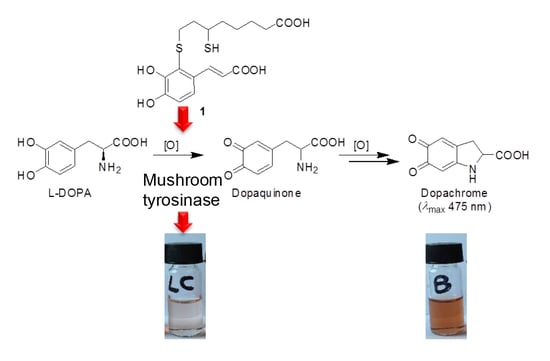
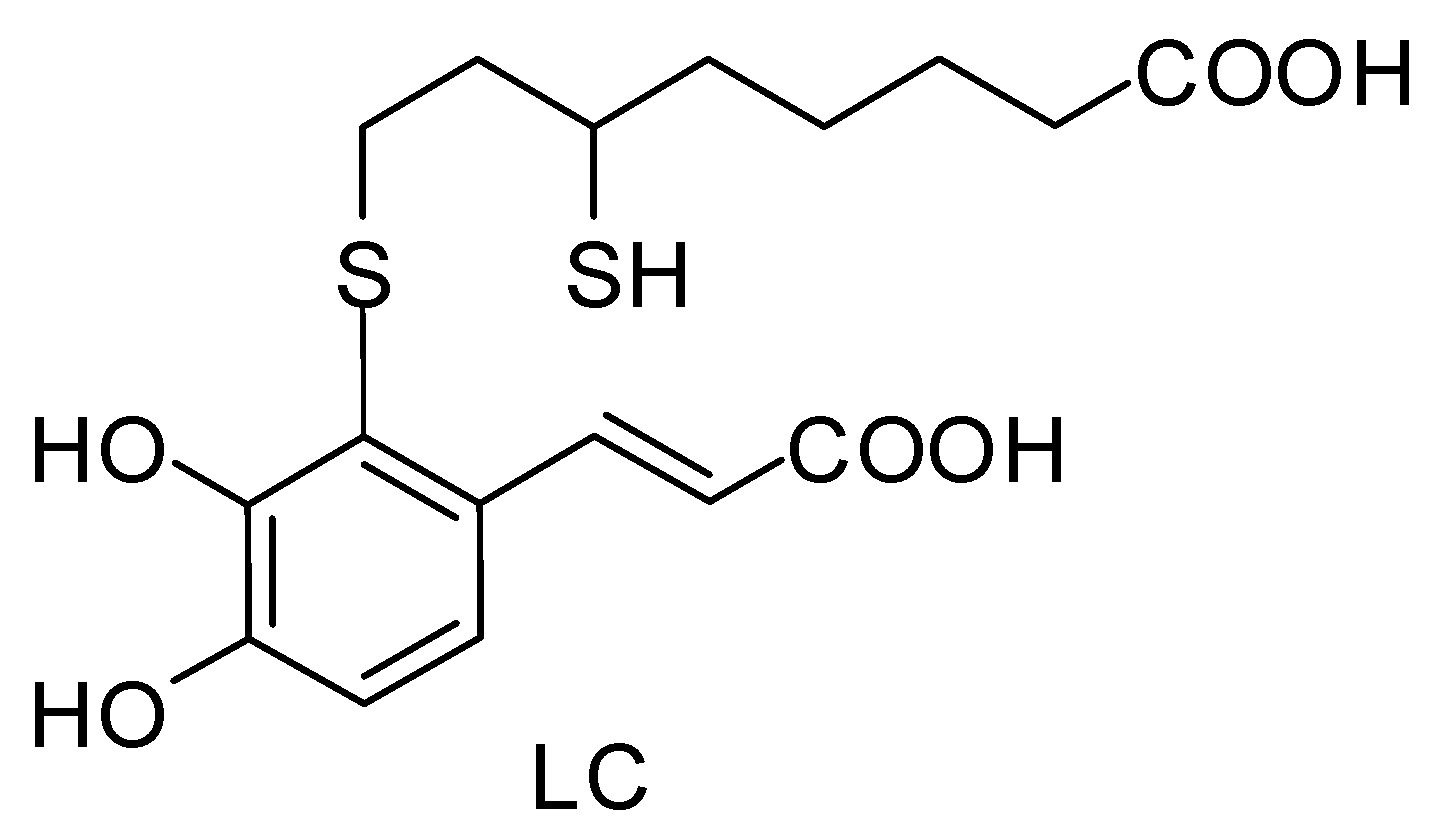
2-S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of 2-S-Lipoylcaffeic Acid
2.4. Mushroom Tyrosinase Inhibition Assay
2.5. Investigation of the Mechanism of Inhibition of Mushroom Tyrosinase Activity
2.6. Cell Viability Assay
3. Results and Discussion
3.1. Preparation of 2-S-Lipoylcaffeic Acid
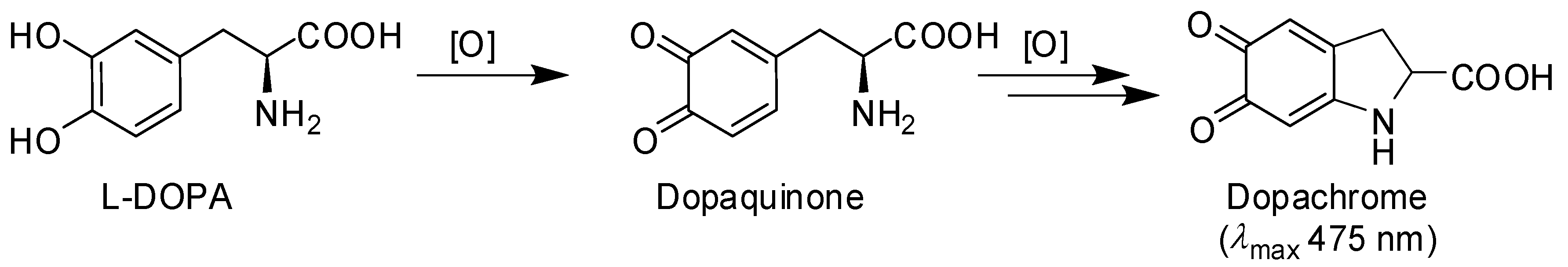
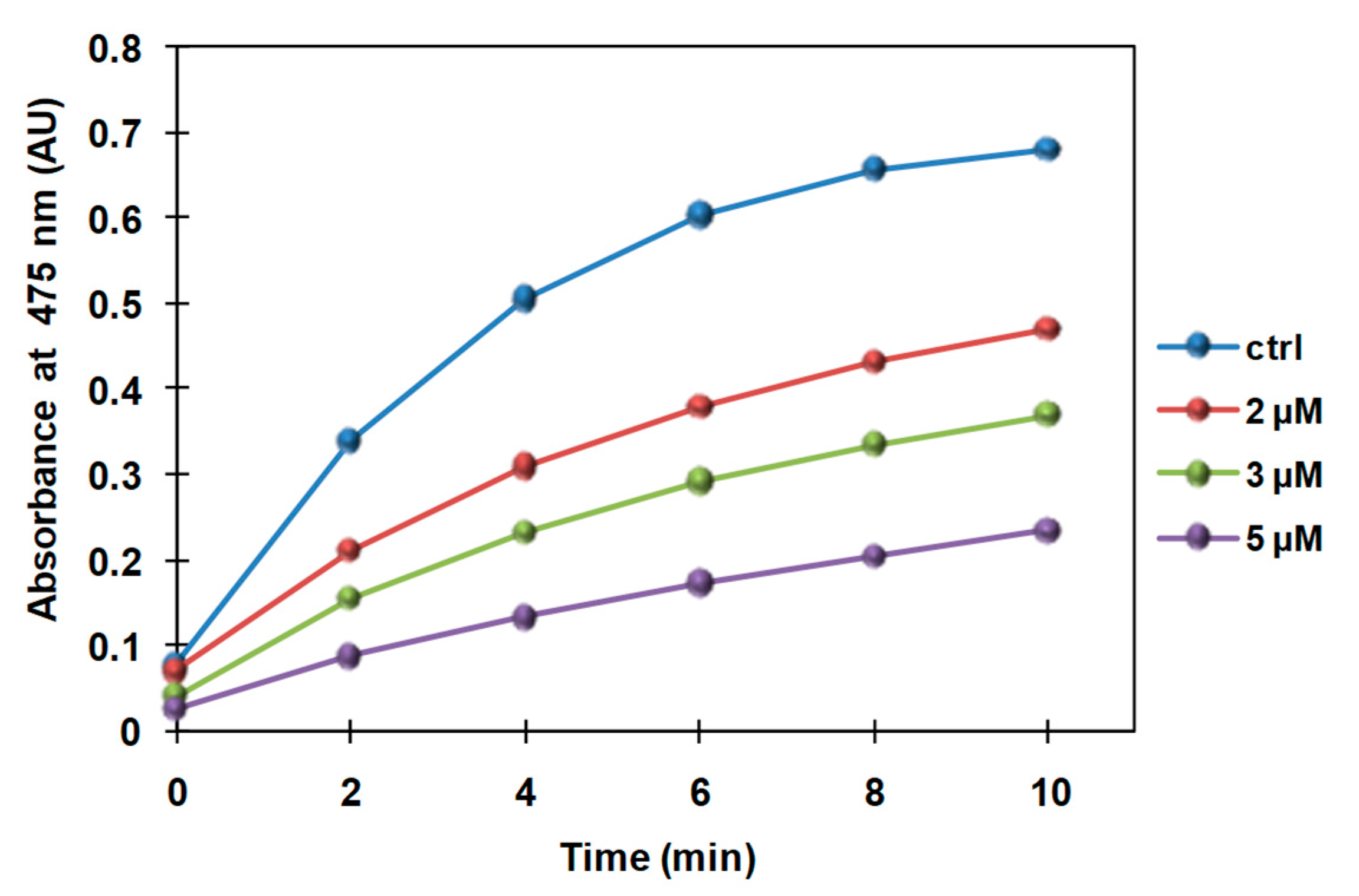
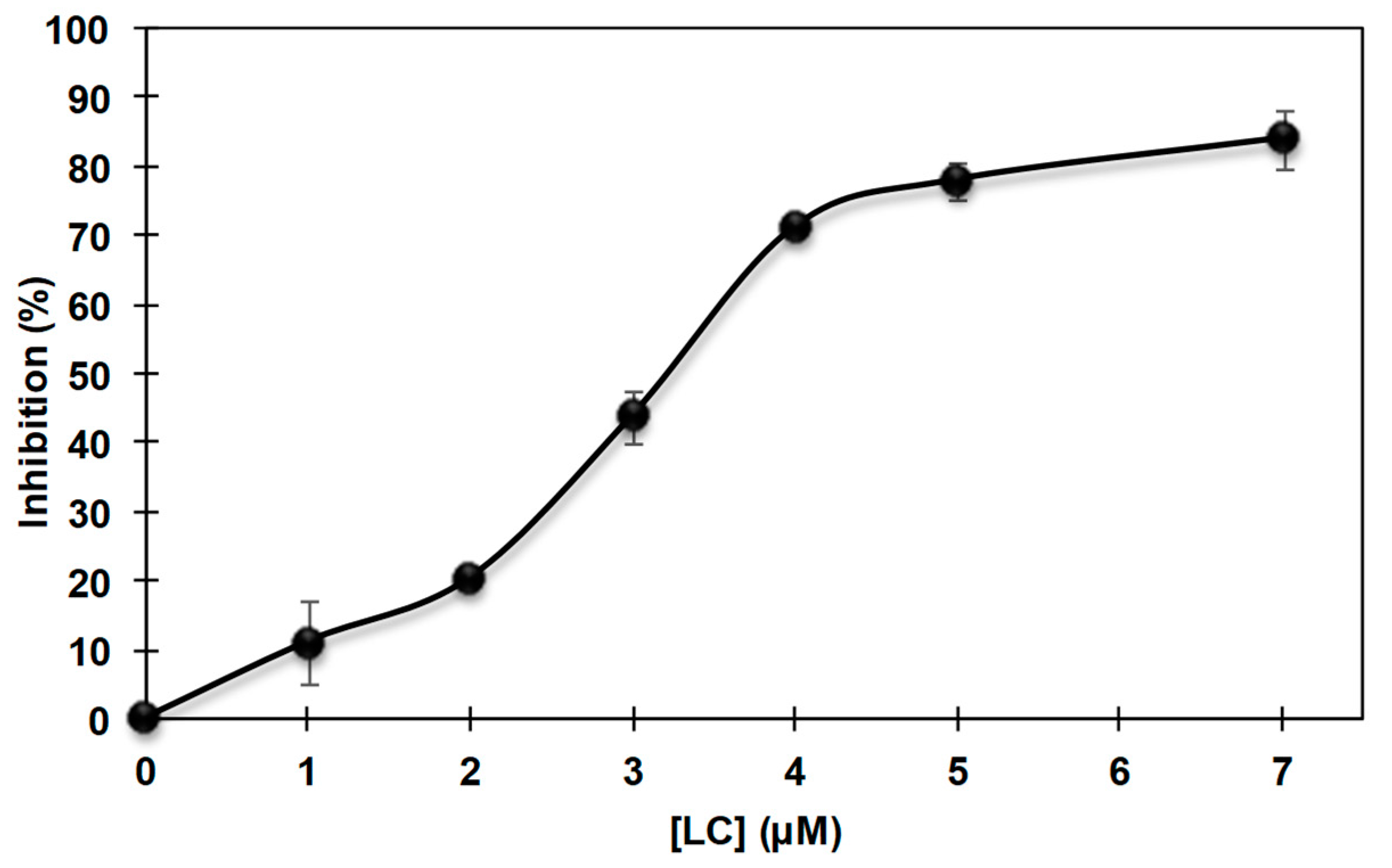
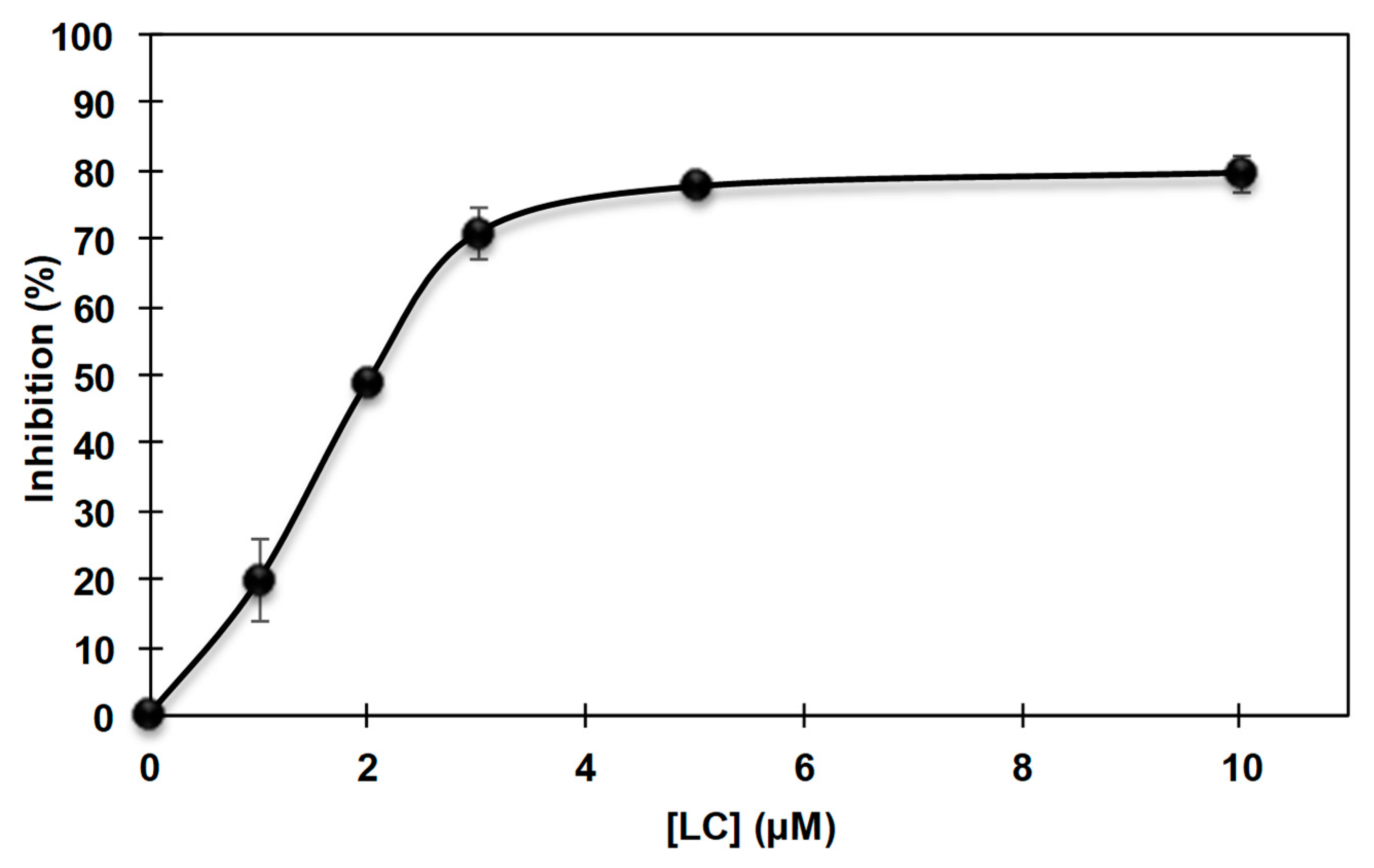
3.2. Inhibition of the Catecholase Activity of Mushroom Tyrosinase by 2-S-Lipoylcaffeic Acid
3.3. Inhibition of the Cresolase Activity of Mushroom Tyrosinase by 2-S-Lipoylcaffeic Acid
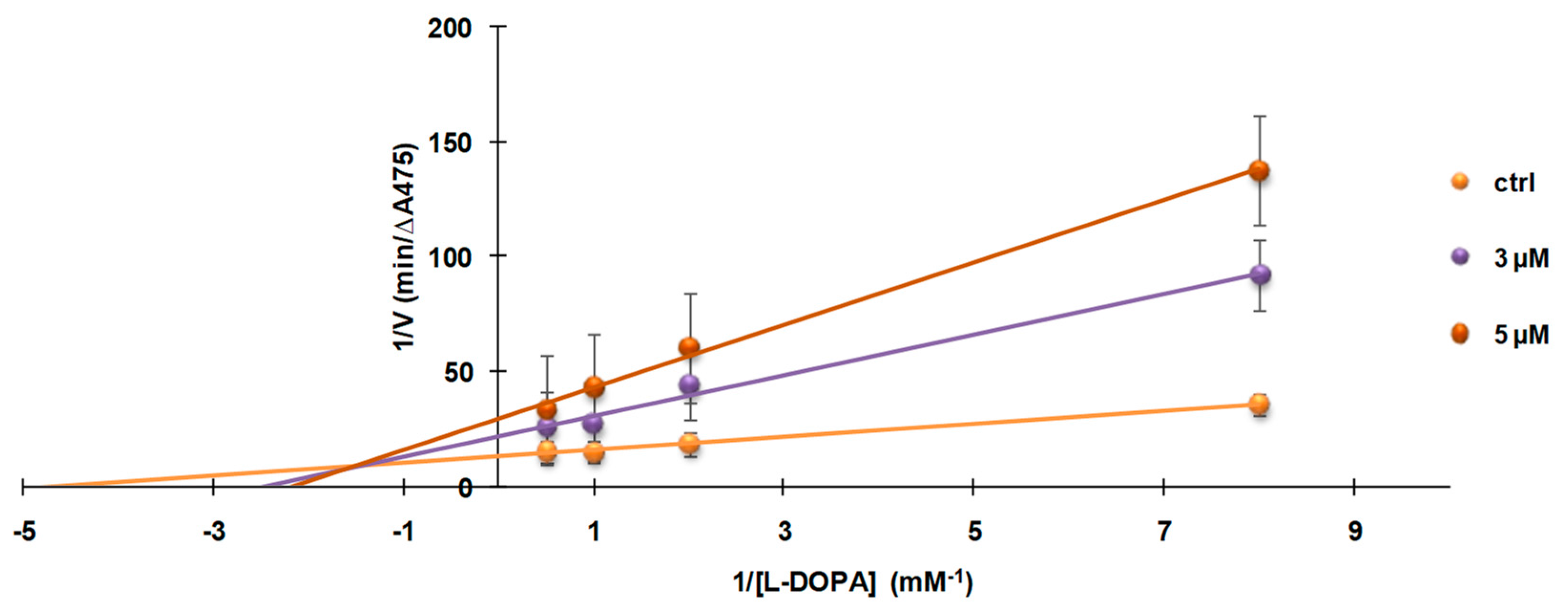
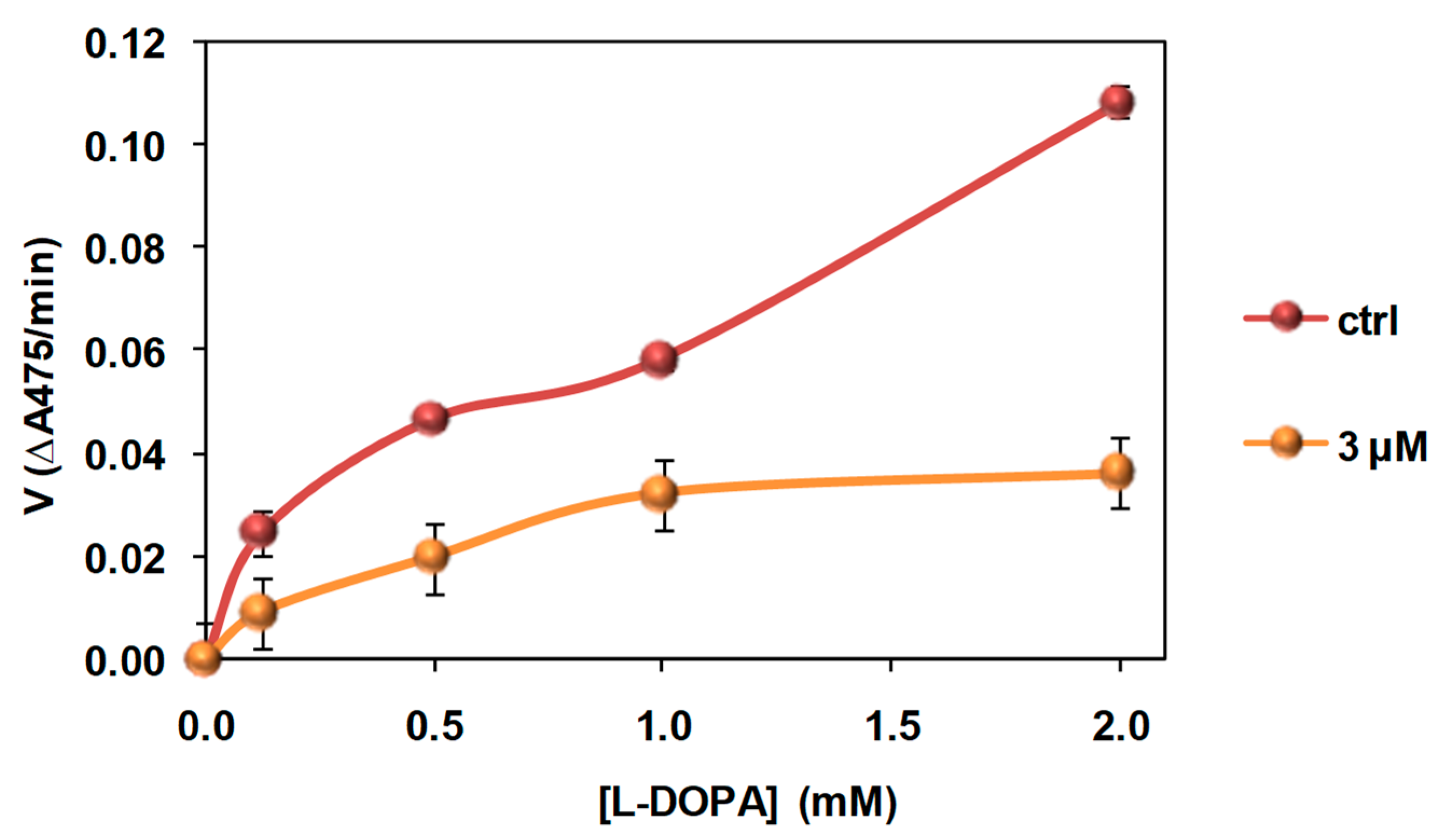
3.4. Investigation of the Mechanism of Inhibition of Mushroom Tyrosinase Activity by 2-S-Lipoylcaffeic Acid
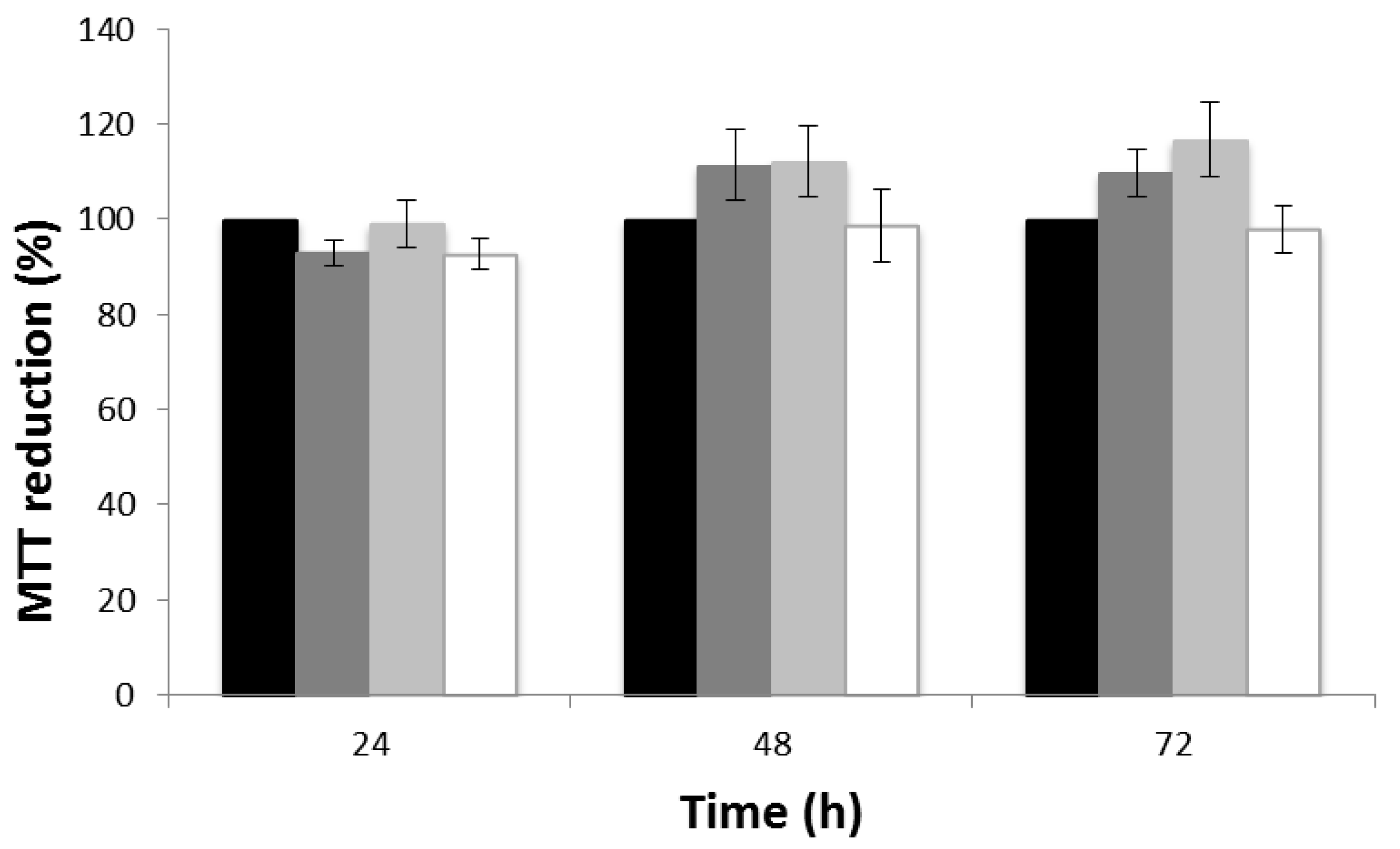
3.5. Cytotoxicity Evaluation
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Slominski, A. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Kovacs, D.; Picardo, M. Mechanisms underlying post-inflammatory hyperpigmentation: Lessons from solar lentigo. Ann. Dermatol. Venereol. 2012, 139 (Suppl. S4), S148–S152. