A New Glass-Forming Electrolyte Based on Lithium Glycerolate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of GlyLix Electrolytes
2.2. Vibrational Spectroscopy Studies
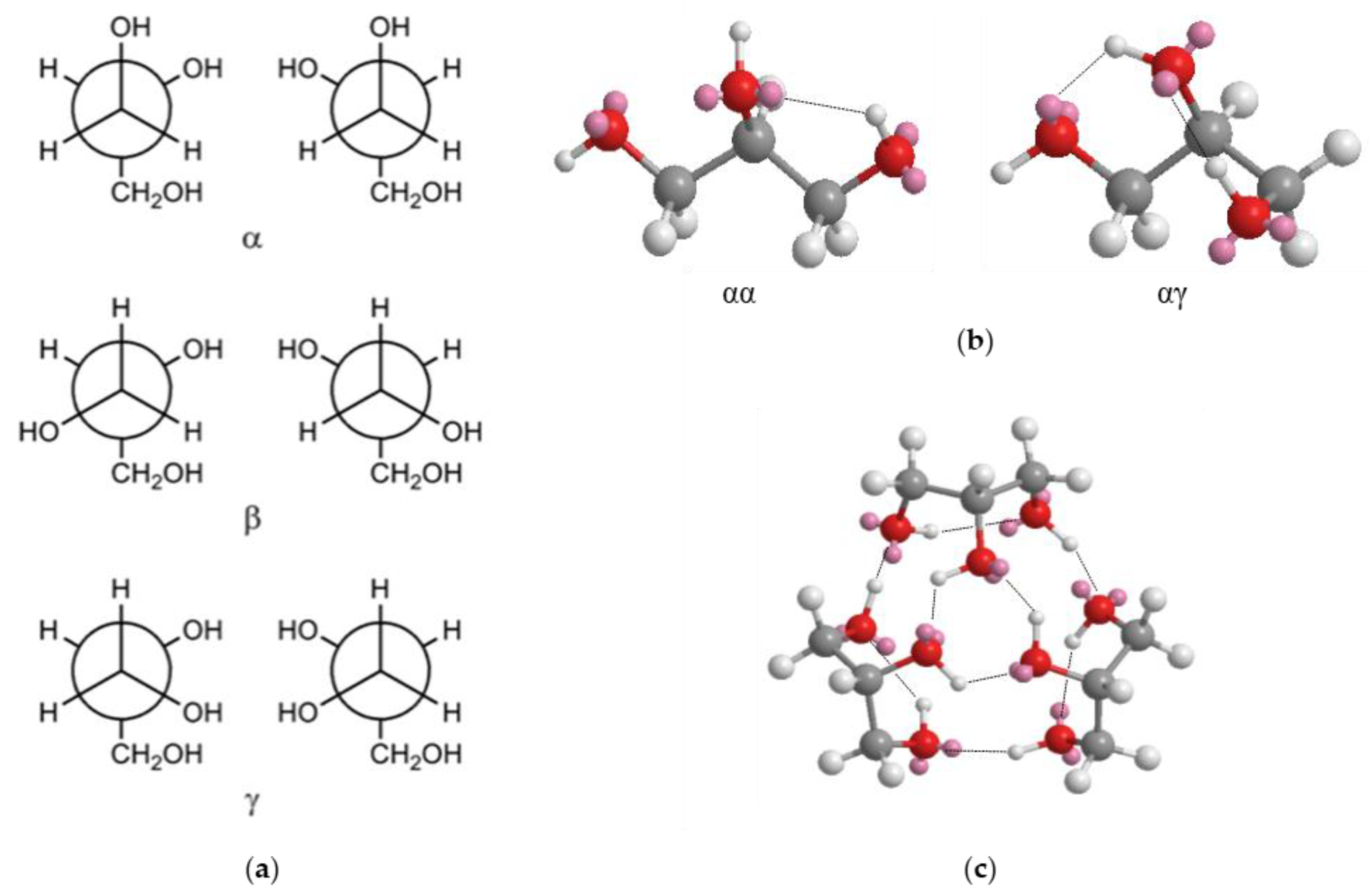
2.2.1. Glycerol Conformations
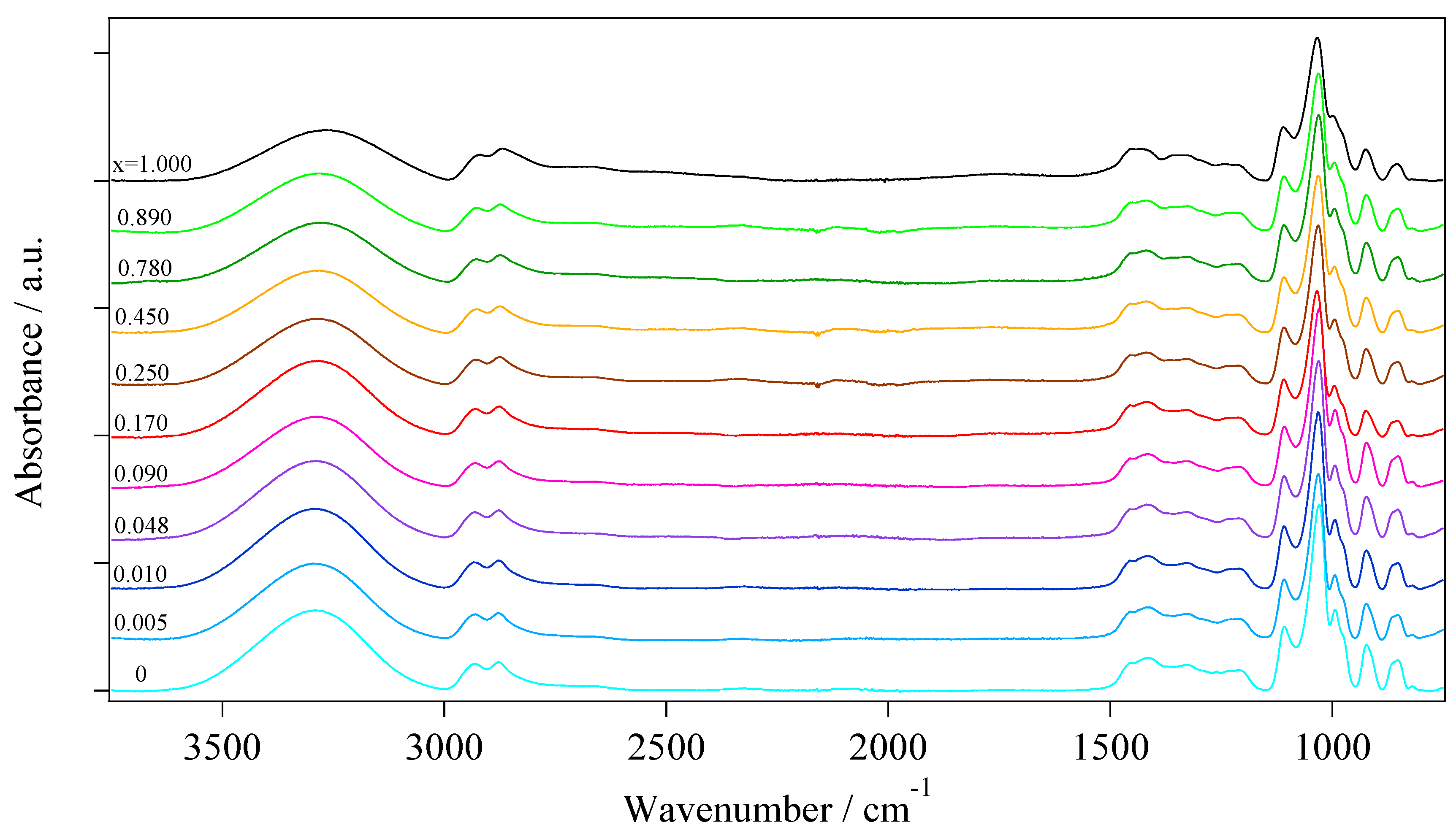
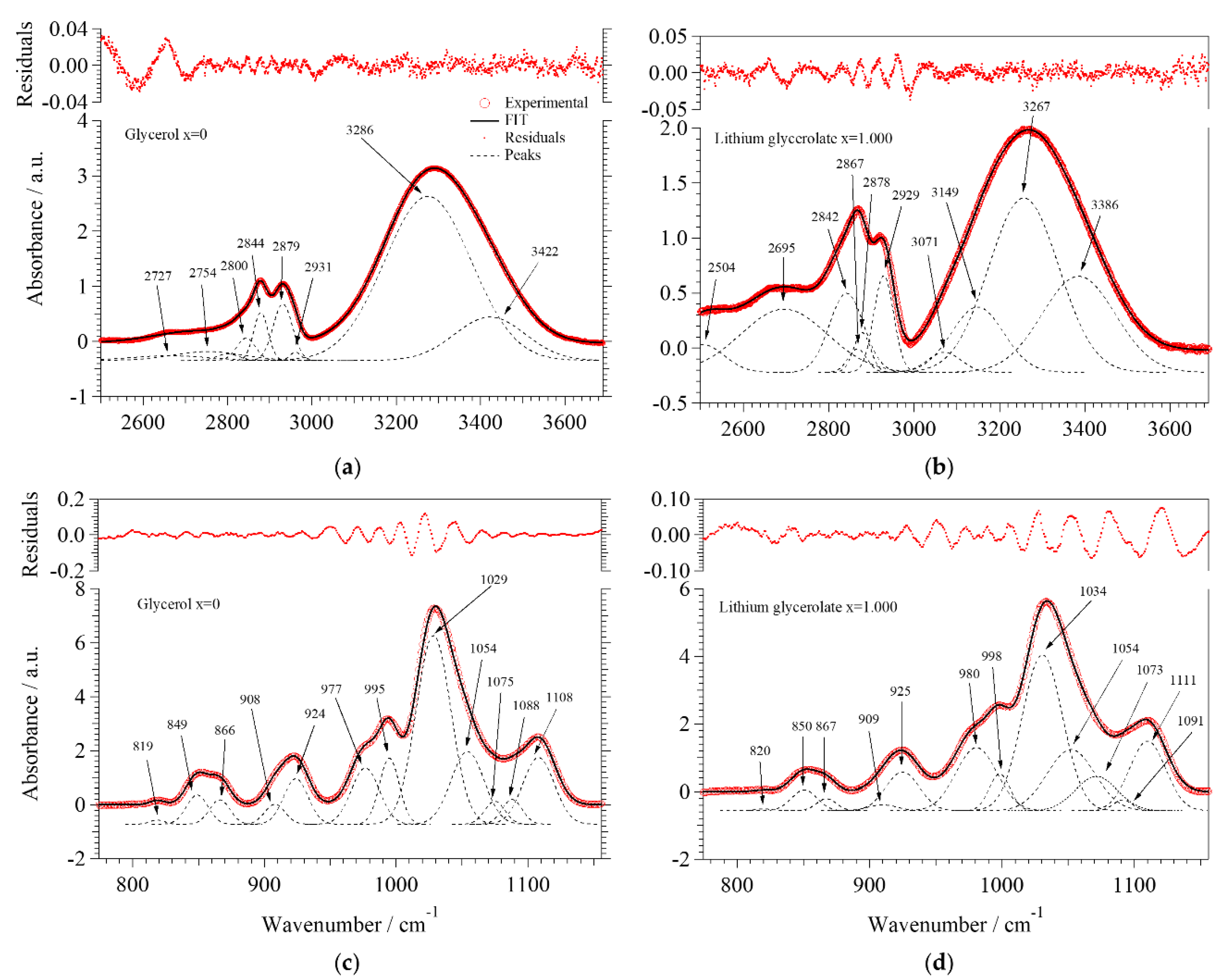
2.2.2. Fourier Transform Infrared Spectroscopy
- Group A: At low concentrations of Li+ (0 ≤ x ≤ 0.090), the areal percentage of the peaks associated to νf(OH) (3386 cm−1) and νHy(OH) (3267 cm−1) varies by no more than 5%. In this group of electrolytes, A3149 and A3071, which are attributed respectively to the νg3Li(OH) and νg2Li(OH) modes, are equal to 0. This suggests that at low values of x, a large number of intra- and inter-molecular hydrogen bonds are present. The coordination geometry remains similar to that of pristine glycerol, i.e., g1 (Figure 2a). Indeed, g1 allows for a higher number of hydrogen bonds with respect to g2 and g3 coordination geometries.
- Group B: In the middle of the concentration range of Li+ (0.170 ≤ x ≤ 0.450), A3386 remains almost constant while A3267 is decreased by ca. 63%, indicating a lower number of hydrogen bonds. A3149 and A3071 appear in the spectra of these samples, reaching values of ca. 30%. The decrease in the number of hydrogen bonds is attributed to the increased number of oxygen atoms involved in the coordination of Li+, as confirmed by the increased intensities of the peaks attributed to the νg3Li(OH) and νg2Li(OH). In samples with 0.170 ≤ x ≤ 0.450, the predominant coordination geometries are g2.1 and g2.2 (Figure 2c,d) due to a lower number of hydrogen bonds and to the formation of lithium coordination by oxygen functionalities.
