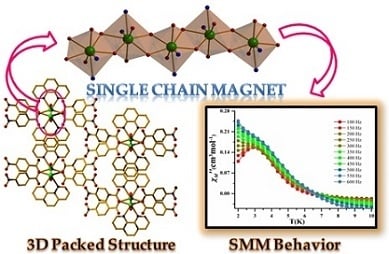
Field Induced Slow Magnetic Relaxation in a Non Kramers Tb(III) Based Single Chain Magnet
Abstract
:1. Introduction
2. Result and Discussion
2.1. Synthesis and Spectral Characterization
2.2. IR Spectroscopy
2.3. PXRD and Thermal Stability
2.4. Magnetic Properties
2.4.1. Direct Current Magnetic Susceptibility
2.4.2. Dynamic Magnetic Measurement
2.5. Theoretical Calculations
2.5.1. Exchange Interaction Calculations
2.5.2. Ab Initio Calculations
2.6. Photoluminescence
3. Materials and Methods
3.1. Starting Materials
3.2. Preparation of the Complex
3.3. X-ray Data Collection and Structure Refinement
3.4. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Wang, Z.M.; Gao, S. Strategies towards single-chain magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.N.; Tang, J.K. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Goswami, S.; Mondal, A.K.; Konar, S. Nanoscopic molecular magnets. Inorg. Chem. Front. 2015, 2, 687–712. [Google Scholar] [CrossRef]
- Dey, B.; Roy, S.; Mondal, A.K.; Santra, A.; Konar, S. Zero Field SMM Behavior and Magnetic Refrigeration in Rare Heterometallic Double Stranded Helicates of Cu2Ln2 (Ln = Dy, Tb, Gd). Eur. J. Inorg. Chem. 2018, 2018, 2429–2436. [Google Scholar] [CrossRef]
- Adhikary, A.; Sheikh, J.A.; Biswas, S.; Konar, S. Synthesis, crystal structure and study of magnetocaloric effect and single molecular magnetic behaviour in discrete lanthanide complexes. Dalton Trans. 2014, 43, 9334–9343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Ishikawa, R.; Breedlove, B.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clerac, R. Single-Chain Magnets and related systems. Struct. Bond. 2015, 164, 143–184. [Google Scholar]
- Mondal, A.K.; Jena, H.S.; Malviya, A.; Konar, S. Lanthanide-Directed Fabrication of Four Tetranuclear Quadruple Stranded Helicates Showing Magnetic Refrigeration and Slow Magnetic Relaxation. Inorg. Chem. 2016, 55, 5237–5244. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A toolbox of building blocks, linkers and crystallisation methods used to generate single-chain magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Liddle, S.T.; van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, A.; Jena, H.S.; Khatua, S.; Konar, S. Synthesis and Characterization of Two Discrete Ln10 Nanoscopic Ladder-Type Cages: Magnetic Studies Reveal a Significant Cryogenic Magnetocaloric Effect and Slow Magnetic Relaxation. Chem. Asian J. 2014, 9, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Parmar, V.; Konar, S. Modulating the Slow Relaxation Dynamics of Binuclear Dysprosium(III) Complexes through Coordination Geometry. Magnetochemistry 2016, 2, 35. [Google Scholar] [CrossRef]
- Bogani, L.; Vindigni, A.; Sessoli, R.; Gatteschi, D. Single chain magnets: Where to from here? J. Mater. Chem. 2008, 18, 4750–4758. [Google Scholar] [CrossRef]
- Jassal, A.K.; Sran, B.S.; Suffren, Y.; Bernot, K.; Pointillart, F.; Cador, O.; Hundal, G. Structural diversity and photo-physical and magnetic properties of dimeric to 1D polymeric coordination polymers of lighter lanthanide(iii) dinitrobenzoates. Dalton Trans. 2018, 47, 4722–4732. [Google Scholar] [CrossRef]