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Carrera, M. New and experimental treatments of cloasma and other hypermelanoses. Dermatol. Clin. 2007, 25, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Popiol, J.; Marona, H. Melanogenesis inhibitors: Strategies for searching for and evaluation of active compounds. Curr. Med. Chem. 2016, 23, 3548–3574. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-Tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Mechanistic aspects of the control of tyrosinase activity. Pigment Cell Res. 1993, 6, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Joo, Y.H.; Baek, H.S.; Park, M.; Kim, J.H.; Shin, H.J.; Park, N.H.; Lee, J.H.; Park, Y.H.; Shin, S.S.; et al. Different effects of five depigmentary compounds, rhododendrol, raspberry ketone, monobenzone, rucinol and AP736 on melanogenesis and viability of human epidermal melanocytes. Exp. Dermatol. 2016, 25, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ojika, M.; Yamashita, T.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of rhododendrol produces 2-methylchromane-6,7-dione, the putative ultimate toxic metabolite: Implications for melanocyte toxicity. Pigment Cell Melanoma Res. 2014, 27, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lin, L.C.; Yang, W.F.; Bordon, J.; Wang, H.M.D. An updated organic classification of tyrosinase inhibitors on melanin biosynthesis. Curr. Org. Chem. 2015, 19, 4–18. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Munoz, J.L.; Berna, J.; Garcia-Molina, F.; Garcia-Ruiz, P.A.; Tudela, J.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Unravelling the suicide inactivation of tyrosinase: A discrimination between mechanisms. J. Mol. Catal. B Enzym. 2012, 75, 11–19. [Google Scholar] [CrossRef]
- Xue, Y.L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Furihata, K.; Sawano, Y.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food Chem. 2011, 59, 6011–6017. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Touaibia, M.; Jean-Francois, J.; Doiron, J. Caffeic acid, a versatile pharmacophore: An overview. Mini Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Chen, C.C.; Lin, P.; You, Y.J.; Chiang, H.M. N-(4-Bromophenethyl) caffeamide inhibits melanogenesis by regulating AKT/glycogen synthase kinase 3 β/microphthalmia-associated transcription factor and tyrosinase-related protein 1/tyrosinase. Curr. Pharm. Biotechnol. 2015, 16, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Yang, J.K.; Choi, H.R.; Park, K.C.; Kim, Y.B.; Lee, Y.S. Synthesis and dual biological effects of hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivatives as tyrosinase inhibitor and antioxidant. Bioorg. Med. Chem. Lett. 2013, 23, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Lee, S.; Choi, H.R.; Park, K.C.; Lee, Y.S. Dual effects of caffeoyl-amino acidyl-hydroxamic acid as an antioxidant and depigmenting agent. Bioorg. Med. Chem. Lett. 2011, 21, 5155–5158. