Synthesis, Characterization and Magnetic Studies of a Tetranuclear Manganese(II/IV) Compound Incorporating an Amino-Alcohol Derived Schiff Base
Abstract
:1. Introduction
2. Results and Discussion
2.1. Discussion
2.2. FT-IR Spectroscopy
2.3. Electronic Spectrum
2.4. Crystal Structure Description
2.5. Electrochemistry
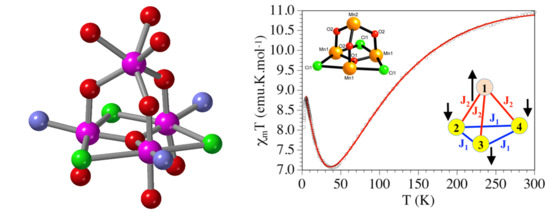
2.6. Magnetic Study
3. Materials and Methods
3.1. Starting Materials
3.2. Syntheses
3.2.1. Synthesis of ((3E)-3-((Z)-4-hydroxy-4-phenylbut-3-en-2-ylideneamino)propane-1,2-diol)
3.2.2. Synthesis of [MnIIMnIV3(μ-Cl)3(µ3-O)(L)3] (1)
3.3. Physical Measurements
3.4. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Reed, C.J.; Agapie, T. Thermodynamics of Proton and Electron Transfer in Tetranuclear Clusters with Mn–OH2/OH Motifs Relevant to H2O Activation by the Oxygen Evolving Complex in Photosystem II. J. Am. Chem. Soc. 2018, 140, 10900–10908. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Nayek, H.P.; Dehnen, S.; Powell, A.K.; Reedijk, J. Trigonal propeller-shaped [MnIII3MIINa] complexes (M = Mn, Ca): Structural and functional models for the dioxygen evolving centre of PS II. Dalton Trans. 2011, 40, 2699–2702. [Google Scholar] [CrossRef] [PubMed]
- Kanady, J.S.; Tsui, E.Y.; Day, M.W.; Agapie, T. A synthetic model of the Mn3Ca subsite of the oxygen-evolving complex in photosystem II. Science 2011, 333, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Gluz, N.; Christou, G. A bioinspired soluble manganese cluster as a water oxidation electrocatalyst with low overpotential. Nat. Catal. 2018, 1, 48–54. [Google Scholar] [CrossRef]
- Stamatatos, T.C.; Foguet-Albiol, D.; Stoumpos, C.C.; Raptopoulou, C.P.; Terzis, A.; Wernsdorfer, W.; Perlepes, S.P.; Christou, G. Initial example of a triangular single-molecule magnet from ligand-induced structural distortion of a [MnIII3O]7+ complex. J. Am. Chem. Soc. 2005, 127, 15380–15381. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.N.; Lan, Y.; AlDamen, M.A.; Zheng, Y.-Z.; Anson, C.E.; Powell, A.K. Effect of ligand substitution on the SMM properties of three isostructural families of double-cubane Mn4Ln2 coordination clusters. Dalton Trans. 2018, 47, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Shopov, D.Y.; Sharninghausen, L.S.; Sinha, S.B.; Borowski, J.E.; Mercado, B.Q.; Brudvig, G.W.; Crabtree, R.H. Synthesis of pyridine-alkoxide ligands for formation of polynuclear complexes. New J. Chem. 2017, 41, 6709–6719. [Google Scholar] [CrossRef]
- Semina, B.K.; Davletshinaa, L.N.; Seibertb, M.; Rubina, A.B. Creation of a 3Mn/1Fe cluster in the oxygen-evolving complex of photosystem II and investigation of its functional activity. J. Photochem. Photobiol. B Biol. 2018, 178, 192–200. [Google Scholar] [CrossRef]
- Paul, S.; Neese, F.; Pantazis, D.A. Structural models of the biological oxygen-evolving complex: Achievements, insights, and challenges for biomimicry. Green Chem. 2017, 19, 2309–2325. [Google Scholar] [CrossRef]
- Alaimo, A.A.; Koumousi, E.S.; Cunha-Silva, L.; McCormick, L.J.; Teat, S.J.; Psycharis, V.; Raptopoulou, C.P.; Mukherjee, S.; Li, C.; Das Gupta, S.; et al. Structural Diversities in Heterometallic Mn-Ca Cluster Chemistry from the Use of Salicylhydroxamic Acid: {MnIII4Ca2}, {MnII/III6Ca2}, {MnIII/IV8Ca}, and {MnIII8Ca2} Complexes with Relevance to Both High- and Low-Valent States of the Oxygen-Evolving Complex. Inorg. Chem. 2017, 56, 10760–10774. [Google Scholar] [CrossRef]