- Jassal, A.K.; Aliaga-Alcalde, N.; Corbella, M.; Aravena, D.; Ruiz, E.; Hundal, G. Neodymium 1D systems: Targeting new sources for field-induced slow magnetization relaxation. Dalton Trans. 2015, 44, 15774–15778. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Le Natur, F.; Cador, O.; Pointillart, F.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Guizouarn, T.; Le Guennic, B.; Bernot, K. Experimental and theoretical evidence that electrostatics governs easy-axis orientation in DyIII-based molecular chains. ChemComm 2014, 50, 13346–13348. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A Family of Rare-Earth-Based Single Chain Magnets: Playing with Anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Hu, P.; Wang, J.; Li, L. Slow magnetic relaxation and field-induced metamagnetism in nitronyl nitroxide-Dy(iii) magnetic chains. Dalton Trans. 2015, 44, 4560–4567. [Google Scholar] [CrossRef]
- Liu, T.-F.; Fu, D.; Gao, S.; Zhang, Y.-Z.; Sun, H.-L.; Su, G.; Liu, Y.-J. An Azide-Bridged Homospin Single-Chain Magnet: [Co(2,2′-bithiazoline)(N3)2]n. J. Am. Chem. Soc. 2003, 125, 13976–13977. [Google Scholar] [CrossRef]
- Bernot, K.; Luzon, J.; Sessoli, R.; Vindigni, A.; Thion, J.; Richeter, S.; Leclercq, D.; Larionova, J.; van der Lee, A. The Canted Antiferromagnetic Approach to Single-Chain Magnets. J. Am. Chem. Soc. 2008, 130, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-B.; Wang, B.-W.; Pan, F.; Wang, Z.-M.; Gao, S. Stringing Oxo-Centered Trinuclear [MnIII3O] Units into Single-Chain Magnets with Formate or Azide Linkers. Angew. Chem. Int. Ed. 2007, 46, 7388–7392. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-Nitronyl Nitroxide Chains as Molecular Magnetic Nanowires. Angew. Chem. Int. Ed. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Miyasaka, H.; Madanbashi, T.; Sugimoto, K.; Nakazawa, Y.; Wernsdorfer, W.; Sugiura, K.-I.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet Behaviour in an Alternated One-Dimensional Assembly of a MnIII Schiff-Base Complex and a TCNQ Radical. Chem. Eur. J. 2006, 12, 7028–7040. [Google Scholar] [CrossRef] [PubMed]
- Clérac, R.; Miyasaka, H.; Yamashita, M.; Coulon, C. Evidence for Single-Chain Magnet Behaviour in a MnIII−NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zuo, J.-L.; Gao, S.; Song, Y.; Zhou, H.-C.; Zhang, Y.-Z.; You, X.-Z. The Observation of Superparamagnetic Behaviour in Molecular Nanowires. J. Am. Chem. Soc. 2004, 126, 8900–8901. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Cangussu, D.; Dul, M.-C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.L.M.; Muñoz, M.C.; Ruiz-García, R.; et al. A Metallacryptand-Based Manganese(II)–Cobalt(II) Ferrimagnet with a Three-Dimensional Honeycomb Open-Framework Architecture. Angew. Chem. 2008, 120, 4279–4284. [Google Scholar] [CrossRef]
- Choi, S.W.; Kwak, H.Y.; Yoon, J.H.; Kim, H.C.; Koh, E.K.; Hong, C.S. Intermolecular Contact-Tuned Magnetic Nature in One-Dimensional 3d−5d Bimetallic Systems: From a Metamagnet to a Single-Chain Magnet. Inorg. Chem. 2008, 47, 10214–10216. [Google Scholar] [CrossRef]
- Harris, T.D.; Bennett, M.V.; Clérac, R.; Long, J.R. [ReCl4(CN)2]2−: A High Magnetic Anisotropy Building Unit Giving Rise to the Single-Chain Magnets (DMF)4MReCl4(CN)2 (M = Mn, Fe, Co, Ni). J. Am. Chem. Soc. 2010, 132, 3980–3988. [Google Scholar] [CrossRef]
