Low Field NMR Determination of pKa Values for Hydrophilic Drugs for Students in Medicinal Chemistry
Abstract
:1. Introduction
2. Results
2.1. Method Adoption
2.2. Student Experimental Results
3. Discussion
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. Prelab Exercise
4.3. Procedure
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zivkovic, A.; Bandolik, J.J.; Skerhut, A.J.; Coesfeld, C.; Prascevic, M.; Zivkovic, L.; Stark, H. Quantitative analysis of multicomponent mixtures of over-the-counter pain killer drugs by low-field NMR spectroscopy. J. Chem. Educ. 2017, 94, 121–125. [Google Scholar] [CrossRef]
- Zivkovic, A.; Bandolik, J.J.; Skerhut, A.J.; Coesfeld, C.; Zivkovic, N.; Raos, M.; Stark, H. Introducing students to NMR methods using low-field 1H NMR spectroscopy to determine the structure and the identity of natural amino acids. J. Chem. Educ. 2017, 94, 115–120. [Google Scholar] [CrossRef]
- Bonjour, J.L.; Pitzer, J.M.; Frost, J.A. Introducing high school students to NMR spectroscopy through percent composition determination using low-field spectrometers. J. Chem. Educ. 2015, 92, 529–533. [Google Scholar] [CrossRef]
- Danieli, E.; Perlo, J.; Blümich, B.; Casanova, F. Small magnets for portable NMR spectrometers. Angew. Chem. Int. Ed. 2010, 49, 4133–4135. [Google Scholar] [CrossRef] [PubMed]
- Periyannan, G.R.; Lawrence, B.A.; Egan, A.E. 1H NMR Spectroscopy-Based Configurational Analysis of Mono- and Disaccharides and Detection of β-Glucosidase Activity: An Undergraduate Biochemistry Laboratory. J. Chem. Educ. 2015, 92, 1244–1249. [Google Scholar] [CrossRef]
