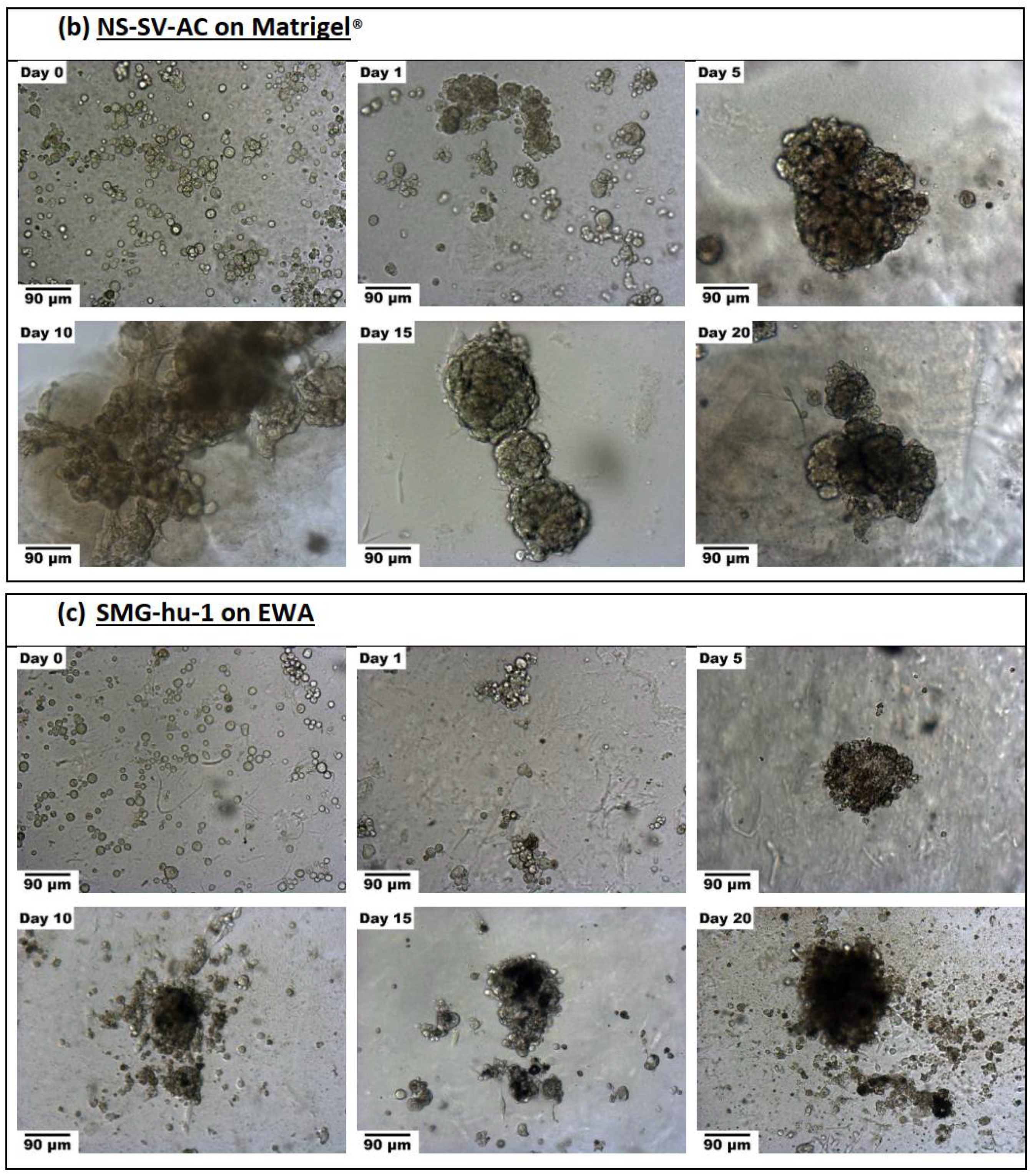
2.3.2. Spheroid Formation
2.4. Statistical Analysis
3. Results
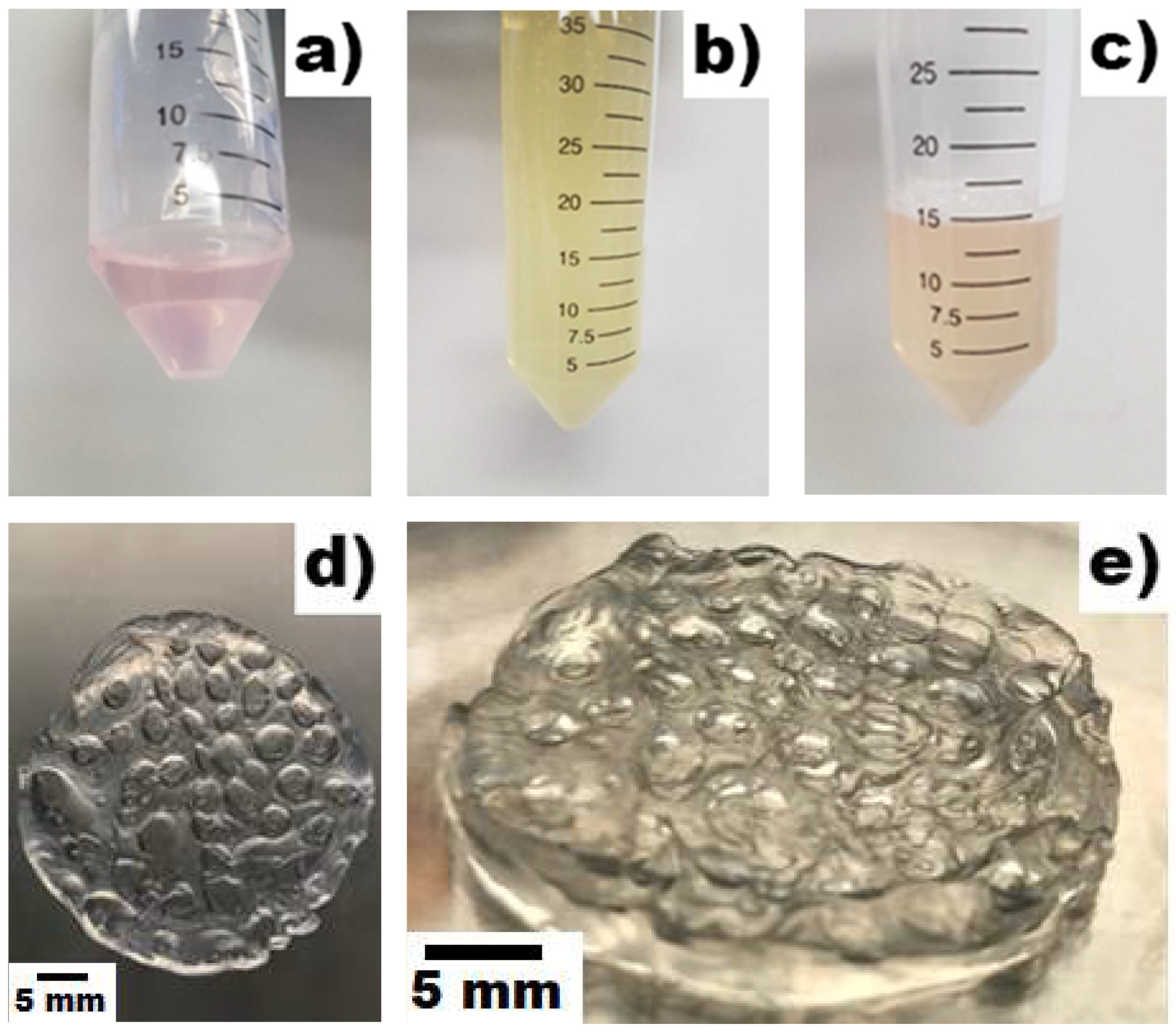
3.1. Scaffold Development
3.2. Physical Tests
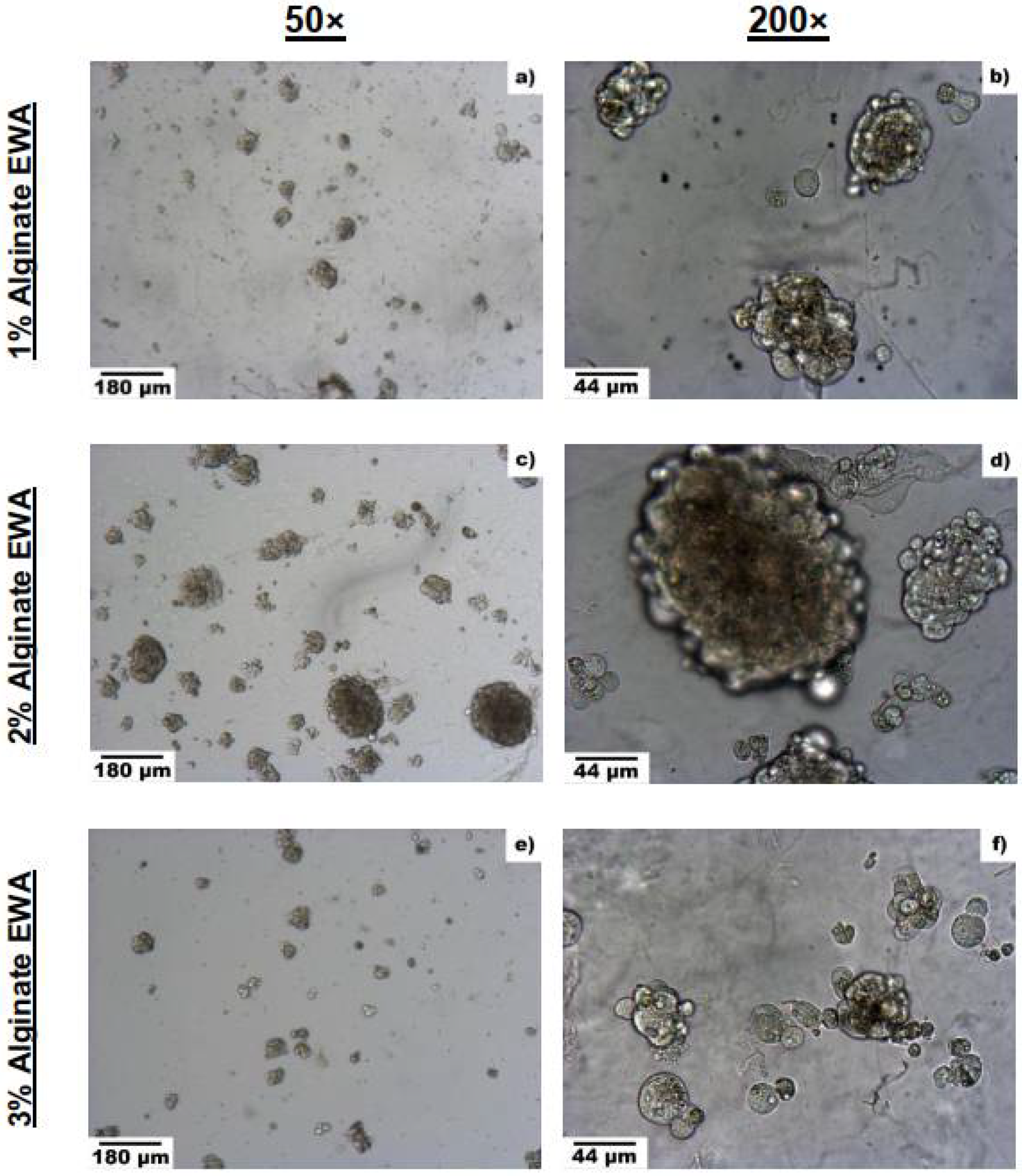
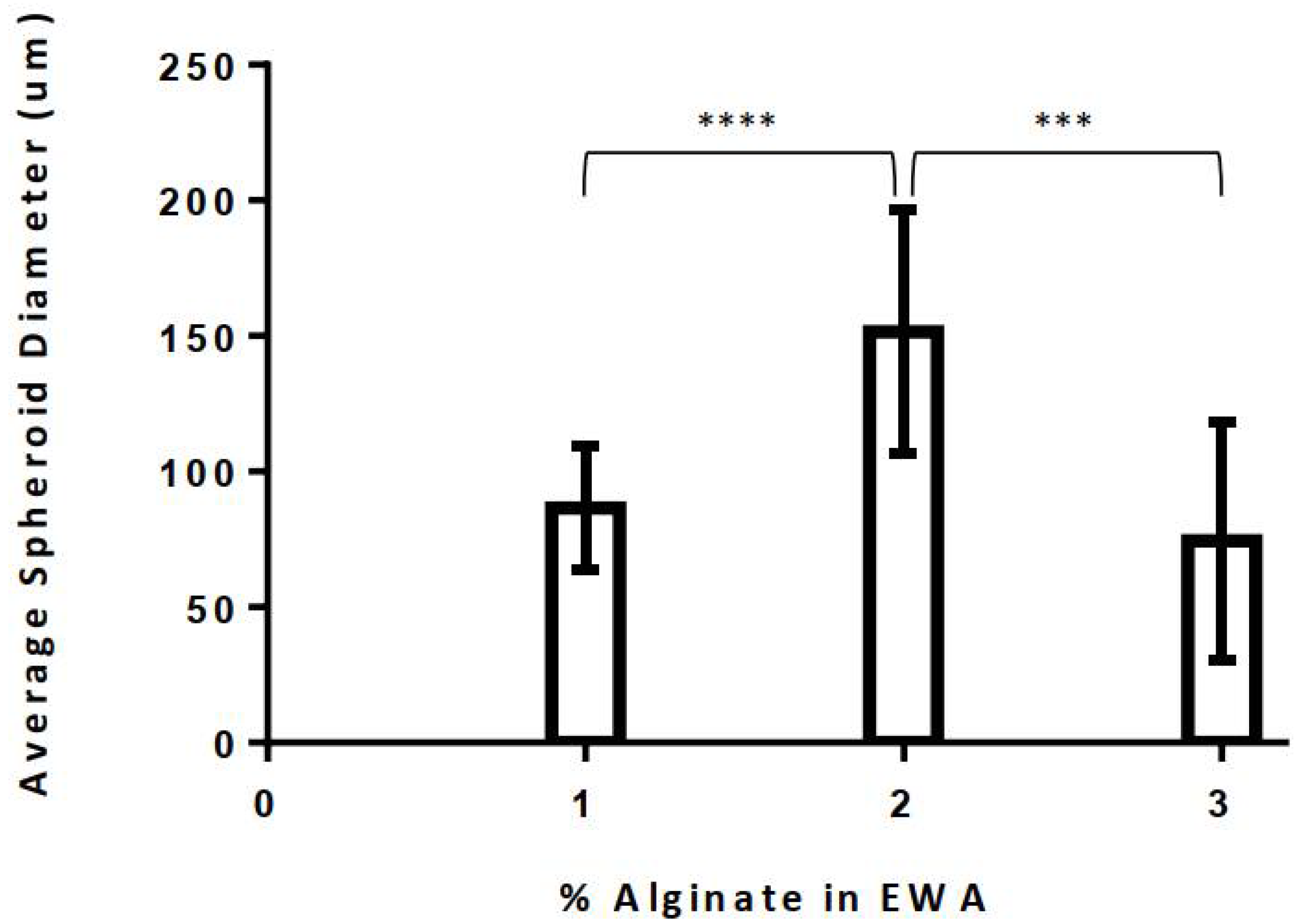
3.2.1. Optimal Alginate Percentage
3.2.2. Degradation Rate
3.3. Biological Tests
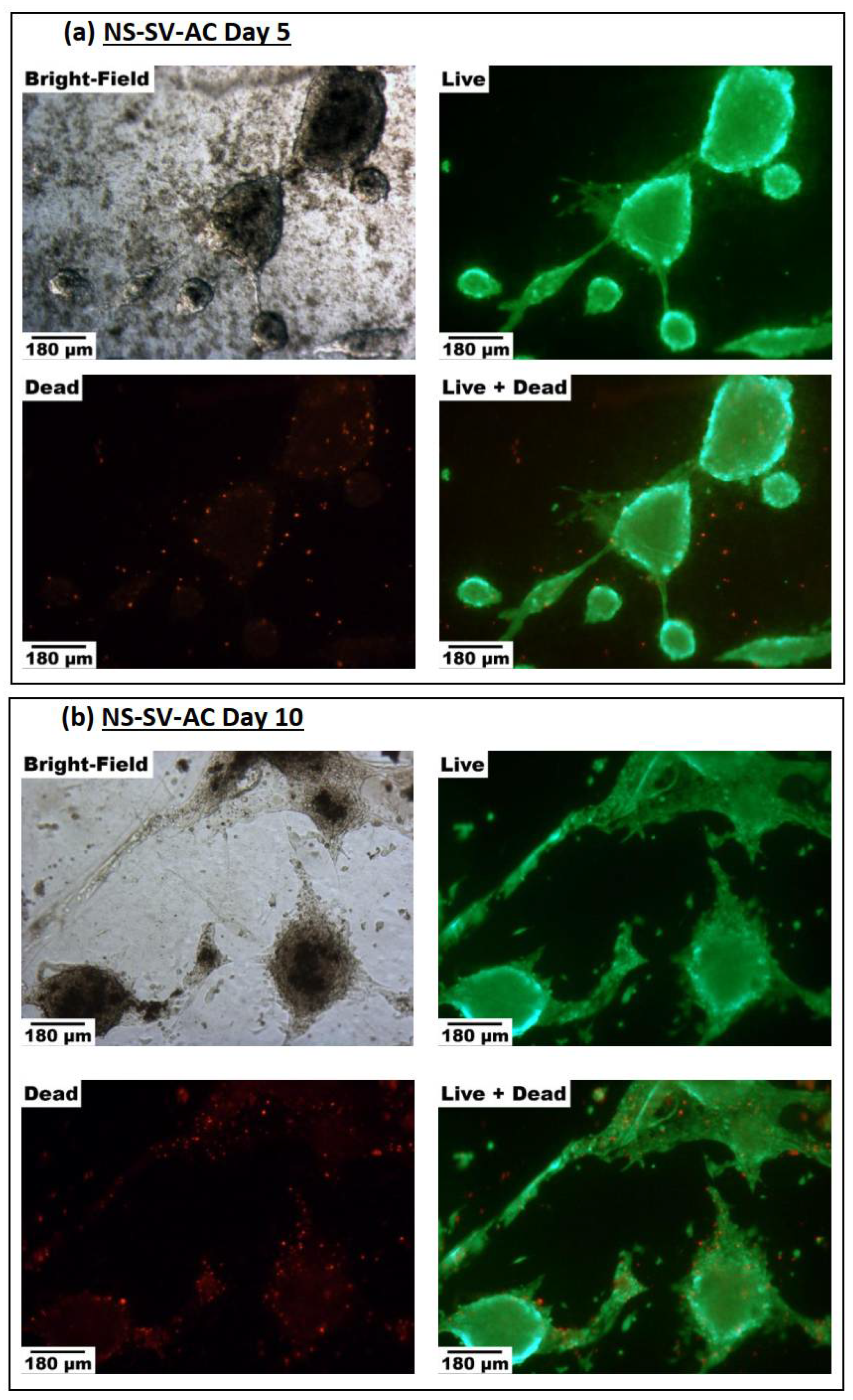
3.3.1. Cell Viability
3.3.2. Spheroid Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beale, T.; Madani, G. Anatomy of the salivary glands. In Seminars in Ultrasound, CT and MRI; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Eliasson, L.; Carlén, A. An update on minor salivary gland secretions. Eur. J. Oral Sci. 2010, 118, 435–442. [Google Scholar] [CrossRef]
