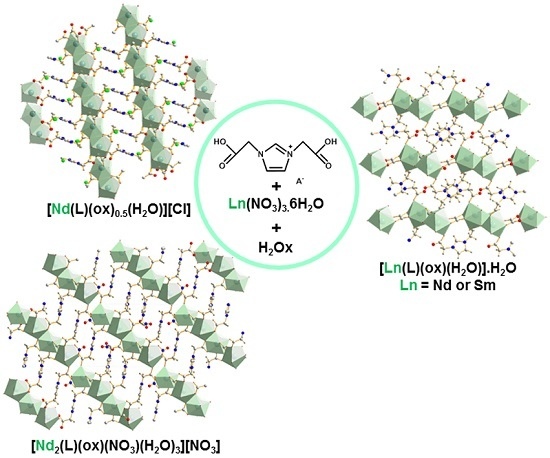
Elaboration of Luminescent and Magnetic Hybrid Networks Based on Lanthanide Ions and Imidazolium Dicarboxylate Salts: Influence of the Synthesis Conditions
Abstract
:1. Introduction
2. Results and Discussion
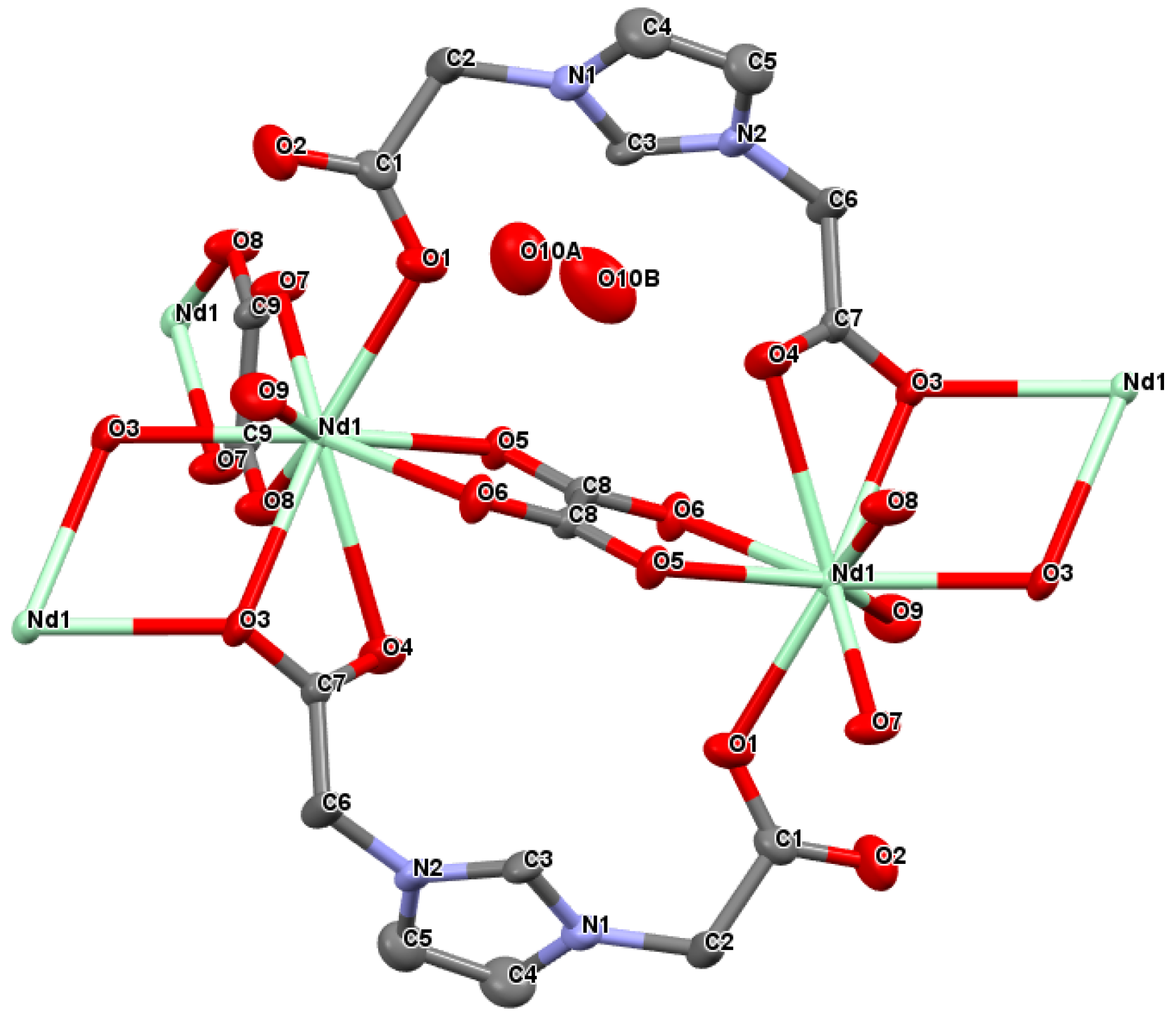
2.1. Crystal Structure