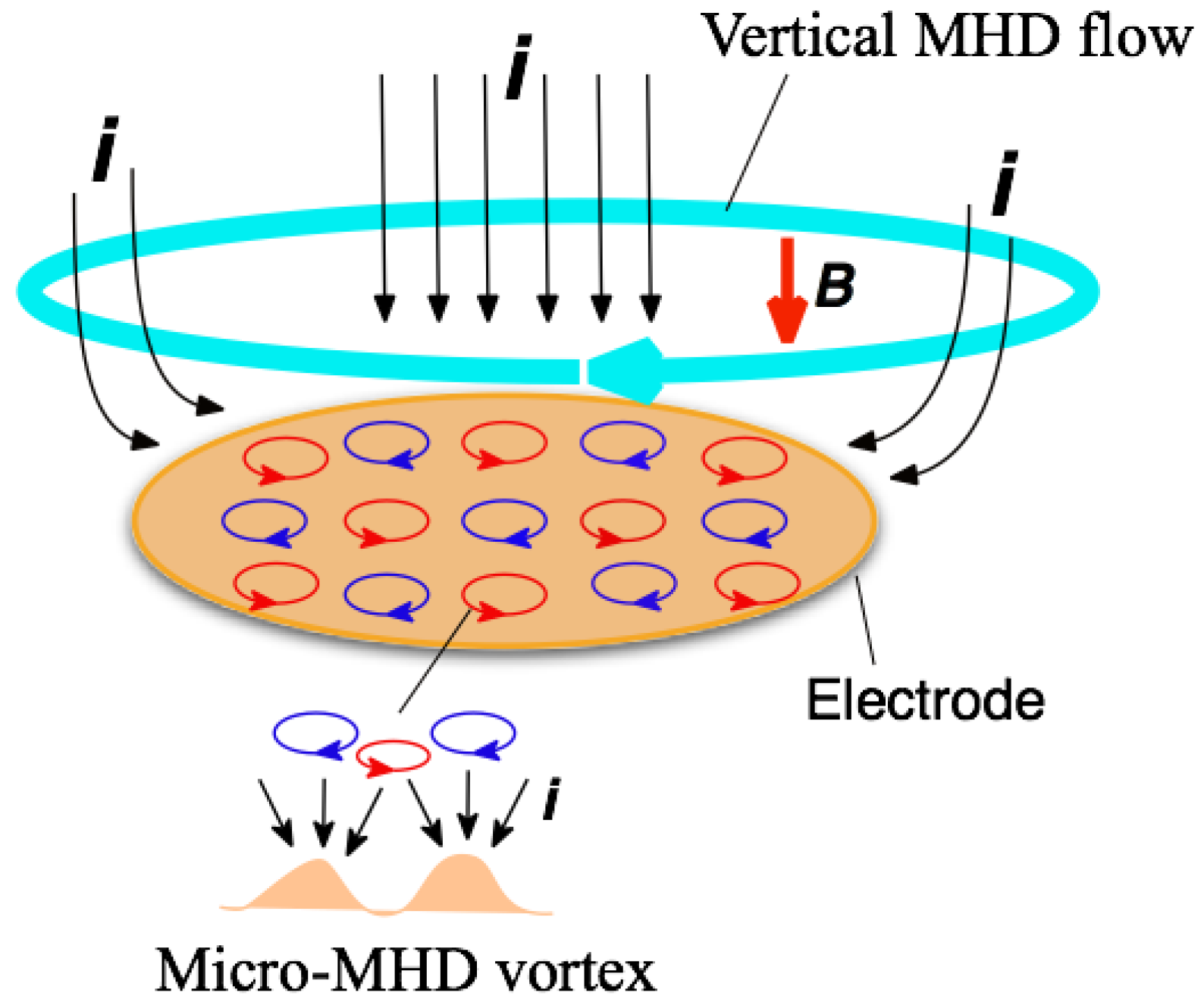
2.1. Effects of Tube Walls on Surface Chirality in MED
The potentiostatic MED of copper films were conducted in a CuSO
4 + H
2SO
4 aqueous solution with the configuration of
Figure 2a. The magnetic field of 5 T was imposed perpendicularly to a working electrode surface and parallel (+5 T) or antiparallel (−5 T) to the ionic currents. The working electrode was embedded in a Teflon
® tube and the height (
h) of tube wall was varied from 2 to 12 mm. In this configuration, the vertical MHD flow is excited around the entrance of tube, and then it penetrates into the inside of tube with damping, as shown in
Figure 2b. Thus, the influence of vertical MHD flows on the film surfaces depends on the wall height.