- Costes, J.-P.; Clemente-Juan, J.M.; Dahan, F.; Milon, J. Unprecedented (Cu2Ln)n Complexes (Ln = Gd3+, Tb3+): A New “Single Chain Magnet”. Inorg. Chem. 2004, 43, 8200–8202. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Zhang, J.; Chen, Y.-M.; Yang, G.-Y. Porous Lanthanide–Organic Open Frameworks with Helical Tubes Constructed from Interweaving Triple-Helical and Double-Helical Chains. Angew. Chem. 2005, 117, 5964–5967. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Robaschik, P.; Fronk, M.; Toader, M.; Klyatskaya, S.; Ganss, F.; Siles, P.F.; Schmidt, O.G.; Albrecht, M.; Hietschold, M.; Ruben, M.; et al. Tuning the magneto-optical response of TbPc2 single molecule magnets by the choice of the substrate. J. Mater. Chem. C 2015, 3, 8039–8049. [Google Scholar] [CrossRef]
- Urdampilleta, M.; Klayatskaya, S.; Ruben, M.; Wernsdorfer, W. Magnetic Interaction Between a Radical Spin and a Single-Molecule Magnet in a Molecular Spin-Valve. ACS Nano 2015, 9, 4458–4464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Wang, J.; Li, L. Unusual Gd–nitronyl nitroxide antiferromagnetic coupling and slow magnetic relaxation in the corresponding Tb analogue. Dalton Trans. 2015, 44, 13890–13896. [Google Scholar] [CrossRef] [PubMed]
- Aguilà, D.; Barrios, L.A.; Luis, F.; Repollés, A.; Roubeau, O.; Teat, S.J.; Aromí, G. Synthesis and Properties of a Family of Unsymmetric Dinuclear Complexes of LnIII (Ln = Eu, Gd, Tb). Inorg. Chem. 2010, 49, 6784–6786. [Google Scholar] [CrossRef]
- Ganivet, C.R.; Ballesteros, B.; de la Torre, G.; Clemente-Juan, J.M.; Coronado, E.; Torres, T. Influence of Peripheral Substitution on the Magnetic Behavior of Single-Ion Magnets Based on Homo- and Heteroleptic TbIII Bis(phthalocyaninate). Chem. Eur. J. 2013, 19, 1457–1465. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A Tetranuclear 3d−4f Single Molecule Magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef]
- Bartolomé, E.; Bartolomé, J.; Arauzo, A.; Luzón, J.; Badía, L.; Cases, R.; Luis, F.; Melnic, S.; Prodius, D.; Shova, S.; Turta, C. Antiferromagnetic single-chain magnet slow relaxation in the {Tb(α-fur)3}n polymer with non-Kramers ions. J. Mater. Chem. C. 2016, 4, 5038–5050. [Google Scholar] [CrossRef]
- Pasca, E.; Roscilde, T.; Evangelisti, M.; Burzurí, E.; Luis, F.; de Jongh, L.J.; Tanase, S. Realization of the one-dimensional anisotropic XY model in a Tb(III)-W(V) chain compound. Phys. Rev. B: Condens. Matter Mater. Phys. B 2012, 85, 184434. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Mei, X.; Hu, P.; Tian, H.; Li, L.; Liao, D.; Sutter, J.-P. Slow magnetic relaxation and antiferromagnetic ordering in a one-dimensional nitronyl nitroxide–Tb(iii) chain. New J. Chem. 2012, 36, 2088–2093. [Google Scholar] [CrossRef]
- Hu, P.; Wang, X.; Ma, Y.; Wang, Q.; Li, L.; Liao, D. A new family of Ln–radical chains (Ln = Nd, Sm, Gd, Tb and Dy): Synthesis, structure, and magnetic properties. Dalton Trans. 2014, 43, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, E.; Bartolomé, J.; Arauzo, A.; Luzón, J.; Cases, R.; Fuertes, S.; Sicilia, V.; Sánchez-Cano, A.I.; Aporta, J.; Melnic, S.; et al. Heteronuclear {TbxEu1−x} furoate 1D polymers presenting luminescent properties and SMM behavior. J. Mater. Chem. 2018, 6, 5286–5299. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Liu, S.-B.; Yang, Y.-L.; Wang, P.-Z.; Zhang, A.-J.; Peng, Y. A Terbium(III)-Complex-Based On–Off Fluorescent Chemosensor for Phosphate Anions in Aqueous Solution and Its Application in Molecular Logic Gates. ACS Appl. Mater. Interfaces. 2015, 7, 4415–4422. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Luminescent chiral lanthanide(iii) complexes as potential molecular probes. Dalton Trans. 2009, 44, 9692–9707. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Vieru, V.; Xu, N.; Gao, C.; Wang, B.