of [Ln(L)(ox)(H2O)]·H2O with Ln = Nd3+ or Sm3+
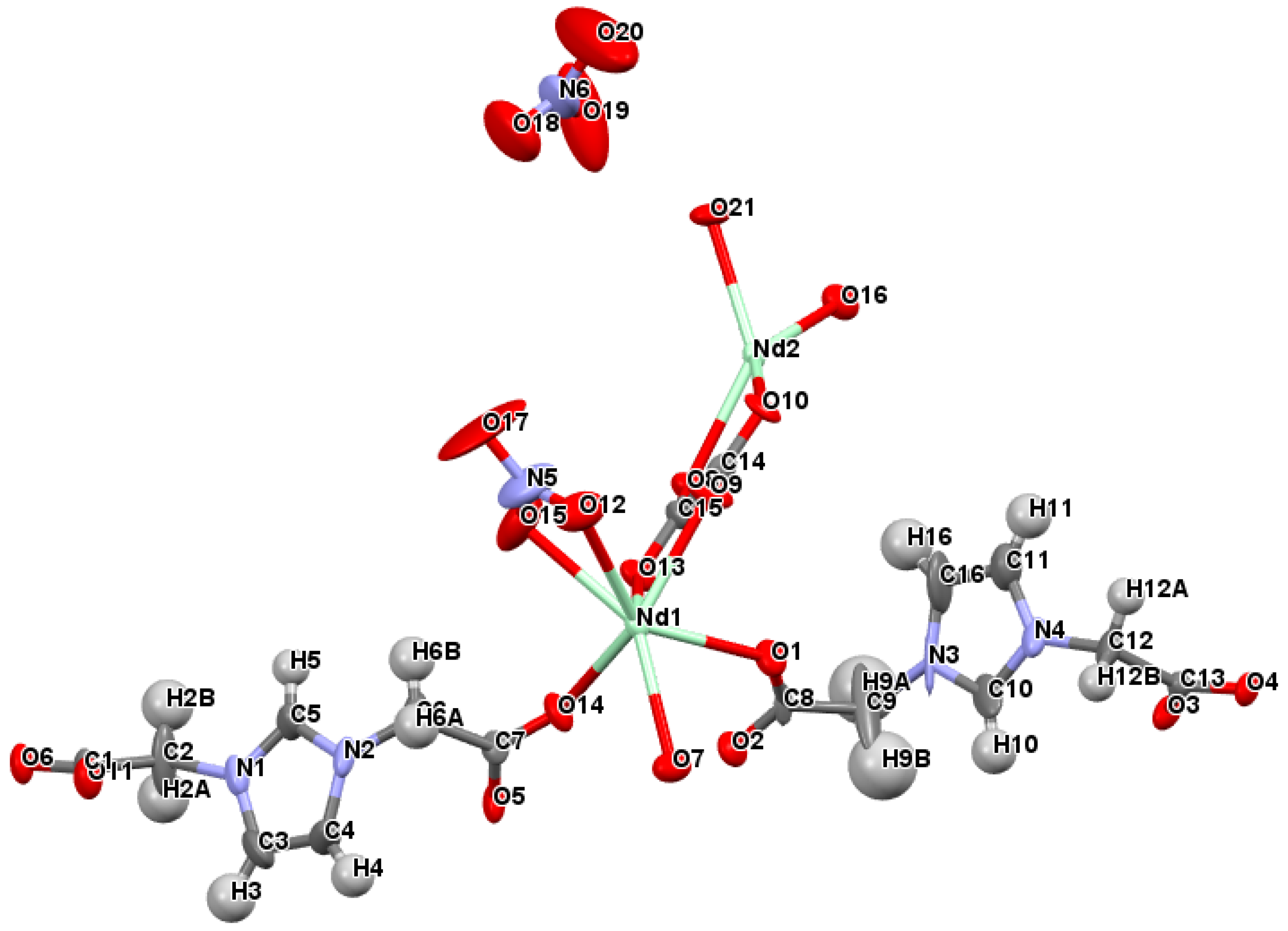