- Group C: In the high Li+ concentration range (0.780 ≤ x ≤ 1.000), A3386 remains almost constant, and A3267 goes back to medium values of areal percentage (up to 64%). Moreover, in this region the areas of νg3Li(OH) and νg2Li(OH) peaks begin to become clearly different, with the former larger than the latter. This evidence is easily explained if we consider that at x ≥ 0.780 the prevailing coordination geometry is g3. This allows for a higher number of hydrogen bonds with respect to g2; at the same time, a significant number of oxygen atoms is coordinating Li+.
- Group A: At low concentrations of Li+ (0 ≤ x ≤ 0.090), the νg1,pri(C–C–O) vibration dominates the spectra, indicating that the majority of Li+ is coordinated by the oxygen functionalities of primary alcohols in the g1 coordination geometry.
- Group B: In the middle of the concentration range of Li+ (0.170 ≤ x ≤ 0.450), we observe a decrease in the area of the peak attributed to νg1,pri(C–C–O) vibration, concurrently with a significant increase of both A1073 (here the effect is significant) and A1111 (here the effect is visible, but less pronounced). Indeed, in this region the main coordination geometries assumed by glycerol molecules are g2.1 and g2.2, with secondary alcohol hydroxyl groups coordinating Li+.
- Group C: Finally, at a high concentration of Li+ (0.780 ≤ x ≤ 1.000), the areas of the peaks attributed to νg1,pri(C–C–O), νg3,pri(C–C–O), and νg3,sec(C–C–O) increase. On the contrary, the areas of the peaks associated to νg2,sec(C–C–O) and νg1,sec(C–C–O) show a minimum for x = 1.000. This demonstrates that at a high concentration of Li+ the dominating coordination geometry for glycerol molecules is g3.