- Morton, J.G.; Joe, C.L.; Stolla, M.C.; Koshland, S.R.; Londergan, C.H.; Schofield, M.H. NMR Determination of Hydrogen Bond Thermodynamics in a Simple Diamide: A Physical Chemistry Experiment. J. Chem. Educ. 2015, 92, 1086–1090. [Google Scholar] [CrossRef]
- Mobley, T.A. NMR kinetics of the SN2 reaction between BuBr and I: An introductory organic chemistry laboratory exercise. J. Chem. Educ. 2015, 92, 534–537. [Google Scholar] [CrossRef]
- Simpson, A.J.; Mitchell, P.J.; Masoom, H.; Liaghati Mobarhan, Y.; Adamo, A.; Dicks, A.P. An oil spill in a tube: An accessible approach for teaching environmental nmr spectroscopy. J. Chem. Educ. 2015, 92, 693–697. [Google Scholar] [CrossRef]
- Gift, A.D.; Stewart, S.M.; Bokashanga, P.K. Experimental Determination of pKa Values by Use of NMR Chemical Shifts, Revisited. J. Chem. Educ. 2012, 89, 1458–1460. [Google Scholar] [CrossRef]
- Manallack, D.T. The pKa Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar] [CrossRef]
- Manallack, D.T.; Prankerd, R.J.; Yuriev, E.; Oprea, T.I.; Chalmers, D.K.; David, K. The significance of acid/base properties in drug discovery. Chem. Soc. Rev. 2014, 42, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Chillistone, S.; Hardman, J.G. Factors affecting drug absorption and distribution. Anaesth. Intensive Care Med. 2014, 15, 309–313. [Google Scholar] [CrossRef]
- Repice, M.D.; Sawyer, R.K.; Hogrebe, M.C.; Brown, P.J.; Luesse, S.B.; Gealy, D.J.; Frey, R.F. Talking Through the Problems: A Study of Discourse in Peer-Led Small Groups. Chem. Educ. Res. Pract. 2016, 17, 555–568. [Google Scholar] [CrossRef]