- Melnic, S.; Shova, S.; Benniston, A.C.; Waddell, P.G. Evolution of manganese–calcium cluster structures based on nitrogen and oxygen donor ligands. CrystEngComm 2017, 19, 3674–3681. [Google Scholar] [CrossRef]
- Mondal, D.; Majee, M.C. Synthesis and Structural Characterization of a New High-valent Bis(oxo)-bridged Manganese(IV) Complex and its Catechol Oxidase Activity. Inorg. Chim. Acta 2017, 465, 70–77. [Google Scholar] [CrossRef]
- Zhang, S.; Hua, Y.; Chen, Z.; Zhang, S.; Hai, H. Manganese trinuclear clusters based on Schiff base: Synthesis, characterization, magnetic and electrochemiluminescence properties. Inorg. Chim. Acta 2018, 471, 530–536. [Google Scholar] [CrossRef]
- Savva, M.; Skordi, K.; Fournet, A.D.; Thuijs, A.E.; Christou, G.; Perlepes, S.P.; Papatriantafyllopoulou, C.; Tasiopoulos, A.J. Heterometallic MnIII4Ln2 (Ln = Dy, Gd, Tb) Cross-Shaped Clusters and Their Homometallic MnIII4MnII2 Analogues. Inorg. Chem. 2017, 56, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.A.; Abboud, K.A.; Christou, G. New mixed-valence MnII4MnIV clusters from an unusual ligand transformation. Polyhedron 2017, 122, 71–78. [Google Scholar] [CrossRef]
- Vaddypally, S.; Jovinelli, D.J.; McKendry, I.G.; Zdilla, M.J. Covalent Metal—Metal-Bonded Mn4 Tetrahedron Inscribed within a Four-Coordinate Manganese Cubane Cluster, as Evidenced by Unexpected Temperature-Independent Diamagnetism. Inorg. Chem. 2017, 56, 3733–3737. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Qiao, X.; Xie, C.-Z.; Ouyang, Y.; Xu, J. Synthesis, characterization, and magnetochemical properties of two Mn4 clusters derived from 2-pyridinecarboxaldehyde Schiff base ligands. J. Coord. Chem. 2017, 70, 1207–1220. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Part B; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE; Version 2.3; University of Barcelona: Barcelona, Spain; Hebrew University of Jerusalem: Jerusalem, Israel, 2013. [Google Scholar]
- Ding, C.; Gao, C.; Ng, S.; Wang, B.; Xie, Y. Polynuclear Complexes with Alkoxo and Phenoxo Bridges from In Situ Generated Hydroxy-Rich Schiff Base Ligands: Syntheses, Structures, and Magnetic Properties. Chem. Eur. J. 2013, 19, 9961–9972. [Google Scholar] [CrossRef] [PubMed]
- Escuer, A.; Mayans, J.; Font-Bardia, M.; Gorecki, M.; Di Bari, L. Syntheses, structures, and chiroptical and magnetic properties of chiral clusters built from Schiff bases: A novel [MnIIMnIII6NaI2] core. Dalton Trans. 2017, 46, 6514–6517. [Google Scholar] [CrossRef] [PubMed]
- Mayans, J.; Font-Bardia, M.; Escuer, A. Triple Halide Bridges in Chiral MnII2MnIII6NaI2 Cages: Structural and Magnetic Characterization. Inorg. Chem. 2018, 57, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thorp, H.H. Bond Valence Sum Analysis of Metal-Ligand Bond Lengths in Metalloenzymes and Model Complexes. 2. Refined Distances and Other Enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Brown, I.D. VALENCE: A program for calculating bond valences. J. Appl. Cryst. 1996, 29, 479–489. [Google Scholar] [CrossRef]
- Goodson, P.A.; Oki, A.R.; Glerup, J.; Hodgson, D.J. Design, synthesis, and characterization of bis(μ-oxo)dimanganese(III,III) complexes. Steric and electronic control of redox potentials. J. Am. Chem. Soc. 1990, 112, 6248–6254. [Google Scholar] [CrossRef]