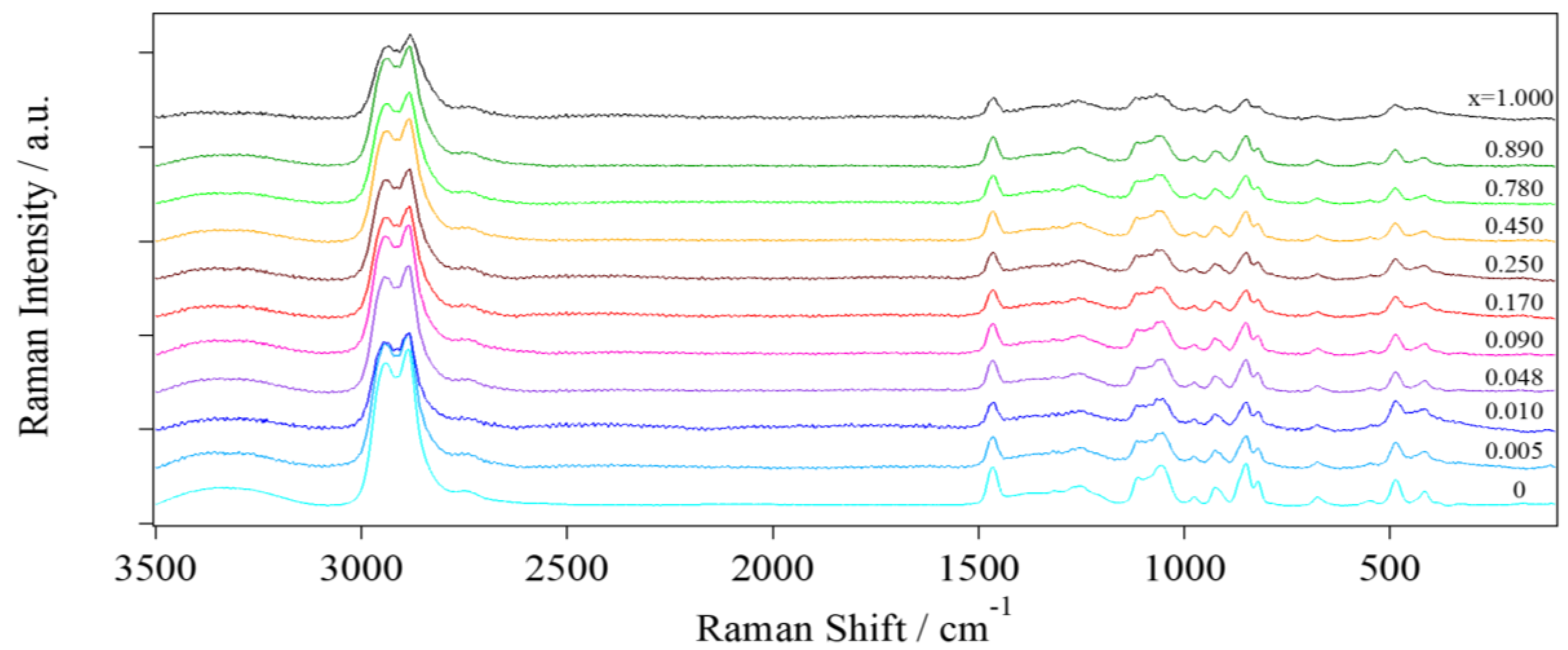
2.2.3. Micro-Raman Spectroscopy
- Group A: At low concentrations of Li+ (0 ≤ x ≤ 0.090), A2945, attributed to the νg1,a(CH) vibration, has the highest value among all the other peaks. Again, it is confirmed that at these concentrations of Li+ the predominant coordination geometry taken by glycerol molecules is g1.
- Group B: In the middle of the concentration range of Li+ (0.170 ≤ x ≤ 0.450), it appears that the area attributed to the CH stretching of g1 conformation decreases, while the highest increase is observed for A2966 and A2909. The latter are attributed to νg2,a(CH) and νg2,s(CH), respectively, with a maximum at x = 0.250. In this concentration region, glycerol molecules prefer g2.1 and g2.2 coordination geometries.
- Group C: Finally, at a high concentration of Li+ (0.780 ≤ x ≤ 1.000), a further decrease of the area attributed to νg1,a(CH) vibration is observed. Differently