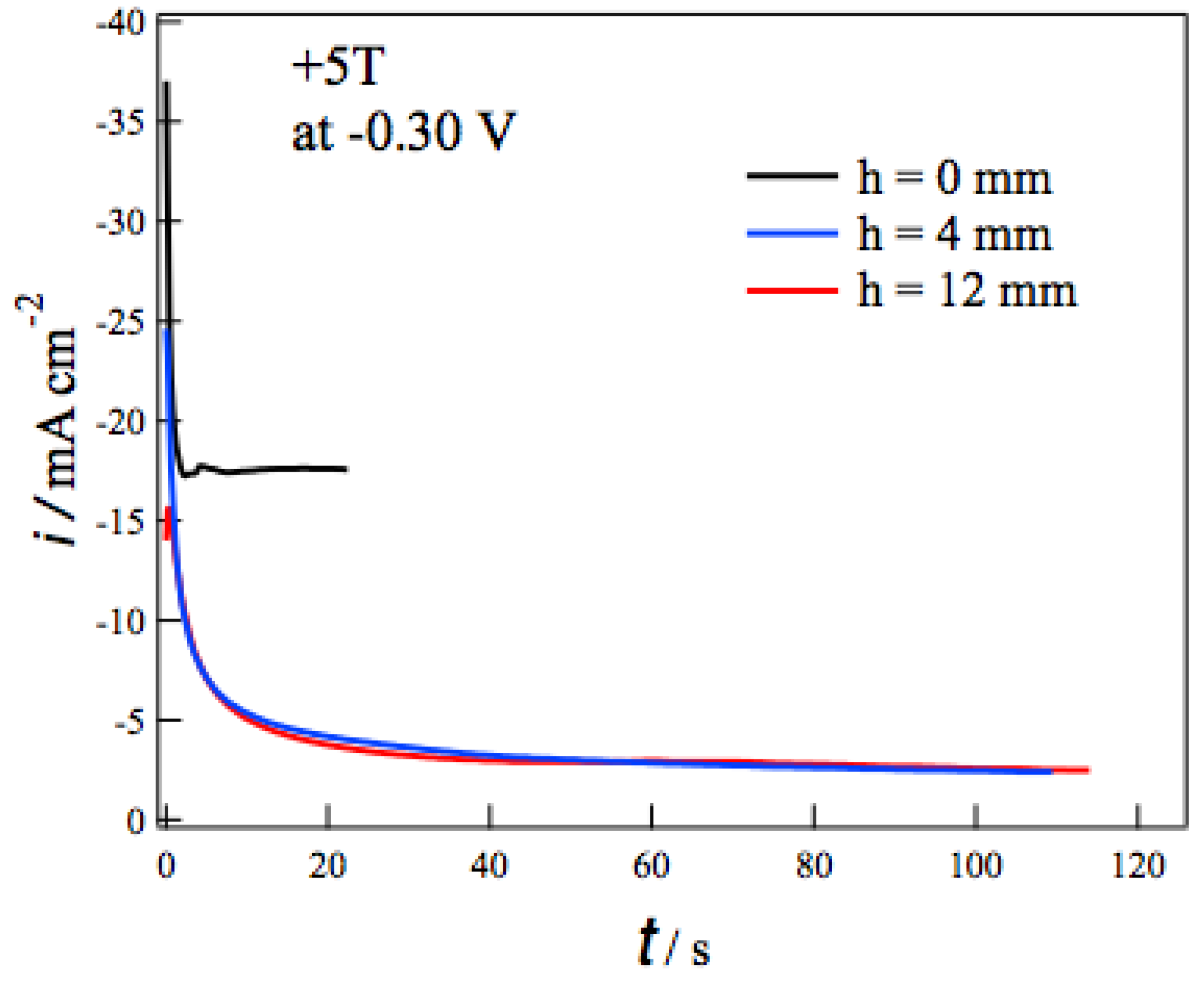
Figure 3 shows the current-time (
i-
t) curves during the MED at −0.3 V in +5 T without (
h = 0 mm) and with the tube wall at
h = 4 and 12 mm. In each
i-t curve the deposition current decreases promptly at the initial 10 seconds and then reaches constant values. The self-organized state like
Figure 1 would be formed in such a steady state [
13]. Comparing
i-t curves at different
h, the tube walls drastically reduce the deposition currents. The tube walls suppress the vertical MHD flow effectively and result in the reduction of the mass-transfer to the electrode surface. It should be noted that the deposition currents at
h = 4 mm are almost the same as those at
h = 12 mm. This fact suggests that the penetrating vertical MHD flow does not reach the electrode surface even at
h = 4 mm. Similar
h dependence of
i-t curves was observed at deposition potentials from −0.05 to −0.45 V.