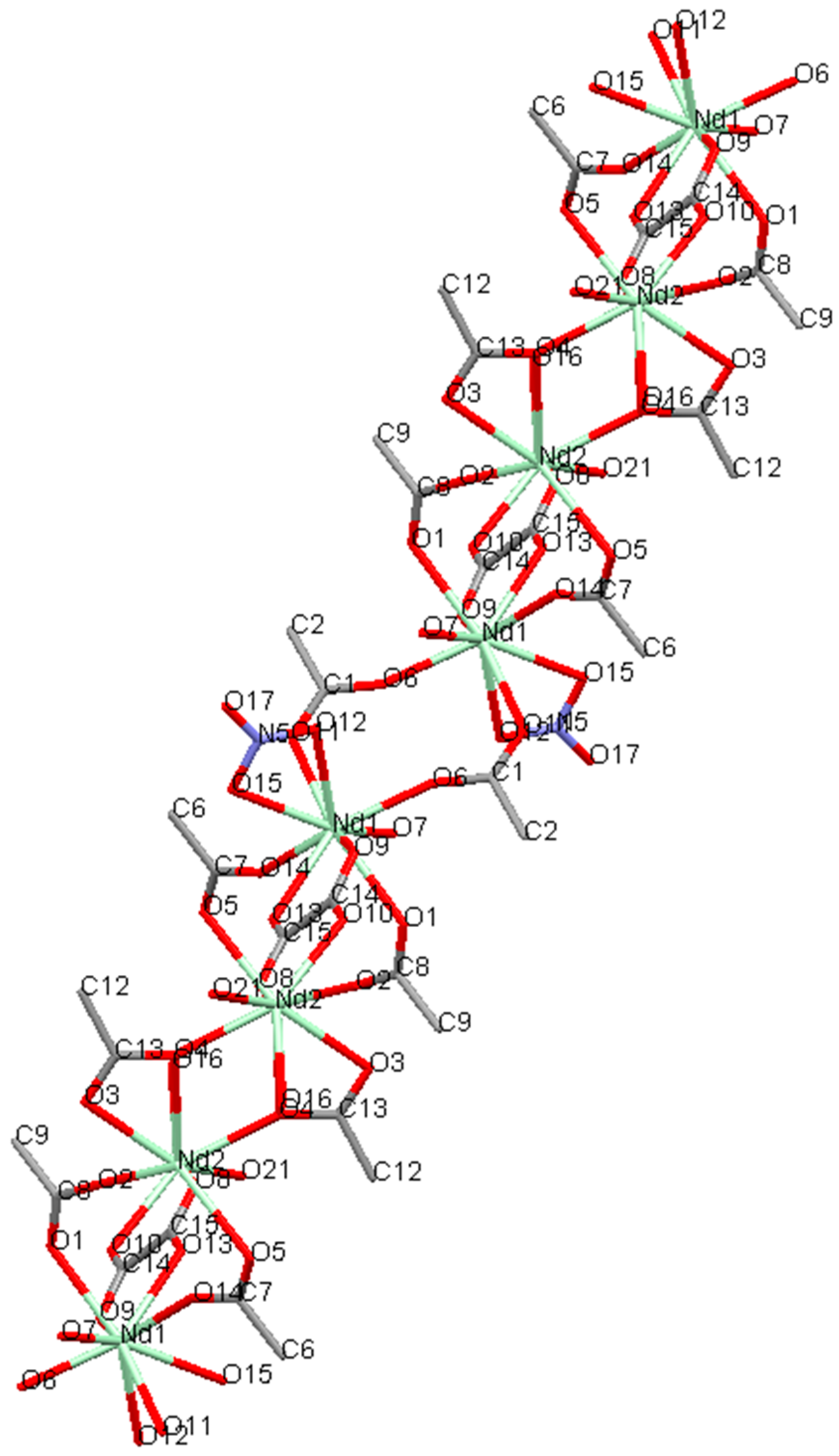
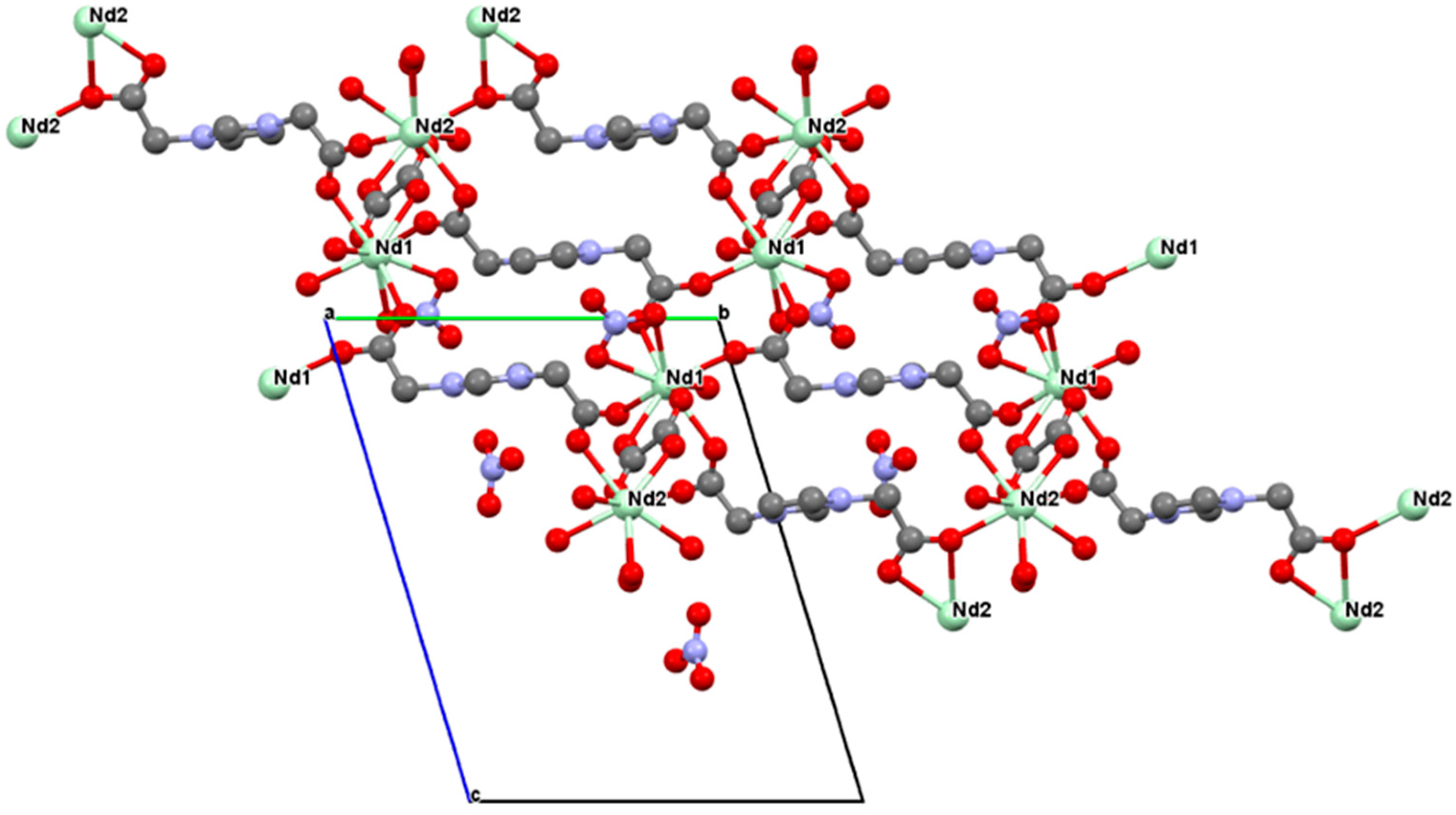
2.2. Crystal Structure of [Nd2(L)(ox)(NO3)(H2O)3][NO3]