from Group B, a significant increase of A2831 is observed, attributed to νg3,s(CH). This indicates that in this concentration range of Li+, the number of glycerol molecules taking g3 coordination geometry is increased.
2.3. Thermal Studies
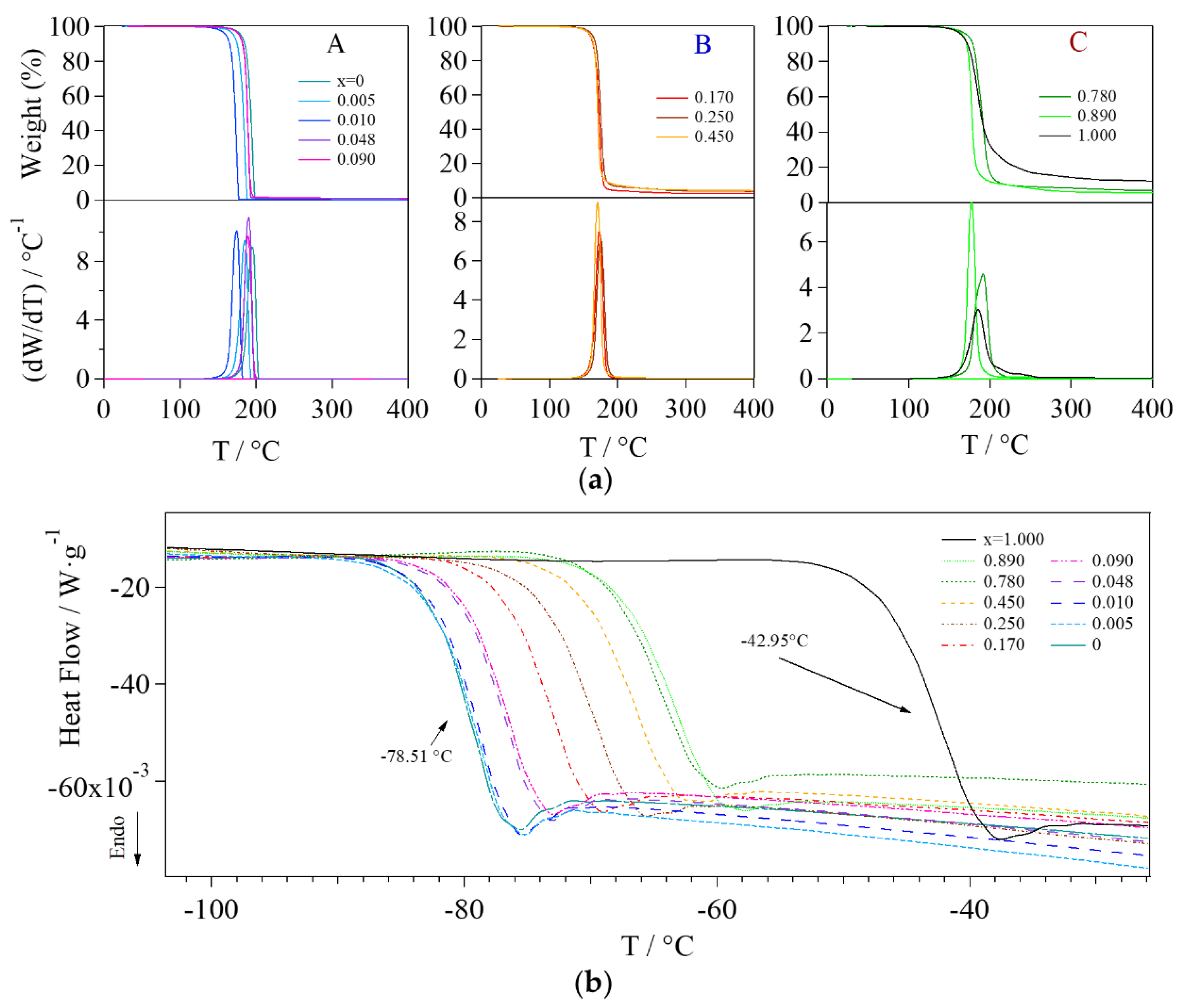
2.3.1. Thermo-Gravimetric Analysis (TGA)
2.3.2. Modulated Differential Scanning Calorimetry (MDSC) Studies
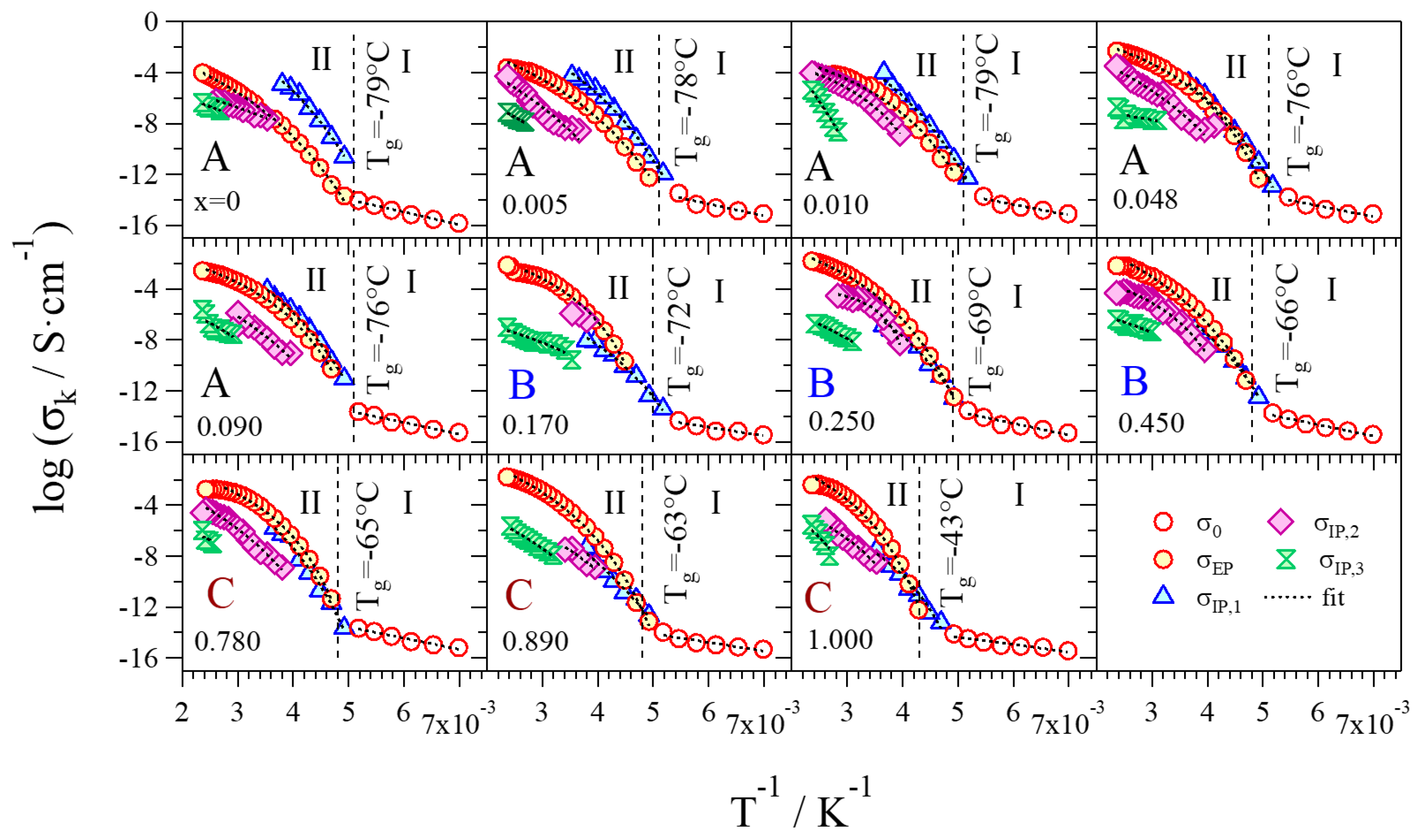
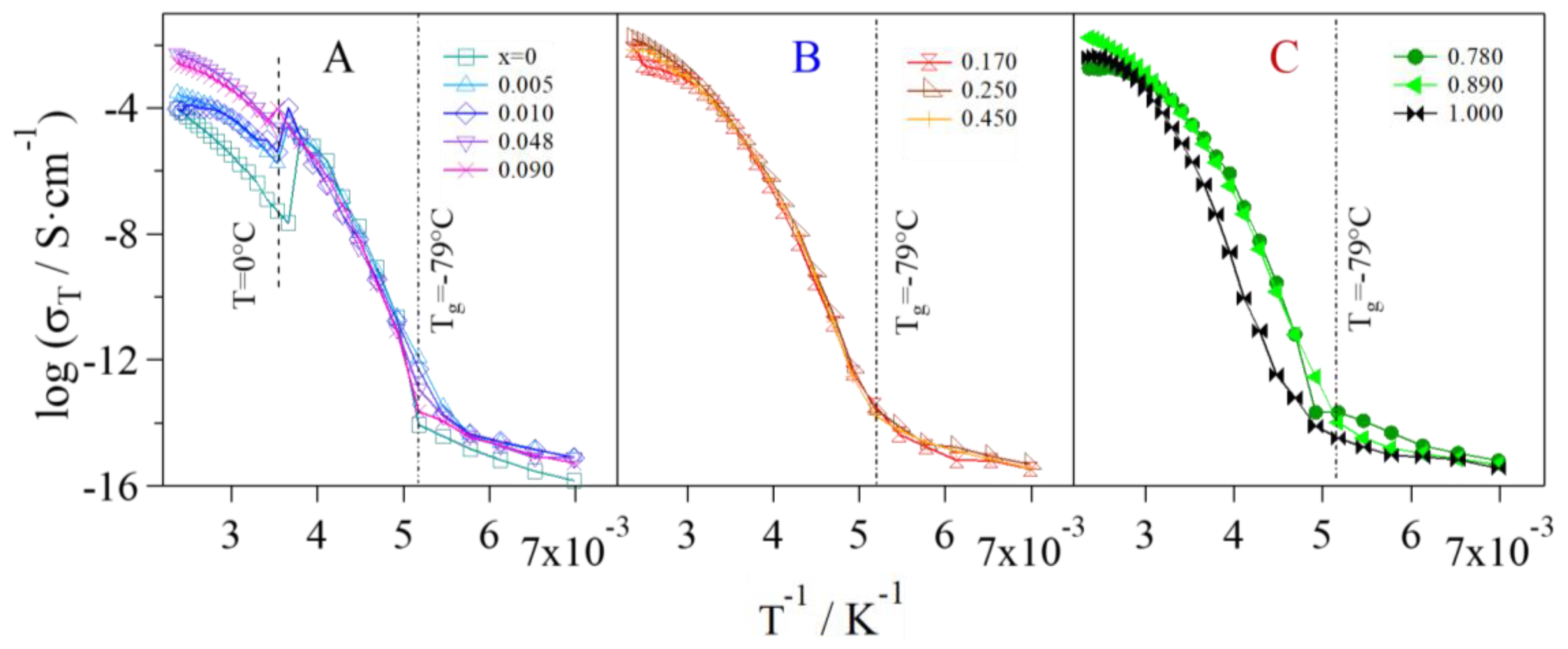
2.4. Broadband Electrical Spectroscopy
2.4.1. Polarization Phenomena
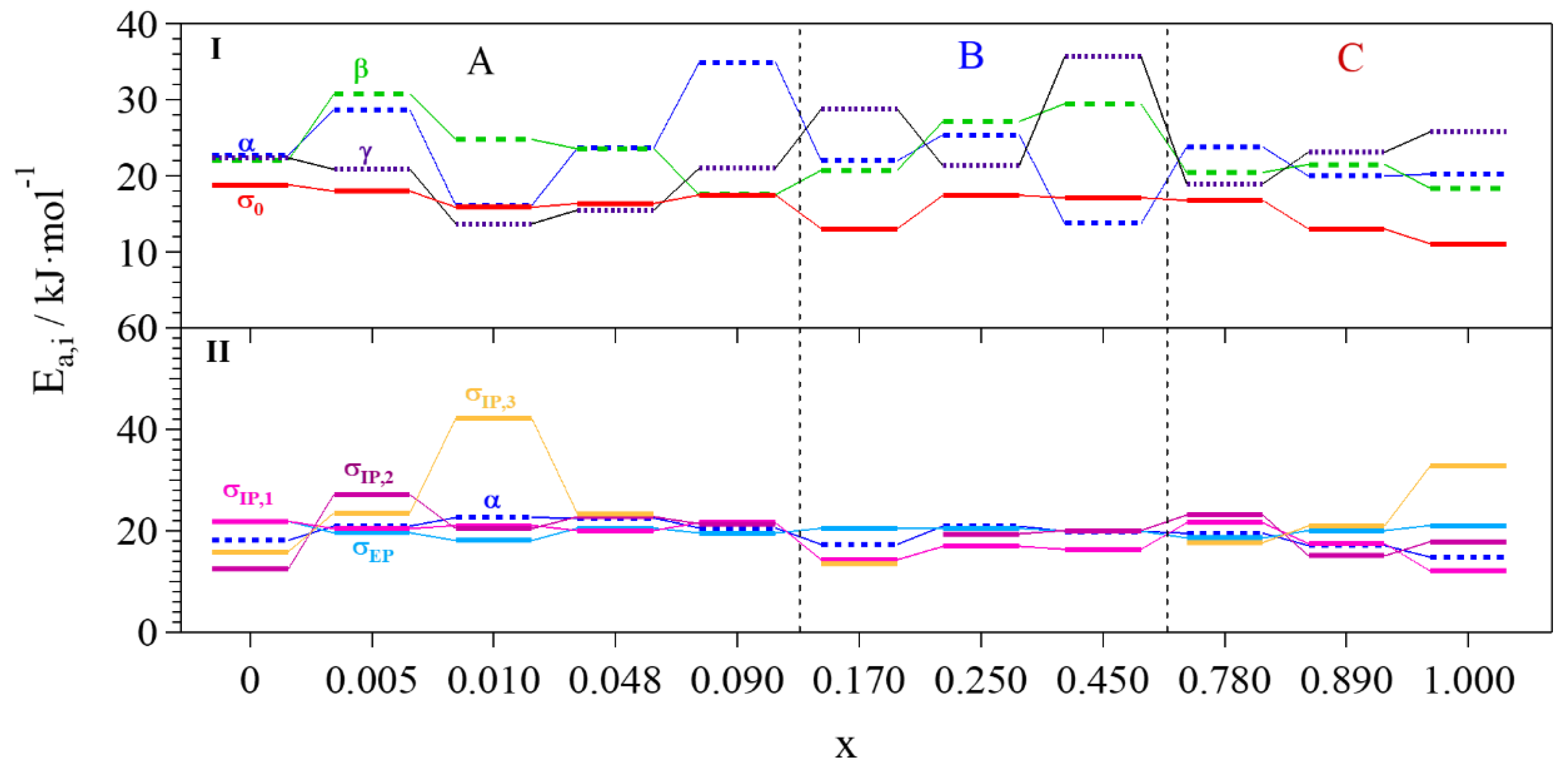
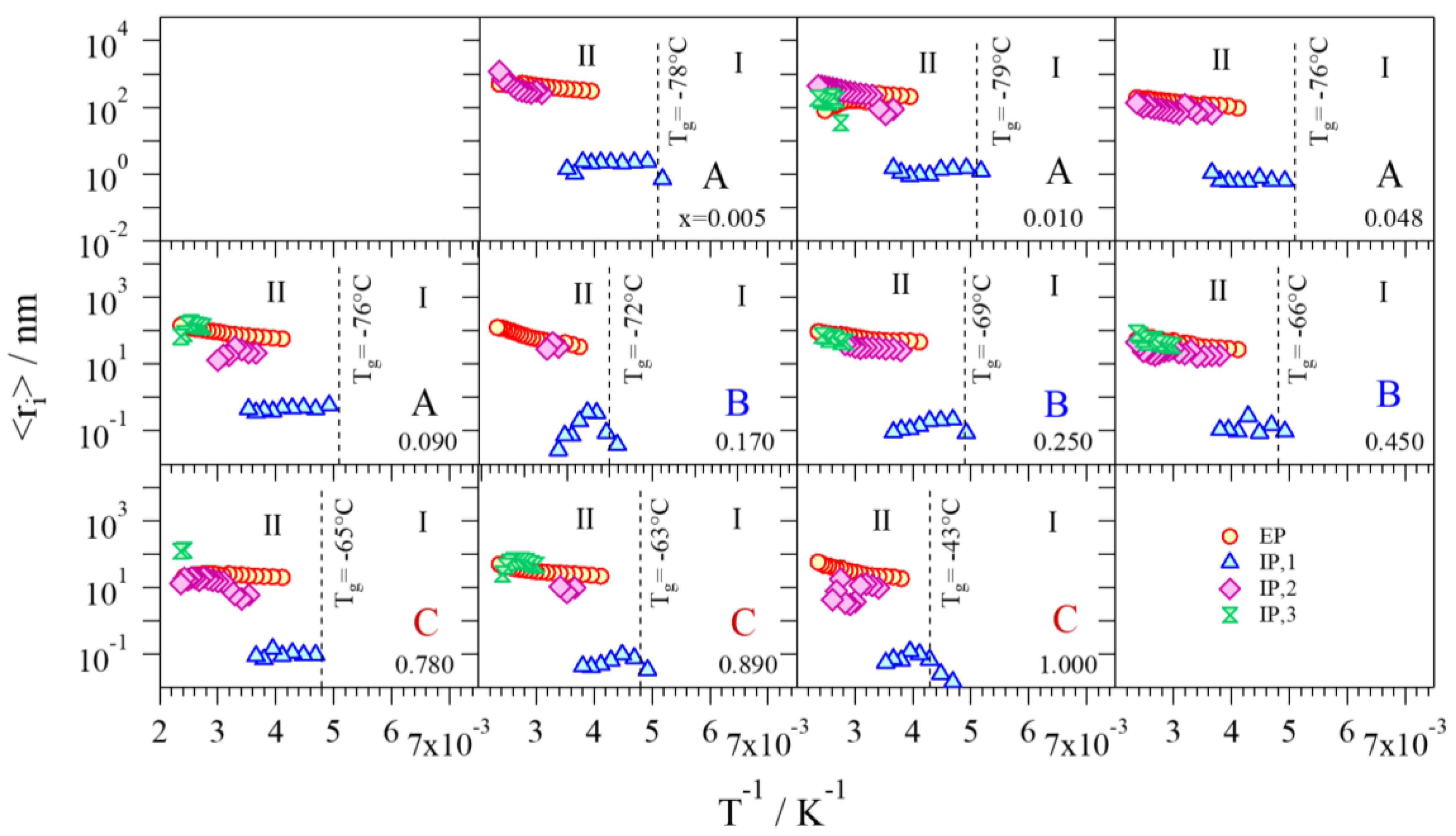
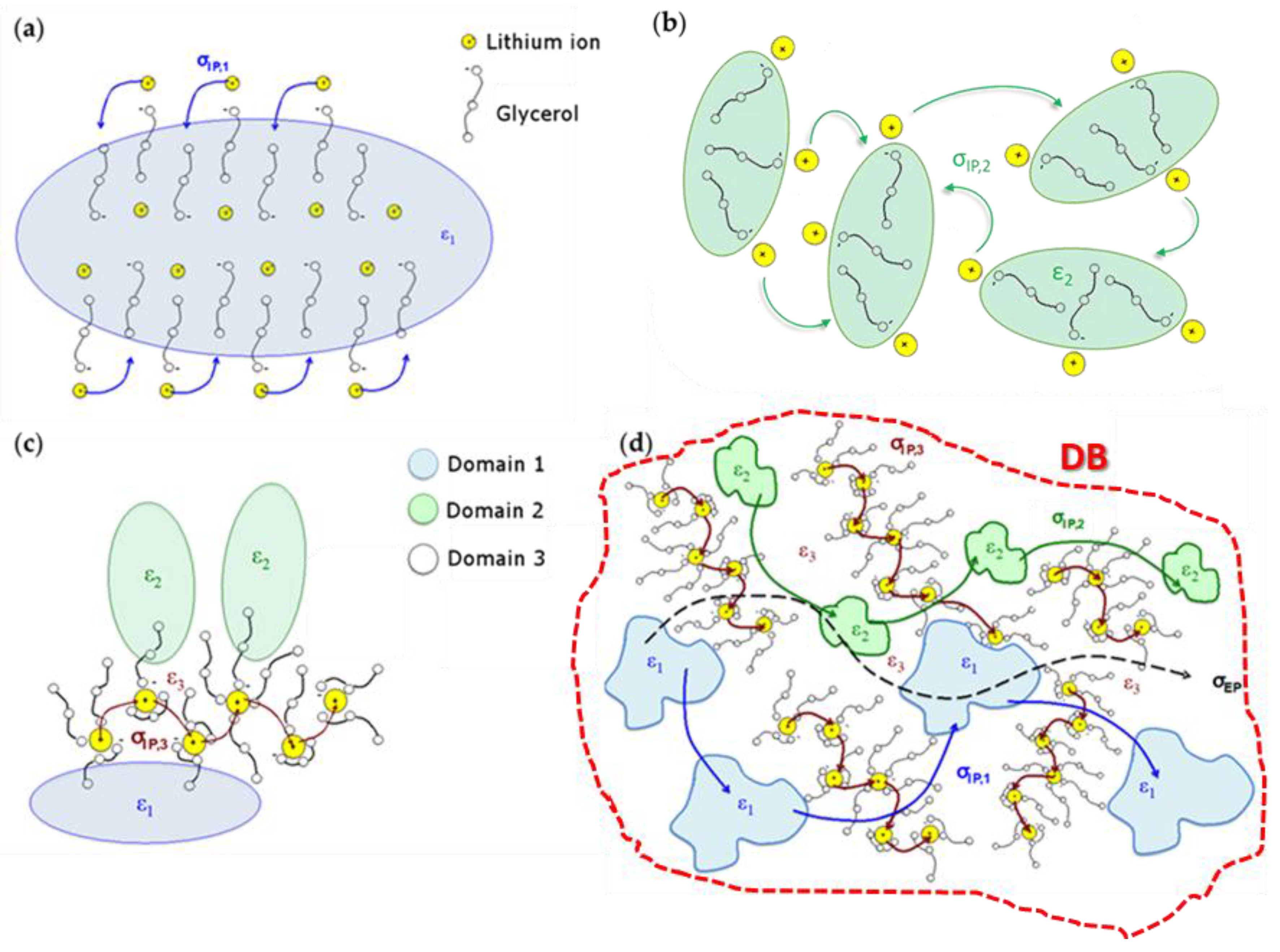
- Group A: Pristine glycerol and electrolytes with a low content of Li+ (x ≤ 0.090) show the lowest values of overall conductivity. At low concentrations of Li+, glycerol molecules are in the αα conformation and coordinate Li+ through the g1 geometry, where the highest number of intra- and inter-molecular hydrogen bonds is achieved. Thus, the presence of these interactions gives rise to glycerol structures that hinder the migration of Li+. Furthermore, a low concentration of charge carriers decreases the overall conductivity. In Group A, a conductivity drop is observed at ca. 0 °C, even if MDSC measurements do not reveal any thermal transition. This phenomenon can be explained by considering that, at these concentrations of Li+ and in this particular temperature range (Tg ≤ T ≤ 0 °C), the structure assumed by the glycerol molecules facilitates the σIP,1 polarization, making it the component providing the highest contribution to the overall conductivity (Figure 9) and perturbing the log σT vs T−1 curve behavior. This effect decreases as the content of Li+ is increased. At temperatures higher than 0 °C, the main contribution to the overall conductivity is mostly provided by the electrode polarization.