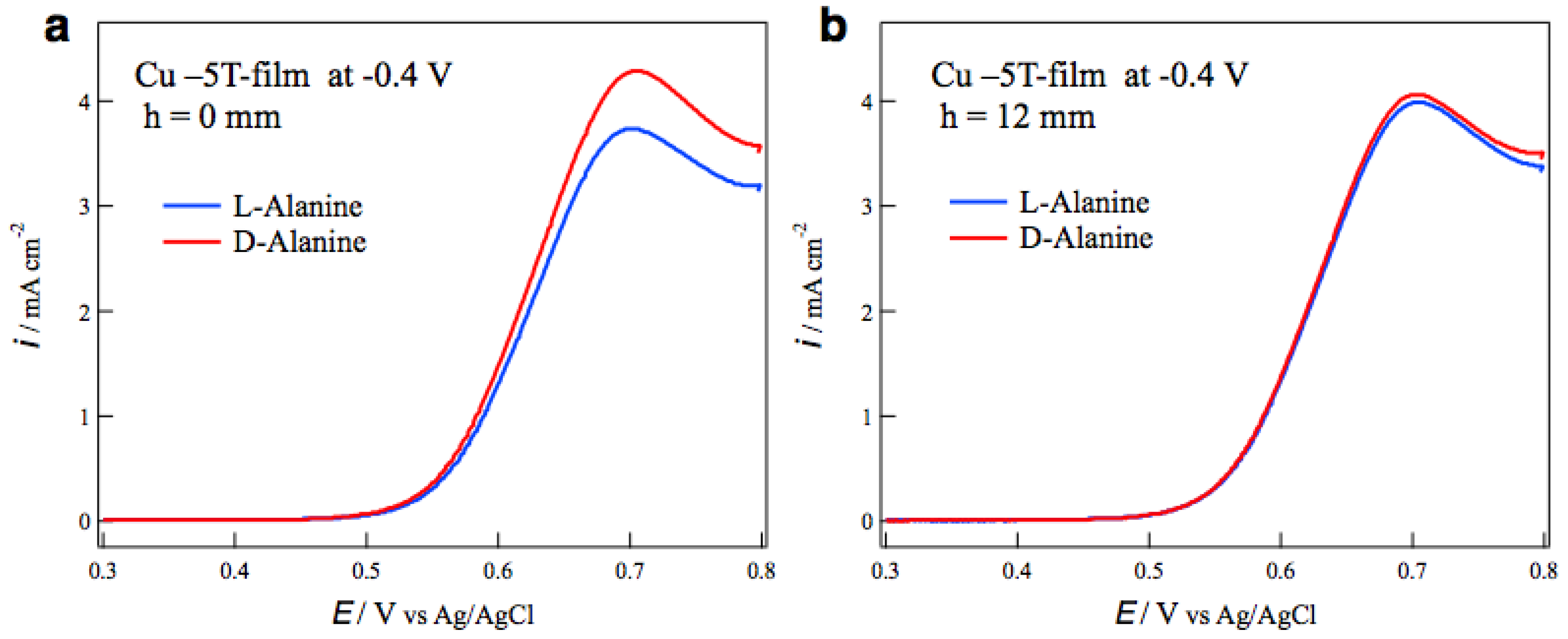
The surface chirality of MED films was examined by the measurements of voltammograms of alanine enantiomers on the MED film electrodes in a NaOH aqueous solution. The most typical result of tube wall effect on the surface chirality is seen in
Figure 4.
Figure 4a shows the voltammograms of L- and D-alanines on the MED −5T-films prepared at −0.4 V without tube wall. The peaks around 0.7 V correspond to the oxidation currents of alanine molecules [
14], and the peak currents show chiral behavior for the enantiomers. The peak current of D-alanine is greater than that of L-alanine, namely this MED film exhibits D-active chirality.