- Humphrey, P.S.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Ra, J.C.; Shin, I.S.; Jang, Y.H.; An, H.-Y.; Choi, J.-S.; Kim, W.C.; Kim, Y.-M. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS ONE 2013, 8, e71167. [Google Scholar] [CrossRef] [Green Version]
- Bayetto, K.; Logan, R.M. Sjögren’s syndrome: A review of aetiology, pathogenesis, diagnosis and management. Aust. Dent. J. 2010, 55, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2009, 2, 303–307. [Google Scholar]
- Westhoff, G.; Zink, A. Epidemiologie des primären Sjögren-Syndroms. Z. Rheumatol. 2010, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Cornec, D.; Jamin, C.; Pers, J.O. Sjogren’s syndrome: Where do we stand, and where shall we go? J. Autoimmun. 2014, 51, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Joraku, A.; Sullivan, C.A.; Yoo, J.J.; Atala, A. Tissue Engineering of Functional Salivary Gland Tissue. Laryngoscope 2005, 115, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagami, H.; Wang, S.; Hai, B. Restoring the function of salivary glands. Oral Dis. 2008, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, J.; Brunner, D.; de Smet, K.; Svenningsen, A.F.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of chemically defined cell culture media—Replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Nogawa, H.; Mizuno, T. Mesenchymal control over elongating and branching morphogenesis in salivary gland development. J. Embryol. Exp. Morphol. 1981, 66, 209–221. [Google Scholar] [CrossRef]
- Patel, V.N.; Rebustini, I.T.; Hoffman, M.P. Salivary gland branching morphogenesis. Differentiation 2006, 74, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- Yang, Z.; Xiong, H.-R. Culture conditions and types of growth media for mammalian cells. In Biomedical Tissue Culture; IntechOpen: London, UK, 2012. [Google Scholar]