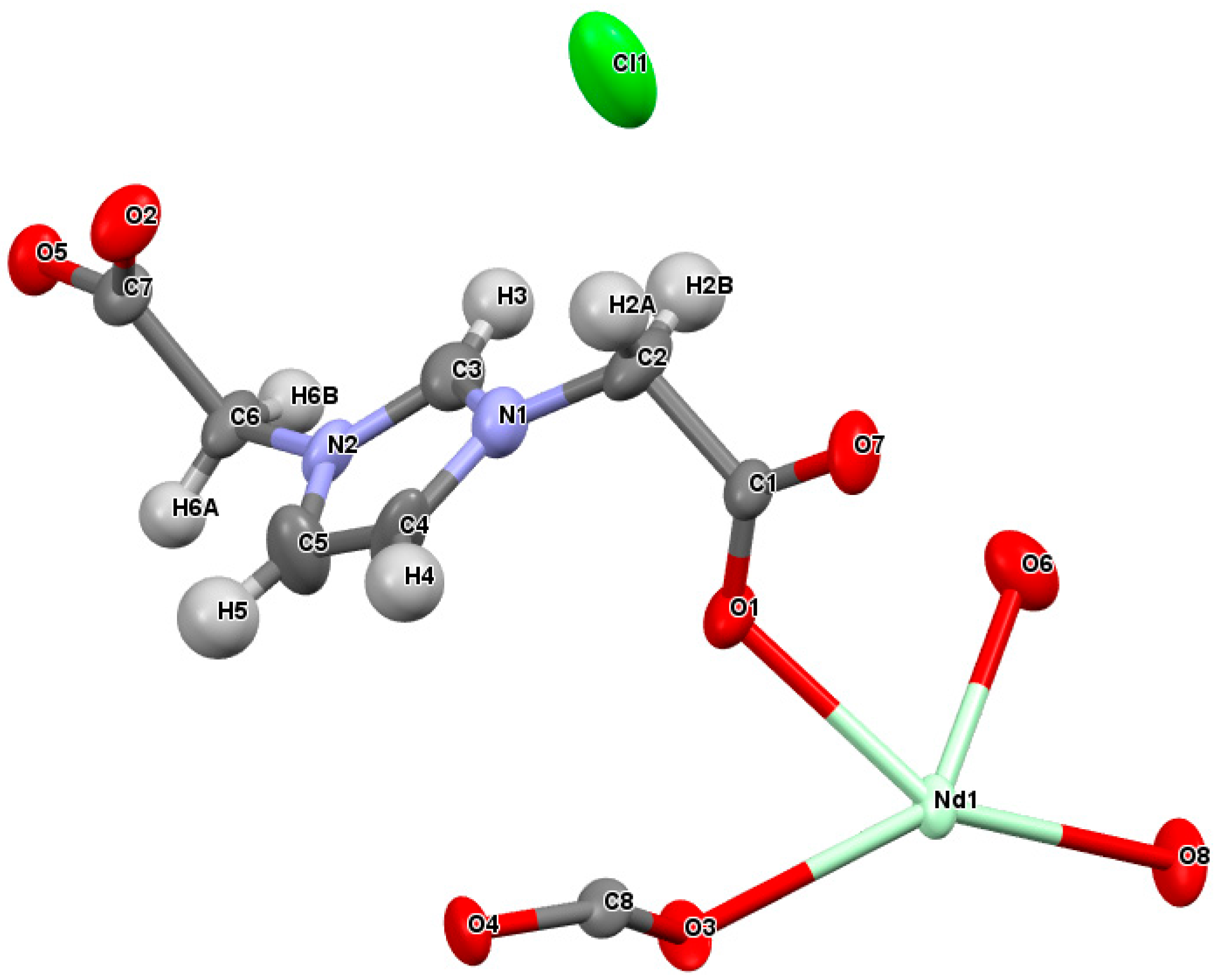
2.3. Crystal Structure of [Nd(L)(ox)0.5(H2O)2][Cl]