- Group B: In this concentration region the highest values of overall conductivity are reached. In particular, GlyLi0.250 shows the highest lithium single-ion room temperature conductivity value, i.e., 1.99 × 10−4 at 30 °C. This value is in line with those present in the literature for lithium-ion-conducting polymer electrolytes. This makes the proposed electrolytes extremely interesting for application in Li-ion batteries [19,35]. Furthermore, this electrolyte is characterized by (i) an impressive high-temperature conductivity of 1.55 × 10−2 S∙cm−1 at 150 °C; and (ii) a significant low-temperature conductivity, that is higher than 10−6 S∙cm−1 at −10 °C, thanks to the glass-forming behavior of the glycerol matrix. In this concentration range of Li+, the g2 conformation geometry assumed by glycerol molecules does not facilitate the σIP,1 polarization. Indeed, (i) the conductivity of this event strongly decreases; (ii) σEP becomes the most contributing component to the overall conductivity at T > Tg; and (iii) the conductivity drop at 0 °C is no longer detected.
- Group C: Similar to Group B, the electrolytes belonging to this concentration region demonstrate high values of room temperature conductivity (σT > 10−4 S∙cm−1). Nevertheless, GlyLi shows a decrease in conductivity, especially in the Tg ≤ T ≤ 30 °C temperature range. This is probably the result of three concurring effects: (i) as already demonstrated by MDSC measurements, the introduction of a high number of Li+ ions makes the structure of the glycerol host more rigid, hindering the migration process of Li+; (ii) the g3 conformation geometry, which is assumed by glycerol molecules in this concentration region, strongly decreases the σIP,1 polarization contribution and thus lowers the overall conductivity; and (iii) the presence of a huge number of Li+ ions increases the probability to entrap lithium ions into the tetrahedral coordination structures of the glycerol molecules.
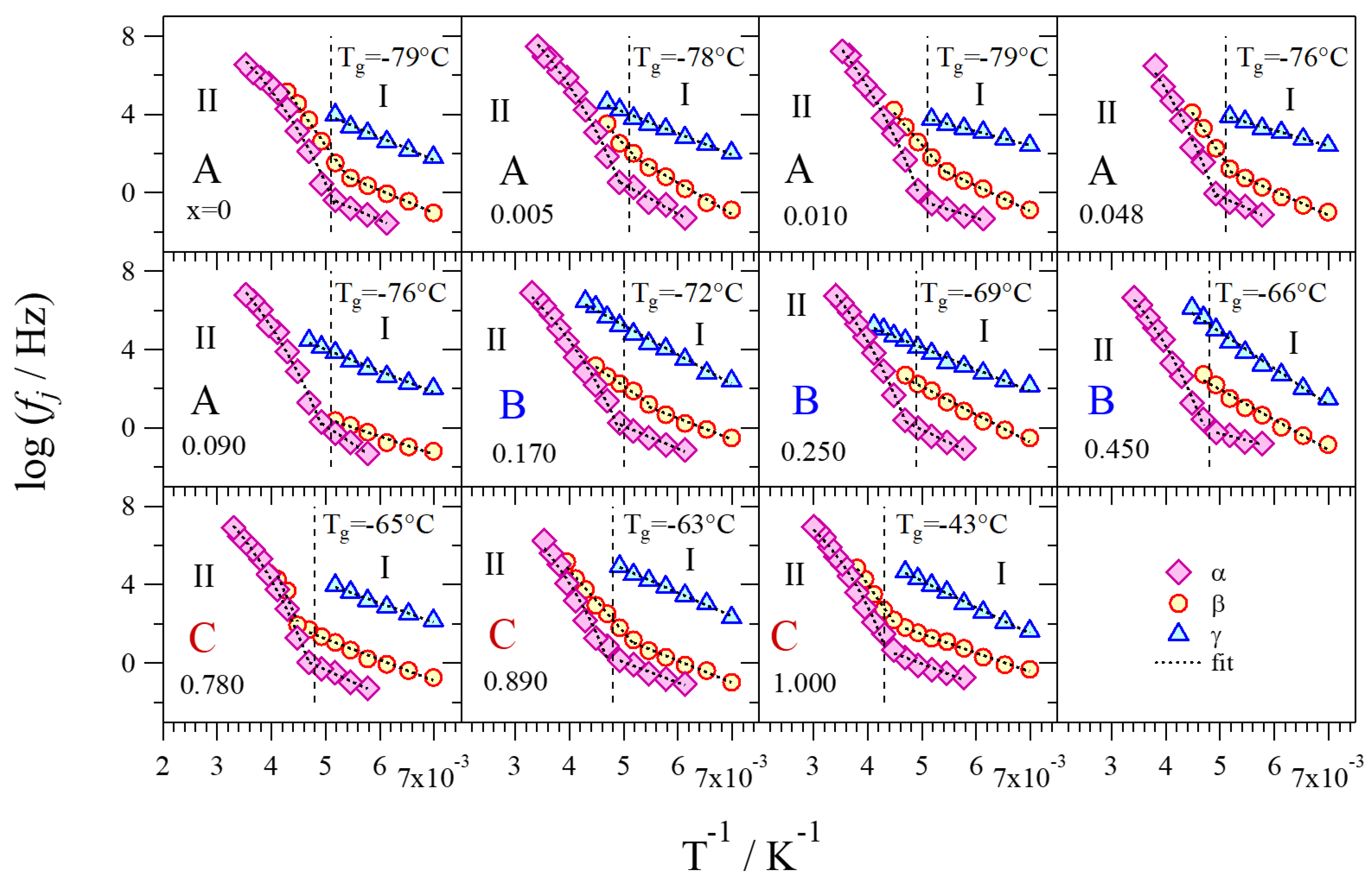
2.4.2. Relaxation Events
2.4.3. Activation Energies
2.4.4. Diffusion Coefficients and Average Charge Migration Distance
2.5. Conduction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Chemical and Structural Characterizations
3.3. Thermal Characterizations
3.4. Electric Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Di Noto, V.; Lavina, S.; Giffin, G.A.; Negro, E.; Scrosati, B. Polymer electrolytes: Present, past and future. Electrochim. Acta 2011, 57, 4–13. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Deng, Y.; Eames, C.; Fleutot, B.; David, R.; Chotard, J.N.; Suard, E.; Masquelier, C.; Islam, M.S. Enhancing the Lithium Ion Conductivity in Lithium Superionic Conductor (LISICON) Solid Electrolytes through a Mixed Polyanion Effect. ACS Appl. Mater. Interfaces 2017, 9, 7050–7058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarascon, J.M.; Guyomard, D. New electrolyte compositions stable over the 0 to 5 V voltage range and compatible with the Li1+xMn2O4/carbon Li-ion cells. Solid State Ion. 1994, 69, 293–305. [Google Scholar] [CrossRef]
- Canepa, P.; Dawson, J.A.; Sai Gautam, G.; Statham, J.M.; Parker, S.C.; Islam, M.S. Particle Morphology and Lithium Segregation to Surfaces of the Li7La3Zr2O12 Solid Electrolyte. Chem. Mater. 2018, 30, 3019–3027. [Google Scholar] [CrossRef]
- Dawson, J.A.; Canepa, P.; Famprikis, T.; Masquelier, C.; Islam, M.S. Atomic-Scale Influence of Grain Boundaries on Li-Ion Conduction in Solid Electrolytes for All-Solid-State Batteries. J. Am. Chem. Soc. 2018, 140, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Bertasi, F.; Negro, E.; Vezzù, K.; Nawn, G.; Pagot, G.; Di Noto, V. Single-Ion-Conducting Nanocomposite Polymer Electrolytes for Lithium Batteries Based on Lithiated-Fluorinated-Iron Oxide and Poly(ethylene glycol) 400. Electrochim. Acta 2015, 175, 113–123. [Google Scholar] [CrossRef]
- Bertasi, F.; Vezzù, K.; Giffin, G.A.; Nosach, T.; Sideris, P.; Greenbaum, S.; Vittadello, M.; Di Noto, V. Single-ion-conducting nanocomposite polymer electrolytes based on PEG400 and anionic nanoparticles: Part 2. Electrical characterization. Int. J. Hydrogen Energy 2014, 39, 2884–2895. [Google Scholar] [CrossRef]
- Bertasi, F.; Vezzù, K.; Negro, E.; Greenbaum, S.; Di Noto, V. Single-ion-conducting nanocomposite polymer electrolytes based on PEG400 and anionic nanoparticles: Part 1. Synthesis, structure and properties. Int. J. Hydrogen Energy 2014, 39, 2872–2883. [Google Scholar] [CrossRef]
- Bonino, F.; Croce, F.; Panero, S. Electrochemical characterization of an ambient temperature rechargeable Li battery based on low molecular weight polymer electrolyte. Solid State Ion. 1994, 70–71, 654–657. [Google Scholar] [CrossRef]
- Pagot, G.; Bertasi, F.; Vezzù, K.; Nawn, G.; Pace, G.; Nale, A.; Di Noto, V. Correlation between Properties and Conductivity Mechanism in Poly(vinyl alcohol)-based Lithium Solid Electrolytes. Solid State Ion. 2018, 320, 177–185. [Google Scholar] [CrossRef]
- Howell, F.S.; Moynihan, C.T.; Macedo, P.B. Electrical Relaxations in Mixtures of Lithium Chloride and Glycerol. Bull. Chem. Soc. Jpn. 1984, 57, 652–661. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-Forming Liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef]