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Ohnishi, K.; Komiya, T.; Imai, K. Synthetic search for cosmetic ingredients: Preparations, tyrosinase inhibitory and antioxidant activities of caffeic amides. J. Oleo Sci. 2002, 51, 19–27. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; d’Ischia, M. 5-S-Lipoylhydroxytyrosol, a multidefense antioxidant featuring a solvent-tunable peroxyl radical-scavenging 3-thio-1,2-dihydroxybenzene motif. J. Org. Chem. 2013, 78, 9857–9864. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Verotta, L.; Goya, L.; Ramos, S.; Martin, M.A.; Bravo, L.; Napolitano, A.; d’Ischia, M. Synthesis and bioactivity profile of 5-S-lipoylhydroxytyrosol-based multidefense antioxidants with a sizeable (poly)sulfide chain. J. Agric. Food Chem. 2013, 61, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Panzella, L.; Pezzella, A.; Napolitano, A.; d’Ischia, M. Reaction of dihydrolipoic acid with juglone and related naphthoquinones: Unmasking of a spirocyclic 1,3-dithiane intermediate en route to naphtho[1,4]dithiepines. Tetrahedron 2010, 66, 3912–3916. [Google Scholar] [CrossRef]
- De Lucia, M.; Panzella, L.; Pezzella, A.; Napolitano, A.; d’Ischia, M. Plant catechols and their S-glutathionyl conjugates as antinitrosating agents: Expedient synthesis and remarkable potency of 5-S-glutathionylpiceatannol. Chem. Res. Toxicol. 2008, 21, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; De Lucia, M.; Napolitano, A.; d’Ischia, M. The first expedient entry to the human melanogen 2-S-cysteinyldopa exploiting the anomalous regioselectivity of 3,4-dihydroxycinnamic acid-thiol conjugation. Tetrahedron Lett. 2007, 48, 7650–7652. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Oxidative conjugation of chlorogenic acid with glutathione. Structural characterization of addition products and a new nitrite-promoted pathway. Bioorg. Med. Chem. 2003, 11, 4797–4805. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Nitrite-mediated decarboxylative conjugation of caffeic acid with glutathione under mildly acidic conditions. Bioorg. Med. Chem. Lett. 2002, 12, 3547–3550. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. α-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–235. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Naito, K.; Hatani, T.; Okada, T.; Tehara, T. Evidence for covalent lipoyl adduction with DOPAquinone following tyrosinase-catalyzed oxidation. Biochem. Biophys. Res. Commun. 