Figure 4b shows the voltammograms of alanine on the MED films prepared with tube wall at
h = 12 mm in the same MED conditions as those in
Figure 4a. The voltammograms are almost coincident between the enantiomers, namely this MED film has an achiral surface.
To evaluate the chirality in voltammograms, an enantiomeric excess (
ee) ratio can be defined as
where
ipL and
ipD represent the peak currents of L- and D-alanines, respectively. The positive sign of
ee ratio represents L-activity, and the negative one represents D-activity.
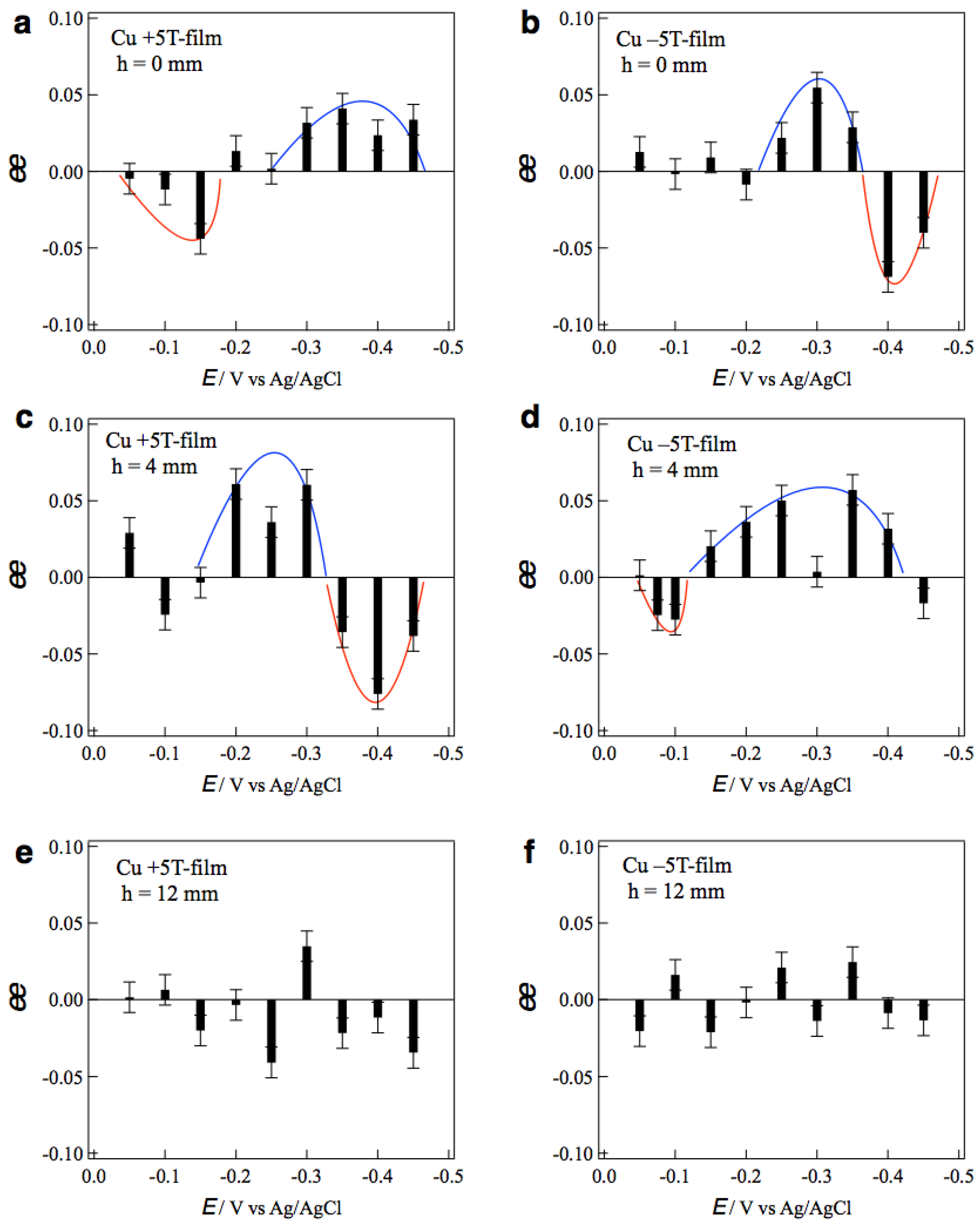
Figure 5a,b show the
ee ratio profiles of the +5T-film and −5T-film electrodes, respectively, prepared without tube wall at various deposition potentials. The +5T-film exhibits D-activity in the potentials of 0.0~−0.15 V, and L-activity in the more negative potentials of −0.30~−0.45 V (
Figure 5a). On the contrary, the −5T-film exhibits L-activity at −0.25~−0.35 V and D-activity at −0.40~−0.45 V. These results demonstrate that the chiral sign depends on both the deposition potential and the polarity of magnetic field.