- Lide, D.R.; Milne, G.W.A. Handbook of Data on Organic Compounds, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1994; ISBN 9780849304453. [Google Scholar]
- Van Duyne, R.P.; Reilley, C.N. Low-Temperature Electrochemistry II. Evaluation of Rate Constants and Activation Parameters for Homogeneous Chemical Reactions Coupled to Charge Transfer. Anal. Chem. 1972, 44, 153–158. [Google Scholar] [CrossRef]
- Macedo, P.B.; Moynihan, C.T.; Bose, R. Role of Ionic Diffusion in Polarization in Vitreous Ionic Conductors. Phys. Chem. Glasses 1972, 13, 171–179. [Google Scholar]
- Bastiansen, O. Intra-Molecular Hydrogen Bonds in Ethylene Glycol, Glycerol, and Ethylene Chlorohydrin. Acta Chem. Scand. 1949, 3, 415–421. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Della Valle, R.G.; Califano, S. Glycerol condensed phases part I. A molecular dynamics study. Phys. Chem. Chem. Phys. 1999, 1, 871–877. [Google Scholar] [CrossRef]
- Di Noto, V.; Vittadello, M.; Greenbaum, S.G.; Suarez, S.; Kano, K.; Furukawa, T. A New Class of Lithium Hybrid Gel Electrolyte Systems. J. Phys. Chem. B 2004, 108, 18832–18844. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Califano, S. Glycerol condensed phases Part II.A molecular dynamics study of the conformational structure and hydrogen bonding. Phys. Chem. Chem. Phys. 1999, 1, 879–885. [Google Scholar] [CrossRef]
- Chelli, R.; Gervasio, F.L.; Gellini, C.; Procacci, P.; Cardini, G.; Schettino, V. Density Functional Calculation of Structural and Vibrational Properties of Glycerol. J. Phys. Chem. A 2000, 104, 5351–5357. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zhang, J.W.; Zhao, C.C.; Du, Z.Y. Tetrahedrally coordinated lithium(I) and zinc(II) carboxylate-phosphinates based on tetradentate 2-carboxyethyl(phenyl)phosphinate ligand. Inorg. Chim. Acta 2014, 414, 121–126. [Google Scholar] [CrossRef]
- Liddel, U.; Becker, E.D. Infra-red spectroscopic studies of hydrogen bonding in methanol, ethanol, and t-butanol. Spectrochim. Acta 1957, 10, 70–84. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: Cambridge, MA, USA, 1991; ISBN 978–0-12-451160-6. [Google Scholar]
- Mendelovici, E.; Frost, R.L.; Kloprogge, T. Cryogenic Raman spectroscopy of glycerol. J. Raman Spectrosc. 2000, 31, 1121–1126. [Google Scholar] [CrossRef]
- Perova, T.S.; Christensen, D.H.; Rasmussen, U.; Vij, J.K.; Nielsen, O.F. Far-infrared spectra of highly viscous liquids: Glycerol and triacetin (glycerol triacetate). Vib. Spectrosc. 1998, 18, 149–156. [Google Scholar] [CrossRef]
- Zietlow, J.P.; Cleveland, F.F.; Meister, A.G. Substituted methanes. III. Raman spectra, assignments, and force constants for some trichloromethanes. J. Chem. Phys. 1950, 18, 1076–1080. [Google Scholar] [CrossRef]
- Dou, B.; Dupont, V.; Williams, P.T.; Chen, H.; Ding, Y. Thermogravimetric kinetics of crude glycerol. Bioresour. Technol. 2009, 100, 2613–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.L.; Zhang, X.H.; Wei, R.J.; Wang, Q.; Fan, Z.Q.; Qi, G.R. Inhibitory effect of hydrogen bonding on thermal decomposition of the nanocrystalline cellulose/poly(propylene carbonate) nanocomposite. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Angell, C.A. Entropy and fragility in supercooling liquids. J. Res. Natl. Inst. Stand. Technol. 1997, 102, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Claudy, P.; Jabrane, S.; Létoffé, J.M. Annealing of a glycerol glass: Enthalpy, fictive temperature and glass transition temperature change with annealing parameters. Thermochim. Acta 1997, 293, 1–11. [Google Scholar] [CrossRef]
- Di Noto, V.; Giffin, G.A.; Vezzù, K.; Piga, M.; Lavina, S. Broadband Dielectric Spectroscopy: A Powerful Tool for the Determination of Charge Transfer Mechanisms in Ion Conductors, Solid State Proton Conductors: Properties and Applications in Fuel Cells; John Wiley & Sons: Chichester, UK, 2012; pp. 109–183. [Google Scholar]
- Kitajima, S.; Bertasi, F.; Vezzù, K.; Negro, E.; Tominaga, Y.; Di Noto, V. Dielectric relaxations and conduction mechanisms in polyether-clay composite polymer electrolytes under high carbon dioxide pressure. Phys. Chem. Chem. Phys. 2013, 15, 16626–16633. [Google Scholar] [CrossRef] [PubMed]
- Tammann, G.; Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Choudhary, S.; Sengwa, R.J. Electrochemical performance of Li+-ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nanoparticles for the lithium ion batteries. Compos. Commun. 2018, 10, 11–17. [Google Scholar] [CrossRef]
- Di Noto, V.; Piga, M.; Giffin, G.A.; Lavina, S.; Smotkin, E.S.; Sanchez, J.Y.; Iojoiu, C. Influence of anions on proton-conducting membranes based on neutralized nafion 117, triethylammonium methanesulfonate, and triethylammonium perfluorobutanesulfonate. 2. electrical properties. J. Phys. Chem. C 2012, 116, 1370–1379. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Schneider, U.; Brand, R.; Loid, A. Glassy dynamics. Contemp. Phys. 2000, 41, 15–36. [Google Scholar] [CrossRef]
- Kudlik, A.; Benkhof, S.; Blochowicz, T.; Tschirwitz, C.; Rössler, E. The dielectric response of simple organic glass formers. J. Mol. Struct. 1999, 479, 201–218. [Google Scholar] [CrossRef]