2006, 343, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Naito, K.; Hatani, T.; Okada, T.; Tehara, T. Modulating effects of a novel skin-lightening agent, α-lipoic acid derivative, on melanin production by the formation of DOPA conjugate products. Bioorg. Med. Chem. 2007, 15, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Santagostino, M.; Sputore, S. A user-friendly entry to 2-iodoxybenzoic acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- Gunsalus, I.C.; Barton, L.S.; Gruber, W. Biosynthesis and structure of lipoic acid derivatives. J. Am. Chem. Soc. 1956, 78, 1763–1766. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Pouysegu, L.; Deffieux, D.; Quideau, S. Synthesis of biologically active catecholic compounds via ortho-selective oxygenation of phenolic compounds using hypervalent iodine(V) reagents. Curr. Org. Synth. 2012, 9, 650–669. [Google Scholar] [CrossRef]
- Guerriero, E.; Sorice, A.; Capone, F.; Costantini, S.; Palladino, P.; d’Ischia, M.; Castello, G. Effects of lipoic acid, caffeic acid and a synthesized lipoyl-caffeic conjugate on human hepatoma cell lines. Molecules 2011, 16, 6365–6377. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lima, N.; Vallverdu-Queralt, A.; Meudec, E.; Mazauric, J.-P.; Sommerer, N.; Bordignon-Luiz, M.T.; Cheynier, V.; Le Guerneve, C. Synthesis, identification, and structure elucidation of adducts formed by reactions of hydroxycinnamic acids with glutathione or cysteinylglycine. J. Nat. Prod. 2016, 79, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment Cell Res. 2002, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Dermatol. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S. The chemistry of melanin: III. Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [PubMed]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kahn, V. Effect of kojic acid on the oxidation of dl-DOPA, norepinephrine, and dopamine by mushroom tyrosinase. Pigment Cell Res. 1995, 8, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Fumo, M.G.; Menichetti, S.; Mugnaini, V.; Pedulli, G.F. Electronic and hydrogen bonding effects on the chain-breaking activity of sulfur-containing phenolic antioxidants. J. Org. Chem. 2006, 71, 6325–6332. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Panzella, L.; Amorati, R.; Capperucci, A.; Pizzo, E.; Napolitano, A.; Menichetti, S.; d’Ischia, M. Resveratrol-based benzoselenophenes with an enhanced antioxidant and chain breaking capacity. Org. Biomol. Chem. 2015, 13, 5757–5764. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A.; Cooksey, C.J.; Johnson, C.I.; Land, E.J.; Latter, A.M.; Ramsden, C.A. Melanogenesis-targeted anti-melanoma pro-drug development: Effect of side-chain variations on the cytotoxicity of tyrosinase-generated ortho-quinones in a model screening system. Eur. J. Cancer 1997, 33, 135–143. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Land, E.J.; Ramsden, C.A.; Riley, P.A. Tyrosinase-mediated cytotoxicity of 4-substituted phenols: Quantitative structure-thiol-reactivity relationships of the derived o-quinones. Anticancer Drug Des. 1995, 10, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.; Haghbeen, K.; Fazli, M. Non-specific binding sites help to explain mixed inhibition in mushroom tyrosinase activities. Eur. J. Med. Chem. 2016, 122, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micillo, R.; Pistorio, V.; Pizzo, E.; Panzella, L.; Napolitano, A.; D’Ischia, M. 2-S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation. Biomimetics 2017, 2, 15. https://doi.org/10.3390/biomimetics2030015
Micillo R, Pistorio V, Pizzo E, Panzella L, Napolitano A, D’Ischia M. 2-S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation. Biomimetics. 2017; 2(3):15. https://doi.org/10.3390/biomimetics2030015
Chicago/Turabian StyleMicillo, Raffaella, Valeria Pistorio, Elio Pizzo, Lucia Panzella, Alessandra Napolitano, and Marco D’Ischia. 2017. "2-S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation" Biomimetics 2, no. 3: 15. https://doi.org/10.3390/biomimetics2030015