The potential dependence of chirality implies that the rate-limiting step is responsible for the chiral sign. The voltammogram of Cu
2+ at 5 T showed that the rate-limiting step changes around −0.3 V [
15]. The electrode process is a kinetic-controlled at more positive potentials and mass-transfer-controlled at more negative potentials. Such a change in the rate-limiting step corresponds to the change in the chiral signs of MED films. In the mass-transfer-controlled process, the copper ions arriving at the film surface immediately react and deposit there. On the other hand, in the kinetic-controlled process, most copper ions arriving on the film surface cannot deposit instantaneously and diffuse on the surface. The micro-MHD effects on these copper ion behaviors could result in the change in chiral sign. Similar changes in the chiral signs were reported in the galvanostatic MED of Cu [
15], where the chiral sign depended on the deposition currents and schematic explanation was described.
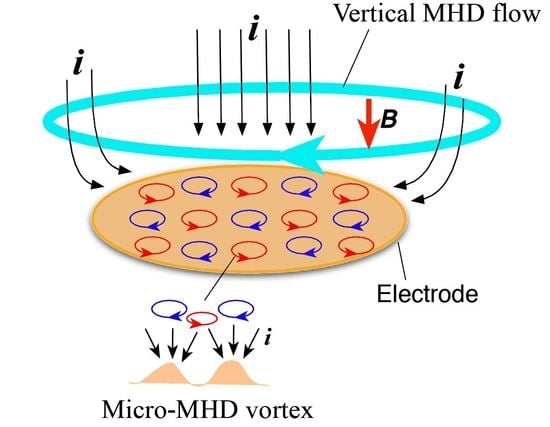
As for the magnetic-field-polarity dependence, the chiral sign is opposite for the reversal of the magnetic field. Such odd magnetic field dependence [
16] can be simply understood by considering the direction of vertical MHD flow. As mentioned before, under the vertical MHD flow, the cyclonic micro-MHD vortices become stable, and the anticyclonic ones become unstable [
10,
11]. The reversal of magnetic field reverses the vertical MHD flow and the stable micro-MHD vortices, as a result, it changes the chiral signs of the MED films.
Figure 5c,d show the
ee profiles of the +5T-film and −5T-film electrodes, respectively, prepared with tube wall at
h = 4 mm. It is surprising that the
ee profile of the +5T-film (
Figure 5c) is almost the same as that of the −5T-film without wall (
Figure 5b), and the profile of the −5T-film (
Figure 5d) is the same as that of the +5T-film without wall (
Figure 5a). These facts imply that the direction of vertical MHD flows around the electrode surface at
h = 4 mm is opposite to that without wall.