- Handloser, C.S.; Chakrabarty, M.R.; Mosher, M.W. Experimental Determination of pKa Values by Use of NMR Chemical Shifts. J. Chem. Ed. 1973, 50, 510–511. [Google Scholar] [CrossRef]
- Krȩzel, A.; Bal, W. A formula for correlating pKa values determined in D2O and H2O. J. Inorg. Biochem. 2004, 98, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ebel, S.; Glaser, E.; Kantelberg, R.; Reyer, B. Auswertung digitaler Titrationskurven nach dem Tubbs-Verfahren. Fresenius’ Z. Anal. Chem. 1982, 312, 604–607. [Google Scholar] [CrossRef]
- Perrin, D.D. Dissociation contants of inorganic acids and bases in aqueous solution. Pure Appl. Chem. 1969, 20, 133–236. [Google Scholar] [CrossRef]
- Becker, C.; Dressman, J.B.; Amidon, G.L.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.; Stavchansky, S.; Barends, D.M. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Isoniazid. J. Pharm. Sci. 2007, 96, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.D.A.D.D.; Da Costa, D.O.; Da Rocha Pita, S.S.; Semaan, F.S. Potentiometric and conductimetric studies of chemical equilibria for pyridoxine hydrochloride in aqueous solutions: Simple experimental determination of pKa values and analytical applications to pharmaceutical analysis. Eclet. Quim. 2010, 35, 81–86. [Google Scholar] [CrossRef]
- Friebolin, H. Ein- und Zweidimensionale NMR-Spektroskopie, 5th ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-33492-6. [Google Scholar]