- Di Noto, V.; Vittadello, M.; Yoshida, K.; Lavina, S.; Negro, E.; Furukawa, T. Broadband dielectric and conductivity spectroscopy of Li-ion conducting three-dimensional hybrid inorganic-organic networks as polymer electrolytes based on poly(ethylene glycol) 400, Zr and Al nodes. Electrochim. Acta 2011, 57, 192–200. [Google Scholar] [CrossRef]
- Cheng, N.-S. Formula for the Viscosity of a Glycerol−Water Mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef] [Green Version]
- Di Noto, V.; Piga, M.; Giffin, G.A.; Vezzù, K.; Zawodzinski, T.A. Interplay between mechanical, electrical, and thermal relaxations in nanocomposite proton conducting membranes based on nafion and a [(ZrO2)·(Ta2O5)0.119] core-shell nanofiller. J. Am. Chem. Soc. 2012, 134, 19099–19107. [Google Scholar] [CrossRef] [PubMed]



| Sample | Li/wt % | nLi/mol·kg−1 | nglycerol/mol·kg−1 | r = nLi/no | x = nLi/nglycerol | y = nGly/nGlyLi |
|---|---|---|---|---|---|---|
| 1 | 7.14 | 10.00 | 10.8 | 0.33 | 1.000 | 0 |
| 2 | 6.30 | 9.67 | 10.2 | 0.30 | 0.890 | 0.12 |
| 3 | 5.55 | 8.47 | 10.3 | 0.26 | 0.780 | 0.28 |
| 4 | 3.30 | 4.92 | 10.5 | 0.15 | 0.450 | 1.22 |
| 5 | 1.83 | 2.69 | 10.7 | 0.082 | 0.250 | 3.00 |
| 6 | 1.23 | 1.79 | 10.7 | 0.055 | 0.170 | 4.88 |
| 7 | 0.68 | 0.99 | 10.8 | 0.030 | 0.090 | 10.1 |
| 8 | 0.36 | 0.52 | 10.8 | 0.016 | 0.048 | 19.8 |
| 9 | 0.08 | 0.11 | 10.8 | 0.0033 | 0.010 | 99.0 |
| 10 | 0.04 | 0.06 | 10.8 | 0.0018 | 0.005 | 200 |
| 11 | 0 | 0 | 10.9 | 0 | 0 | ∞ |
| Glycerol, Gly (x = 0) | Lithium Glycerolate, GlyLi (x = 1.000) | ||||
|---|---|---|---|---|---|
| ωIR 1/cm−1 | ωRaman/cm−1 | ωIR/cm−1 | ωRaman/cm−1 | Assignment 2 | Ref. |
| 3422 (s) | − | 3386 (s) | − | νf(OH) | [19,23,24] |
| 3286 (vs) | 3350 (s) | 3267 (vs) | 3350 (s) | νHy(OH) | [19,23,24] |
| − | − | 3149 (m) | − | νg3Li(OH) | [this work] |
| − | − | 3071 (w) | − | νg2Li(OH) | [this work] |
| − | 2966 (wv) | − | 2967 (w) | νg2,a(CH) | [this work] |
| 2931 (m) | 2945 (vs) | 2929 (m) | 2945 (vs) | νg1,a(CH) | [19,23,24] |
| − | 2909 (w) | − | 2909 (s) | νg2,s(CH) | [this work] |
| − | 2885 (vs) | − | 2885 (vs) | νg1,s(CH) | [19,23,24] |
| 2879 (m) | 2874 (m) | 2878 (m) | 2876 (m) | νg3,a(CH) | [19,23,24] |
| − | − | 2867 (m) | − | νg3,a(CH) | [this work] |
| 2844 (m) | 2831 (s) | 2842 (w) | − | νg3,s(CH) | [this work] |
| 2800 (w) | − | − | − | νg3,s(CH) | [this work] |
| 2754 (vw) | 2741 (vw) | 2695 (vw) | 2736 (w) | νs(CH of C2) | [19,23,24] |
| 2727 (vw) | − | − | − | νs(CH of C2) | [this work] |
| − | − | 2504 (vw) | − | ν(CH) | [this work] |
| − | 1465 (s) | − | 1464 (s) | sr(CH2) | [19,23,24] |
| 1416 (s) | − | 1435 (s) | − | δip(OH),ω(CH2) | [19,23,24] |
| 1323 (m) | 1313 (vw) | 1336 (m) | 1313 (vw) | δip(OH),ω(CH) | [19,23,24] |
| 1210 (w) | 1253 (v) | 1225 (vs) | 1253 (v) | δip(OH),ω(CH) | [19,23,24] |
| 1108 (vs) | 1112 (s) | 1111 (m) | 1112 (s) | νg3,sec(C–C–O) | [19,23,24] |
| 1088 (w) | − | 1091 (w) | − | νg1,sec(C–C–O) | [this work] |
| 1075 (w) | − | 1073 (w) | − | νg2,sec(C–C–O) | [this work] |
| 1054 (m) | − | 1054 (m) | − | νg3,pri(C–C–O) | [this work] |
| 1029 (vs) | 1055 (s) | 1034 (vs) | 1055 (s) | νg1,pri(C–C–O) | [19,23,24] |
| 995 (s) | 975 (w) | 998 (sh) | 975 (w) | νa(CO),T | [19,23,24] |
| 977 (s) | − | 980 (s) | − | νs(CO),T | [this work] |
| 924 (m) | 922 (m) | 925 (m) | 922 (m) | νa(CO),G | [19,23,24] |
| 908 (vw) | − | 909 (vw) | − | νs(CO),G | [this work] |
| 866 (w) | − | 867 (w) | − | νa,pri(C–C–C) | [this work] |
| 849 (m) | 850 (s) | 850 (m) | 850 (s) | νs,pri(C–C–C) | [19,23,24] |
| 819 (vw) | 820 (m) | 820 (vw) | 820 (m) | νpri(C–C–C) | [19,23,24] |
| 639 (m, sh) | 673 (w) | 630 (m, sh) | 673 (w) | δ(C–C–O) | [19,23,24] |
| 550 (s) | 548 (w) | 563 (w) | 548 (w) | φ(C–C) | [19,25] |
| 488 (s) | 484 (m) | 493 (m) | 484 (m) | ρ(C–C–O) | [19,25] |
| 413 (m) | 413 (m) | ρ(C–C–O) | [19,25] | ||
| 327 (vw) | 327 (vw) | φ(C–C), δ(C–C–O) | [19] | ||
| x | Group | Coordination Geometry 1 | Tdec. 2/°C | Tg 3/°C | ΔHer 4/J·g−1 |
|---|---|---|---|---|---|
| 0 | A | g1 | 195.09 | −78.51 | −2.476 |
| 0.005 | A | g1 | 195.03 | −78.22 | −2.267 |
| 0.010 | A | g1 | 190.38 | −78.53 | −2.521 |
| 0.048 | A | g1 | 190.19 | −76.18 | −1.877 |
| 0.090 | A | g1 | 188.95 | −76.17 | −2.057 |
| 0.170 | B | g2.1 and g.2.2 | 172.77 | −72.35 | −2.096 |
| 0.250 | B | g2.1 and g.2.2 | 174.79 | −69.07 | −2.077 |
| 0.450 | B | g2.1 and g.2.2 | 170.14 | −66.15 | −1.84 |
| 0.780 | C | g3 | 191.34 | −64.93 | −1.637 |
| 0.890 | C | g3 | 177.09 | −63.04 | −3.483 |
| 1.000 | C | g3 | 185.42 | −42.95 | −3.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagot, G.; Tonello, S.; Vezzù, K.; Di Noto, V. A New Glass-Forming Electrolyte Based on Lithium Glycerolate. Batteries 2018, 4, 41. https://doi.org/10.3390/batteries4030041
Pagot G, Tonello S, Vezzù K, Di Noto V. A New Glass-Forming Electrolyte Based on Lithium Glycerolate. Batteries. 2018; 4(3):41. https://doi.org/10.3390/batteries4030041
Chicago/Turabian StylePagot, Gioele, Sara Tonello, Keti Vezzù, and Vito Di Noto. 2018. "A New Glass-Forming Electrolyte Based on Lithium Glycerolate" Batteries 4, no. 3: 41. https://doi.org/10.3390/batteries4030041