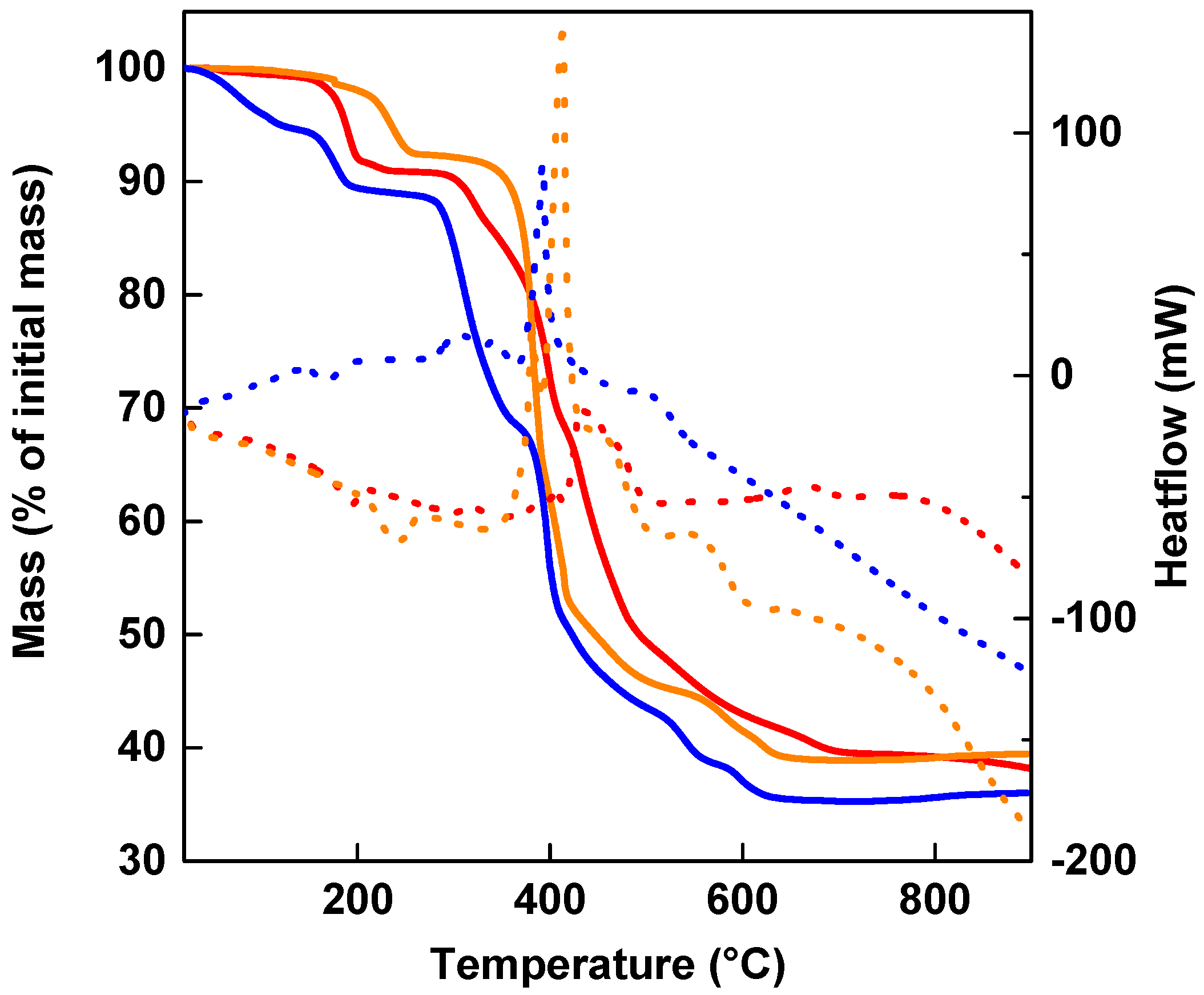
2.4. Thermal Analyses
2.5. UV-Visible-NIR Spectroscopy