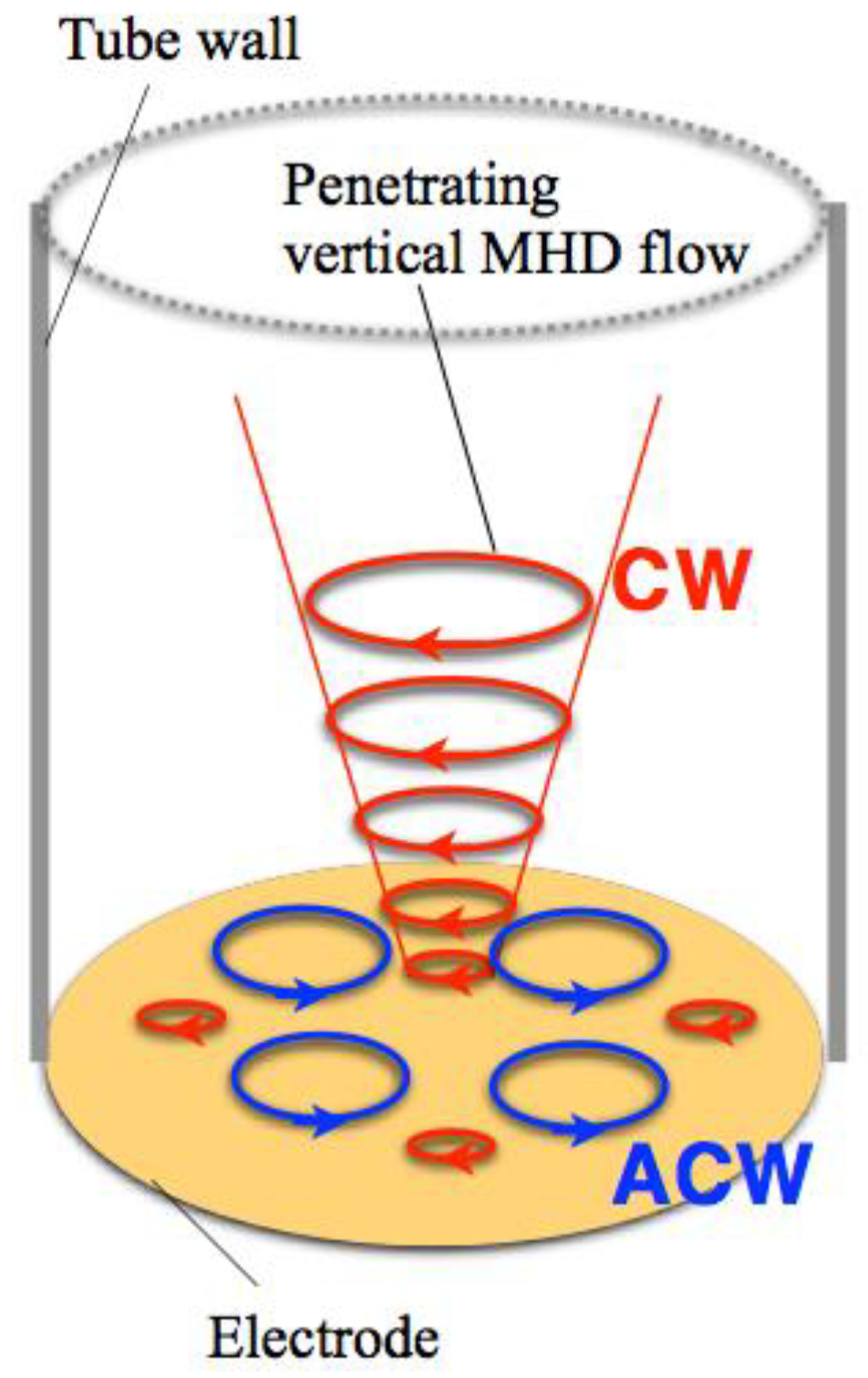
Figure 6 shows a possible scheme for such reversal of vertical MHD flow. The same deposition currents at
h = 4 and 12 mm in
Figure 3 suggest that the penetrating vertical MHD flow at
h = 4 mm does not reach the electrode surface directly. However, when the front of penetrating flow would reach near the electrode surface, the reverse flows could be induced around the penetrating flow such that the adjoining flows never conflict with each other. If the penetrating flow is clockwise (CW), then the anti-clockwise (ACW) flows would be induced. Such induced flows could cause opposite chiral signs. This finding would be useful as a new simple method to control surface chirality.
The effects of tube wall were examined with taller walls.
Figure 5e,f show the
ee profiles of the +5T-film and −5T-film electrodes, respectively, prepared with the tube wall at
h = 12 mm. No clear tendency in chiral signs is seen in both profiles, indicating that surface chirality almost disappeared on both MED films. The penetrating MHD flow could not reach the electrode surface and could not affect the micro-MHD vortices. These results demonstrate that the vertical MHD flow plays the significant role in the chiral surface formation; breaking of the symmetrical state in micro-MHD vortices.
2.2. Effects of Tube Walls on Surface Chirality in MEE
The self-organized MHD vortices like
Figure 1 can be also formed in etching processes in magnetic fields, and the chiral surface formation was reported in the MEE processes [
17]. The influence of tube wall on the surface chirality was examined in the MEE of copper films. The electrode configuration of MEE was the same as that in MED (
Figure 2) except the ionic current direction. The MEE of copper films was conducted galvanostatically in a CuSO
4 + H
2SO
4 aqueous solution in the magnetic fields of ±5 T. The most typical result of tube wall effect is seen in
Figure 7.