-W.; Shi, W.; Chibotaru, L.F.; Gao, S.; Cheng, P.; et al. Coupling Influences SMM Properties for Pure 4 f Systems. Chem. Eur. J. 2018, 24, 6079–6086. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Zhang, S.; Li, R.; Zhang, Y.-Q.; Yang, E.-C.; Zhao, X.-J. A Rare Water and Hydroxyl-Extended One-Dimensional Dysprosium(III) Chain and Its Magnetic Dilution Effect. Inorg. Chem. 2017, 56, 9594–9601. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bauza, A.; Frontera, A.; Schaper, F.; Banik, R.; Purkayastha, A.; Reddy, B.M.; Sridhar, B.; Drew, M.G.B.; Das, S.K.; et al. Structural diversity and non-covalent interactions in Cd(II) and Zn(II) complexes derived from 3,5-dinitrobenzoic acid and pyridine: Experimental and theoretical aspects. Inorg. Chim. Acta 2016, 440, 38–47. [Google Scholar] [CrossRef]
- Roy, S.; Oyarzabal, I.; Vallejo, J.; Cano, J.; Colacio, E.; Bauza, A.; Frontera, A.; Kirillov, A.M.; Drew, M.G.B.; Das, S. Two Polymorphic Forms of a Six-Coordinate Mononuclear Cobalt(II) Complex with Easy-Plane Anisotropy: Structural Features, Theoretical Calculations, and Field-Induced Slow Relaxation of the Magnetization. Inorg. Chem. 2016, 55, 8502–8513. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Bera, S.P.; Mondal, A.; Roy, S.; Dey, B.; Santra, A.; Konar, S. 3D isomorphous lanthanide coordination polymers displaying magnetic refrigeration, slow magnetic relaxation and tunable proton-conduction. Dalton Trans. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J.A.; Goswami, S.; Konar, S. Modulating the magnetic properties by structural modification in a family of Co-Ln (Ln = Gd, Dy) molecular aggregates. Dalton Trans. 2014, 43, 14577–14585. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Adhikary, A.; Jena, H.S.; Konar, S. Lanthanide based coordination polymers chill, relax under magnetic field and also fluoresce. Dalton Trans. 2013, 42, 9813–9817. [Google Scholar] [CrossRef]
- Biswas, S.; Chakraborty, J.; Singh Parmar, V.; Bera, S.P.; Ganguli, N.; Konar, S. Channel-Assisted Proton Conduction Behavior in Hydroxyl-Rich Lanthanide-Based Magnetic Metal–Organic Frameworks. Inorg. Chem. 2017, 56, 4956–4965. [Google Scholar] [CrossRef]
- Dey, A.; Das, S.; Palacios, M.A.; Colacio, E.; Chandrasekhar, V. Single-Molecule Magnet and Magnetothermal Properties of Two-Dimensional Polymers Containing Heterometallic [Cu5Ln2] (Ln = GdIII and DyIII) Motifs. Eur. J. Inorg. Chem. 2018, 1645–1654. [Google Scholar] [CrossRef]
- She, S.; Gong, L.; Wang, B.; Yang, Y.; Lei, Q.; Liu, B.; Su, G. Slow magnetic relaxation in a two-dimensional dysprosium(III) coordination polymer. Inorg. Chem. Commun. 2016, 70, 18–21. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Hu, P.; Cao, J.-F.; Zhao, Y.-H.; Wu, Y.-N.; Zhu, Y.-X.; Su, Y.; Wang, C. Magnetic relaxation in mononuclear Tb and Dy complexes involving chelate nitronyl nitroxide ligand. Polyhedron 2018, 155, 375–381. [Google Scholar] [CrossRef]