- Arora, M. Cell culture media: A review. Mater. Methods 2013, 3, 175. [Google Scholar] [CrossRef]
- Piedimonte, G.; Borghetti, A.F.; Guidotti, G.G. Effect of Cell Density on Growth Rate and Amino Acid Transport in Simian Virus 40-transformed 3T3 Cells. Cancer Res. 1982, 42, 4690. [Google Scholar]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Biotechnology 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Maria, O.M.; Zeitouni, A.; Gologan, O.; Tran, S.D. Matrigel Improves Functional Properties of Primary Human Salivary Gland Cells. Tissue Eng. Part A 2011, 17, 1229–1238. [Google Scholar] [CrossRef]
- Ozdemir, T.; Fowler, E.W.; Hao, Y.; Ravikrishnan, A.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Biomaterials-Based strategies for salivary gland tissue regeneration. Biomater. Sci. 2016, 4, 592–604. [Google Scholar] [CrossRef]
- Kaipparettu, B.A.; Kuiatse, I.; Chan, B.T.-Y.; Kaipparettu, M.B.; Lee, A.V.; Oesterreich, S. Novel egg white–based 3-D cell culture system. BioTechniques 2008, 45, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousseau, Y.; Mollard, S.; Qiu, H.; Richard, L.; Cazal, R.; Nizou, A.; Vedrenne, N.; Rémi, S.; Baaj, Y.; Fourcade, L.; et al. In vitro 3D angiogenesis assay in egg white matrix: Comparison to Matrigel, compatibility to various species, and suitability for drug testing. Lab. Investig. 2014, 94, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Vetsch, J.R.; Paul, G.R.; Rubert, M.; Müller, R. Alginate dependent changes of physical properties in 3D bioprinted cell-laden porous scaffolds affect cell viability and cell morphology. Biomed. Mater. 2019, 14, 065009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pham, H.M.; Munguia-Lopez, J.G.; Kinsella, J.M.; Tran, S.D. The Optimization of a Novel Hydrogel—Egg White-Alginate for 2.5 D Tissue Engineering of Salivary Spheroid-like Structure. Molecules 2020, 25, 5751. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, H.M.; Zhang, Y.; Munguia-Lopez, J.G.; Tran, S.D. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics 2022, 7, 5. https://doi.org/10.3390/biomimetics7010005
Pham HM, Zhang Y, Munguia-Lopez JG, Tran SD. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics. 2022; 7(1):5. https://doi.org/10.3390/biomimetics7010005
Chicago/Turabian StylePham, Hieu M., Yuli Zhang, Jose G. Munguia-Lopez, and Simon D. Tran. 2022. "Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing" Biomimetics 7, no. 1: 5. https://doi.org/10.3390/biomimetics7010005