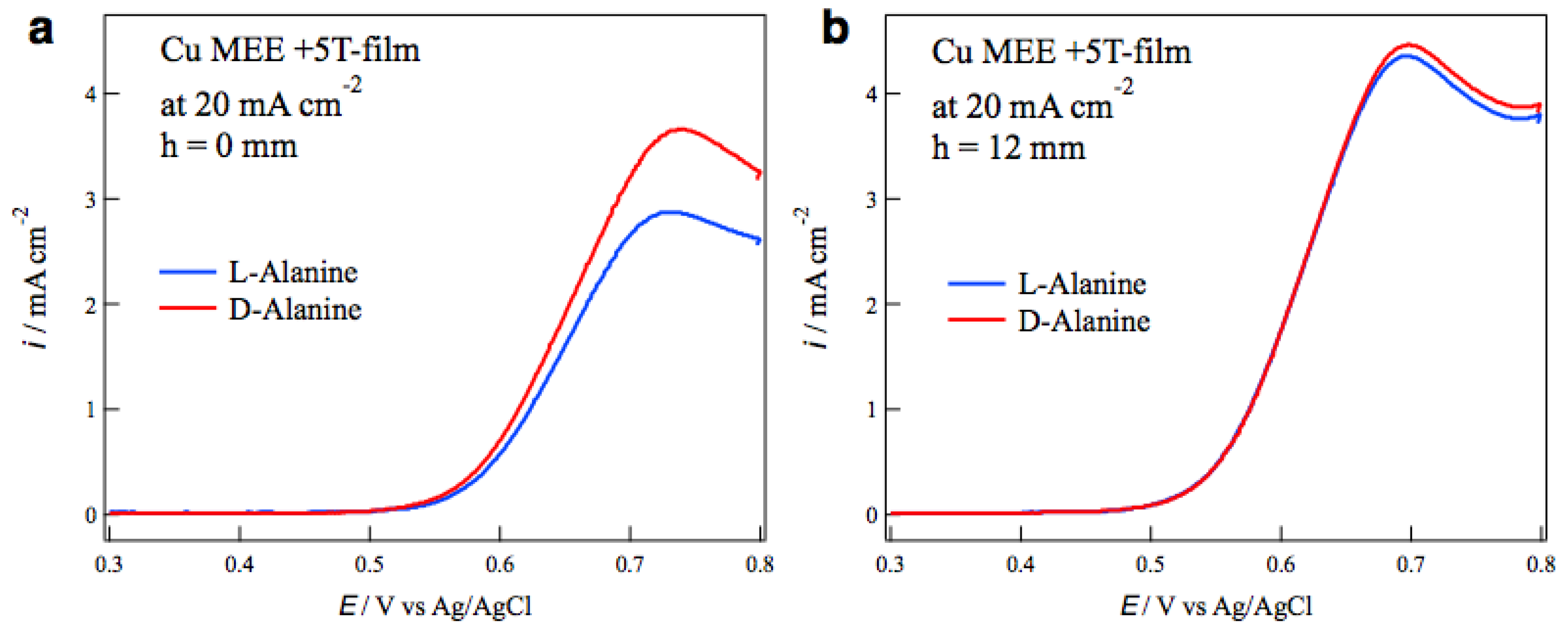
Figure 7a,b show the voltammograms of alanine enantiomers on the MEE +5T-film electrodes prepared without and with tube wall at
h = 12 mm, respectively, at the etching current of 20 mA cm
−2. While the MEE film without tube wall exhibits D-active behavior clearly, the MEE film with tube wall shows achiral behavior.