- Pradeep, C.P.; Htwe, T.; Zacharias, P.S.; Das, S.K. First structurally characterized optically active mononuclear Mn(IV) complex: Synthesis, crystal structure and properties of [MnIVL2] {H2L = S-(−)-2-[(2-hydroxy-1-phenylethylimino)methyl]phenol}. New J. Chem. 2004, 28, 735–739. [Google Scholar] [CrossRef]
- Borrás-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. High-Nuclearity Magnetic Clusters: Generalized Spin Hamiltonian and Its Use for the Calculation of the Energy Levels, Bulk Magnetic Properties, and Inelastic Neutron Scattering Spectra. Inorg. Chem. 1999, 38, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. MAGPACK1 A package to calculate the energy levels, bulk magnetic properties, and inelastic neutron scattering spectra of high nuclearity spin clusters. J. Comput. Chem. 2001, 22, 985–991. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers: New York, NY, USA, 1993. [Google Scholar]
- Stoicescu, L.; Jeanson, A.; Duhayon, C.; Tesouro-Vallina, A.; Boudalis, A.K.; Costes, J.; Tuchagues, J. Synthesis of CdTe Nanocrystals Using Te Nanorods as the Te Source and the Formation of Microtubes with Red Fluorescence. Inorg. Chem. 2007, 46, 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Lecren, L.; Clerac, R. Synthesis, structure and magnetism of new polynuclear transition metal aggregates assembled with Schiff-base ligand and anionic N-donor ligands. J. Mol. Struct. 2008, 890, 339–345. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]






| Bond Lengths (Å) | |||
| Mn1-Cl1 | 2.663(3) | Mn2-O2 | 2.134(5) |
| Mn1-O1 | 1.8873(11) | Mn2-O3 | 2.115(8) |
| Mn1-O2 | 1.892(5) | Mn2-O3 | 2.227(7) |
| Mn1-O11 | 1.890(4) | Mn2-O31 | 2.228(7) |
| Mn1-N7 | 1.984(6) | ||
| Mn1-Cl11 | 2.719(3) | ||
| Bond Angles (°) | |||
| Cl1-Mn1-Cl11 | 164.90(8) | O21-Mn2-O2 | 93.30(18) |
| O1-Mn1-Cl1 | 83.38(6) | O21-Mn2-O3 | 147.6(4) |
| O1-Mn1-Cl11 | 81.83(7) | O22-Mn2-O3 | 116.6(4) |
| O1-Mn1-O2 | 93.2(3) | O2-Mn2-O3 | 74.1(2) |
| O1-Mn1-O11 | 94.3(2) | O32-Mn2-O3 | 85.0(3) |
| O1-Mn1-N7 | 174.7(3) | O31-Mn2-O3 | 85.0(3) |
| O2-Mn1-Cl1 | 91.20(18) | O32-Mn2-O31 | 85.0(3) |
| O2-Mn1-Cl11 | 92.64(17) | Mn1-Cl1-Mn12 | 74.35(8) |
| O2-Mn1-N7 | 82.1(2) | Mn11-O1-Mn12 | 119.00(7) |
| O11-Mn1-Cl1 | 91.87(16) | Mn1-O2-Mn2 | 125.5(2) |
| O11-Mn1-Cl11 | 86.22(16) | ||
| O11-Mn1-O2 | 172.2(2) | ||
| O11-Mn1-N7 | 90.3(2) | ||
| N7-Mn1-Cl1 | 99.1(2) | ||
| N7-Mn1-Cl11 | 95.9(2) | ||
| Geometry | Symmetry | Mn1 | Mn2 |
|---|---|---|---|
| HP-6 | D6h | 33.617 | 36.993 |
| PPY-6 | C5v | 25.913 | 18.084 |
| OC-6 | Oh | 3.592 | 8.406 |
| TPR-6 | D3h | 14.942 | 2.205 |
| JPPY-6 | C5v | 28.178 | 22.087 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandy, M.; Shit, S.; Rosair, G.; Gómez-García, C.J. Synthesis, Characterization and Magnetic Studies of a Tetranuclear Manganese(II/IV) Compound Incorporating an Amino-Alcohol Derived Schiff Base. Magnetochemistry 2018, 4, 57. https://doi.org/10.3390/magnetochemistry4040057
Nandy M, Shit S, Rosair G, Gómez-García CJ. Synthesis, Characterization and Magnetic Studies of a Tetranuclear Manganese(II/IV) Compound Incorporating an Amino-Alcohol Derived Schiff Base. Magnetochemistry. 2018; 4(4):57. https://doi.org/10.3390/magnetochemistry4040057
Chicago/Turabian StyleNandy, Madhusudan, Shyamapada Shit, Georgina Rosair, and Carlos J. Gómez-García. 2018. "Synthesis, Characterization and Magnetic Studies of a Tetranuclear Manganese(II/IV) Compound Incorporating an Amino-Alcohol Derived Schiff Base" Magnetochemistry 4, no. 4: 57. https://doi.org/10.3390/magnetochemistry4040057