- Mondal, P.; Dey, B.; Roy, S.; Bera, S.P.; Nasani, R.; Santra, A.; Konar, S. Field-Induced Slow Magnetic Relaxation and Anion/Solvent Dependent Proton Conduction in Cobalt(II) Coordination Polymers. Cryst. Growth Des. 2018, 18, 6211–6220. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajeshkumar, T.; Rajaraman, G.; Murugavel, R. Is a strong axial crystal-field the only essential condition for a large magnetic anisotropy barrier? The case of non-Kramers Ho(iii) versus Tb(iii ). Dalton Trans. 2018, 47, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Vieru, V.; Ungur, L.; Cemortan, V.; Sukhanov, A.; Baniodeh, A.; Anson, C.E.; Powell, A.K.; Voronkova, V.; Chibotaru, L. Magnetization Blocking in Fe2IIIDy2III Molecular Magnets: Ab initio Calculations and EPR. Chem. Eur. J. 2018, 0. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Wielechowski, D.P.; Vieru, V.; Chilton, N.F.; Moubaraki, B.; Abrahams, B.F.; Chibotaru, L.F.; Murray, K.S. A {CrIII2DyIII2} Single-Molecule Magnet: Enhancing the Blocking Temperature through 3d Magnetic Exchange. Angew. Chem. Int. Ed. 2013, 52, 12014–12019. [Google Scholar] [CrossRef]
- Vieru, V.; Ungur, L.; Chibotaru, L.F. Key Role of Frustration in Suppression of Magnetization Blocking in Single-Molecule Magnets. J. Phys. Chem. Lett. 2013, 4, 3565–3569. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Lanthanide-Containing Molecular and Supramolecular Polymetallic Functional Assemblies. Chem. Rev. 2002, 102, 1897–1928. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Hovinen, J.; Guy, P.M. Bioconjugation with Stable Luminescent Lanthanide(III) Chelates Comprising Pyridine Subunits. Bioconjugate Chem. 2009, 20, 404–421. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Goswami, S.; Konar, S. Influence of the coordination environment on slow magnetic relaxation and photoluminescence behavior in two mononuclear dysprosium(iii) based single molecule magnets. Dalton Trans. 2015, 44, 5086–5094. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Gopakumar, N.; Bünzli, J.C.G.; Scopelliti, R.; Kim, H.K.; Reddy, M.L.P. Brilliant Photoluminescence and Triboluminescence from Ternary Complexes of DyIII and TbIII with 3-Phenyl-4-propanoyl-5-isoxazolonate and a Bidentate Phosphine Oxide Coligand. Inorg. Chem. 2013, 52, 8750–8758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.-Z.; Wu, J.; Lv, D.-Y.; Tang, Y.; Zhu, K.; Wu, J. Lanthanide coordination polymers based on 5-(2′-carboxylphenyl) nicotinate: Syntheses, structure diversity, dehydration/hydration, luminescence and magnetic properties. Dalton Trans. 2013, 42, 4822–4830. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Biswas, S.; Tomar, K.; Konar, S. Tuning the Magnetoluminescence Behavior of Lanthanide Complexes Having Sphenocorona and Cubic Coordination Geometries. Eur. J. Inorg. Chem. 2016, 2016, 2774–2782. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.J.; Howard, A.K.; Puschmann, H. A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with ShelXL. Acta Cryst. C 2015, 27, 30–38. [Google Scholar]









| Complex 1 | Fitting (cm−1) | From Calculations (cm−1) |
|---|---|---|
| Exchange Coupling Parameter (J) | +0.06 | |
|
Crystal Field Parameters (, , ) | (−566, 155, 5.4) | (−356, 78.8, 4.6) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharwar, A.K.; Mondal, A.; Konar, S. Field Induced Slow Magnetic Relaxation in a Non Kramers Tb(III) Based Single Chain Magnet. Magnetochemistry 2018, 4, 59. https://doi.org/10.3390/magnetochemistry4040059
Kharwar AK, Mondal A, Konar S. Field Induced Slow Magnetic Relaxation in a Non Kramers Tb(III) Based Single Chain Magnet. Magnetochemistry. 2018; 4(4):59. https://doi.org/10.3390/magnetochemistry4040059
Chicago/Turabian StyleKharwar, Ajit Kumar, Arpan Mondal, and Sanjit Konar. 2018. "Field Induced Slow Magnetic Relaxation in a Non Kramers Tb(III) Based Single Chain Magnet" Magnetochemistry 4, no. 4: 59. https://doi.org/10.3390/magnetochemistry4040059