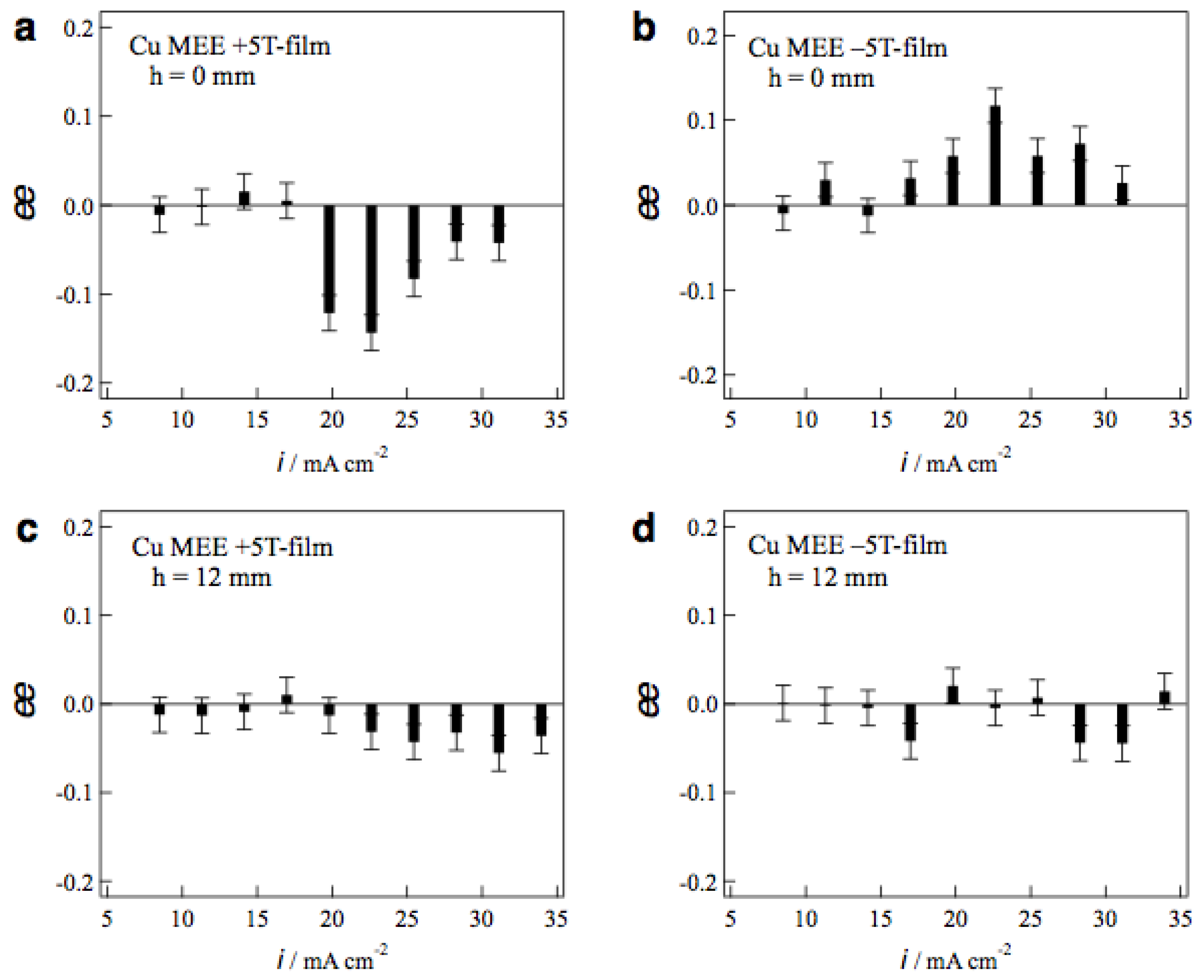
Figure 8a,b show the
ee ratio profiles of MEE +5T-film and −5T-film electrodes, respectively, prepared without tube wall at various etching currents. The MEE +5T-film exhibits D-activity in the etching currents of 20−30 mA cm
−2, and the −5T-film exhibits the L-activity in the same current region. The chiral signs of MEE films represent clear odd magnetic field dependence.
Figure 8c,d shows the
ee ratio profiles of MEE +5T-film and −5T-film electrodes, respectively, prepared with tube wall at
h = 12 mm. The surface chirality almost disappears at whole etching currents on both films. This means that the penetrating vertical MHD flow does not reach the electrode surface at
h = 12 mm and it cannot break the symmetrical self-organized state of micro-MHD vortices.
The large ee ratios (~0.2) were observed occasionally in the MEE experiments with tube wall at h = 2 and 4 mm, however, the reproducibility of such large values was poor. In these conditions, the penetrating vertical MHD flow could reach the electrode surfaces directly and make strong influence on the micro-MHD vortices. However, such strong influence never produces stable self-organized states, resulting in the lack of reproducibility.