- Tubbs, C.F. Determination of Potentiometric Titration Inflection Point by Concentric Arcs Method. Anal. Chem. 1954, 26, 1670. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
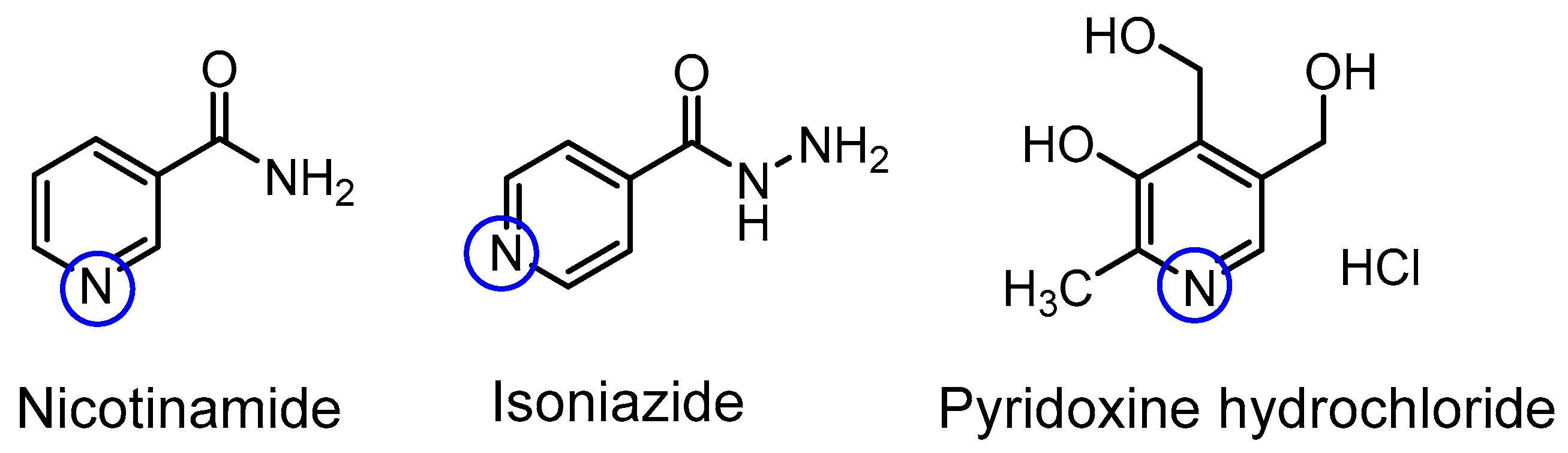
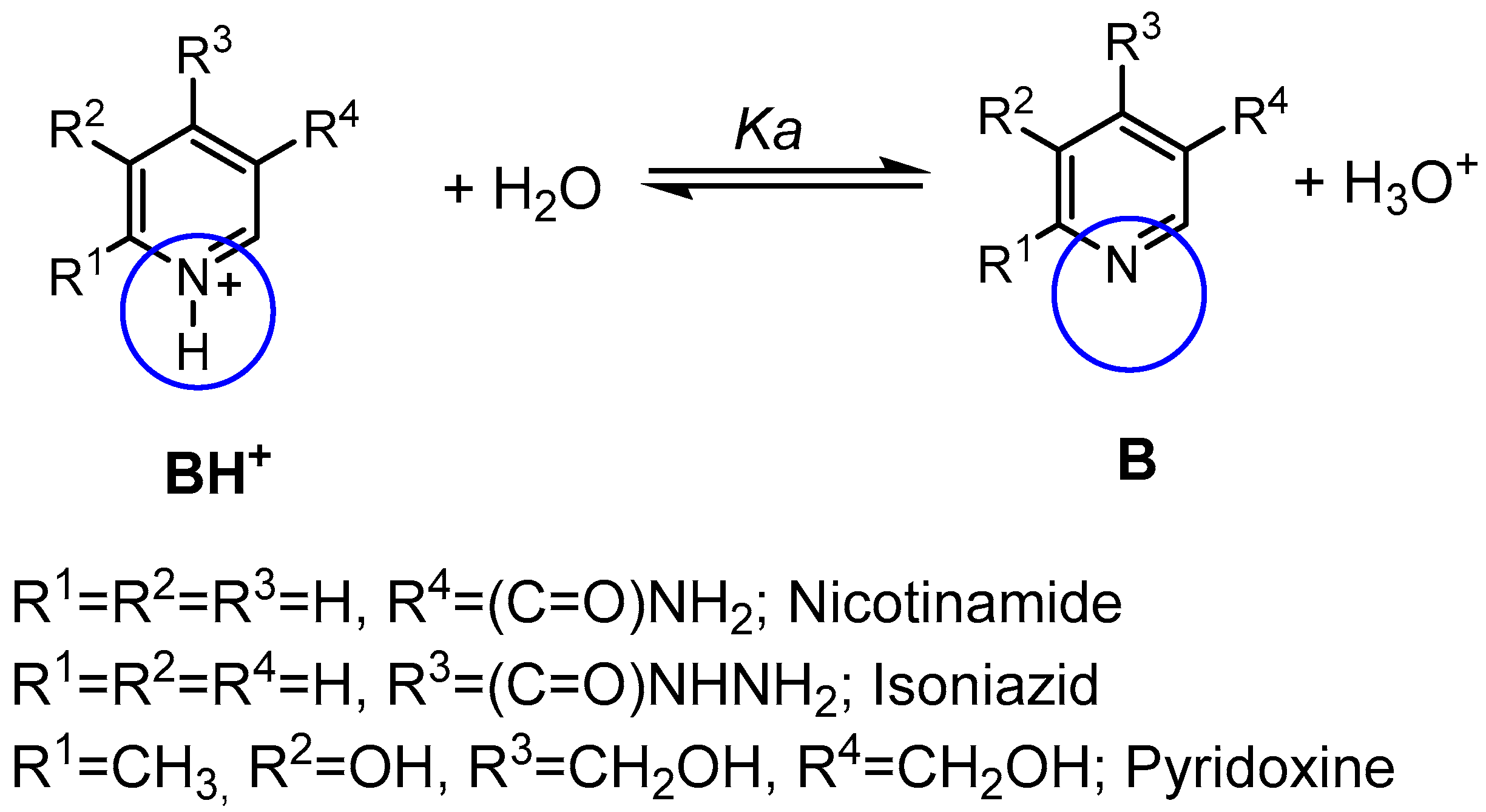
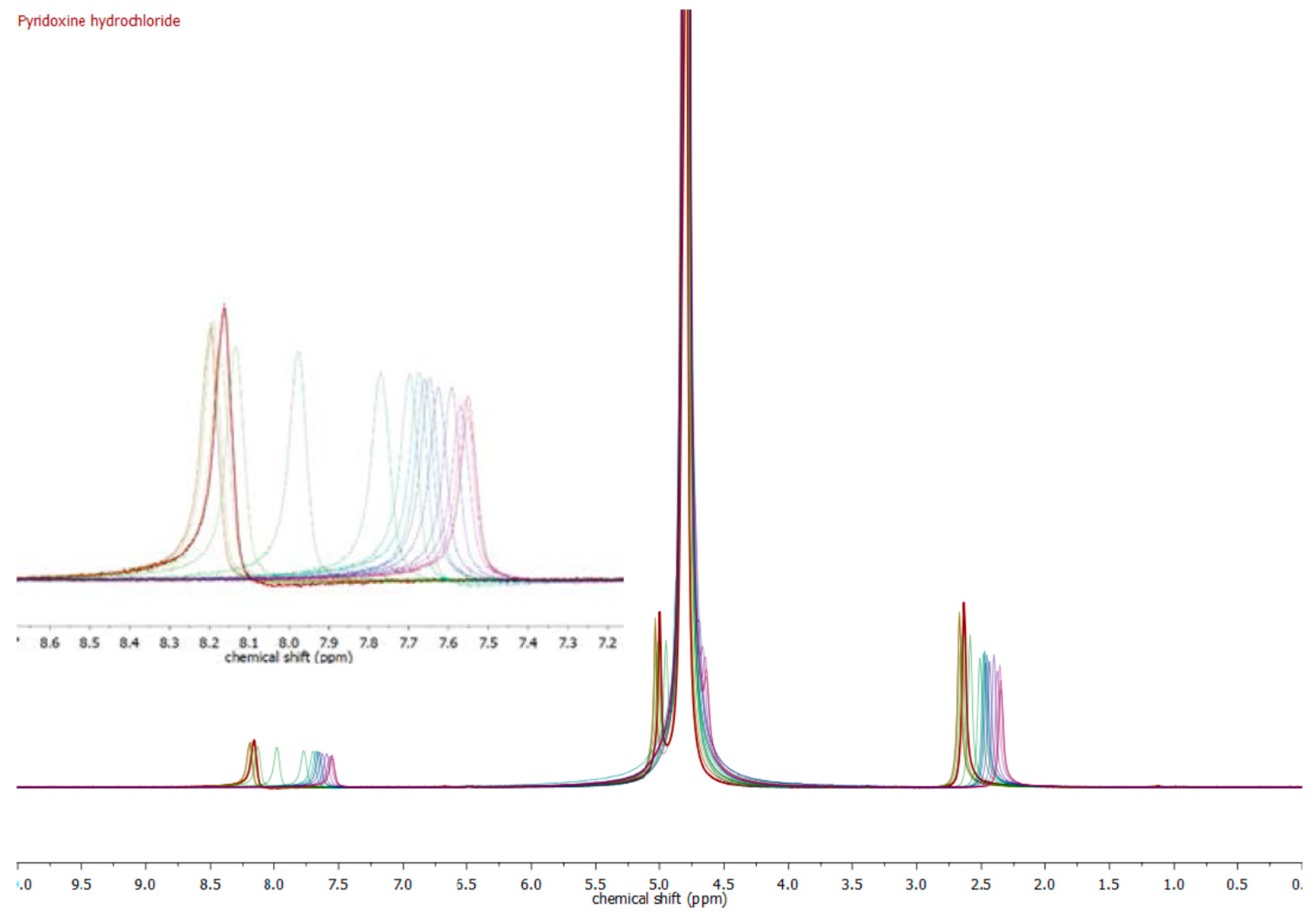

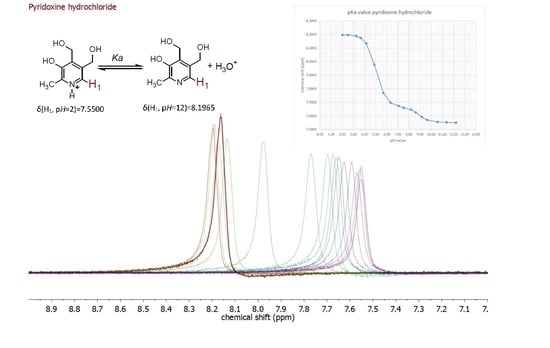
| Compound | pKa (NMR Method) b | pKa c (Literature) | pKa (Web-Based Calculation) d |
|---|---|---|---|
| Nicotinamide | 3.54 ± 0.02 | 3.35 [17] | 3.60 |
| Isoniazid | 3.65 ± 0.19 | 3.50 [18] | 3.20 |
| Pyridoxine a | 5.24 ± 0.15 | 5.20 [19] | 5.60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, A.; Bandolik, J.J.; Skerhut, A.J.; Coesfeld, C.; Raos, M.; Zivkovic, N.; Nikolic, V.; Stark, H. Low Field NMR Determination of pKa Values for Hydrophilic Drugs for Students in Medicinal Chemistry. Magnetochemistry 2017, 3, 29. https://doi.org/10.3390/magnetochemistry3030029
Zivkovic A, Bandolik JJ, Skerhut AJ, Coesfeld C, Raos M, Zivkovic N, Nikolic V, Stark H. Low Field NMR Determination of pKa Values for Hydrophilic Drugs for Students in Medicinal Chemistry. Magnetochemistry. 2017; 3(3):29. https://doi.org/10.3390/magnetochemistry3030029
Chicago/Turabian StyleZivkovic, Aleksandra, Jan Josef Bandolik, Alexander Jan Skerhut, Christina Coesfeld, Miomir Raos, Nenad Zivkovic, Vlastimir Nikolic, and Holger Stark. 2017. "Low Field NMR Determination of pKa Values for Hydrophilic Drugs for Students in Medicinal Chemistry" Magnetochemistry 3, no. 3: 29. https://doi.org/10.3390/magnetochemistry3030029