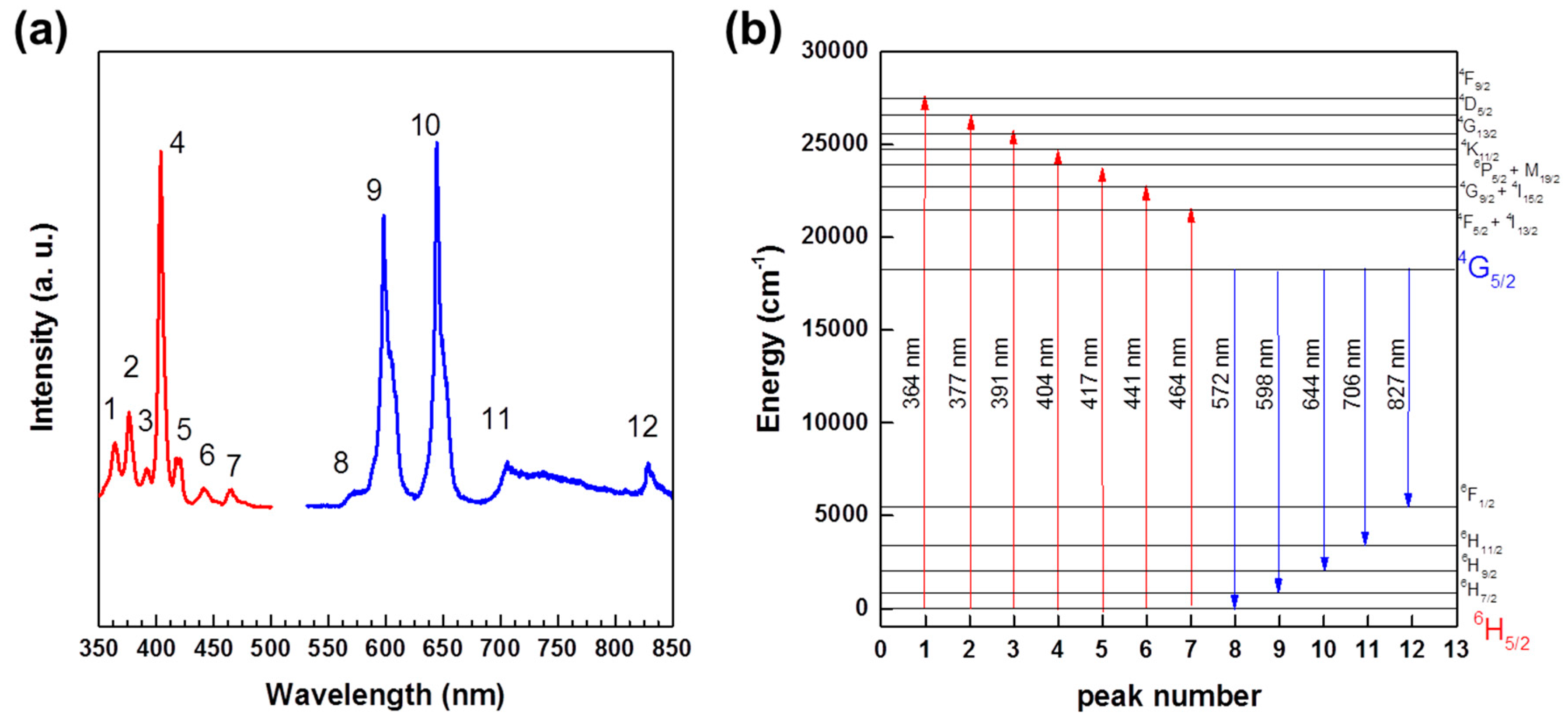
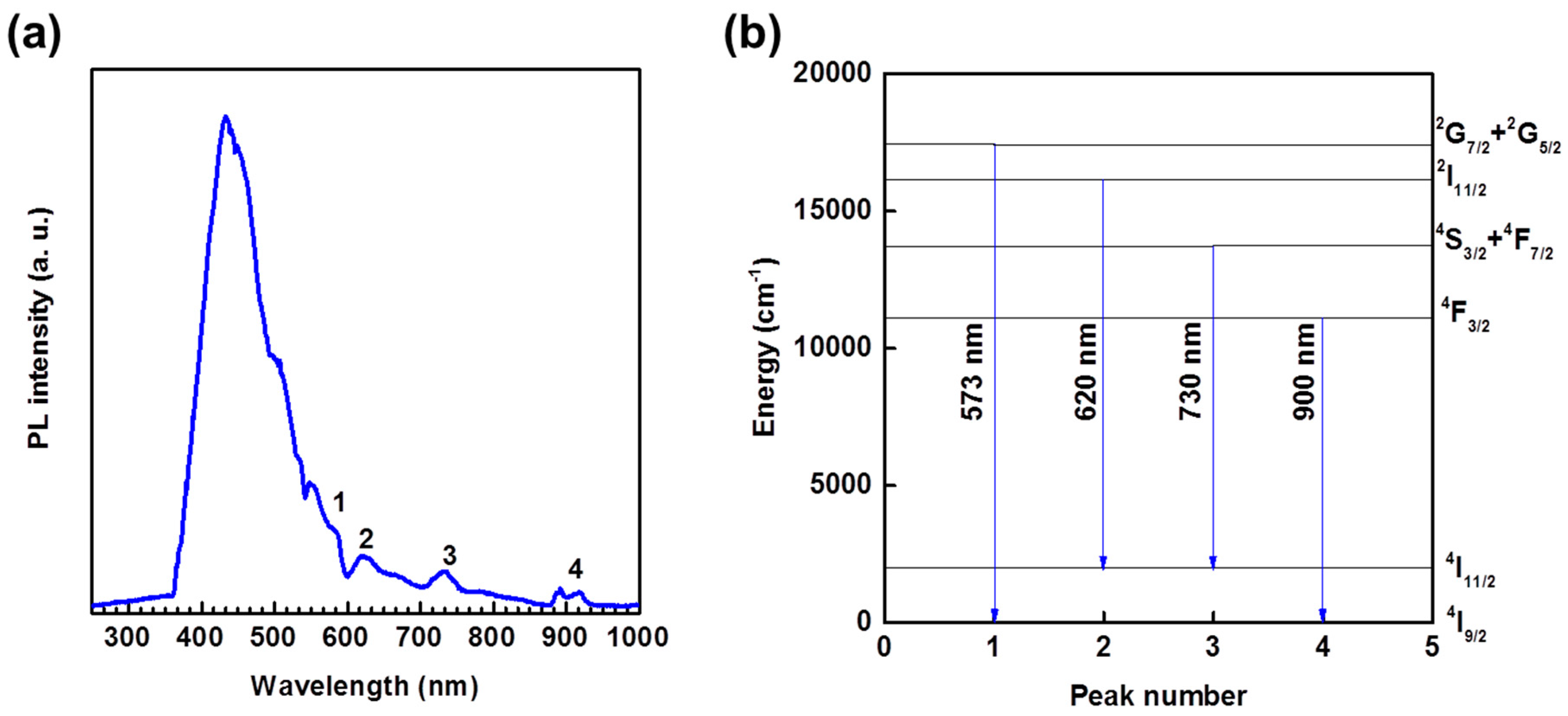
2.6. Luminescent Properties
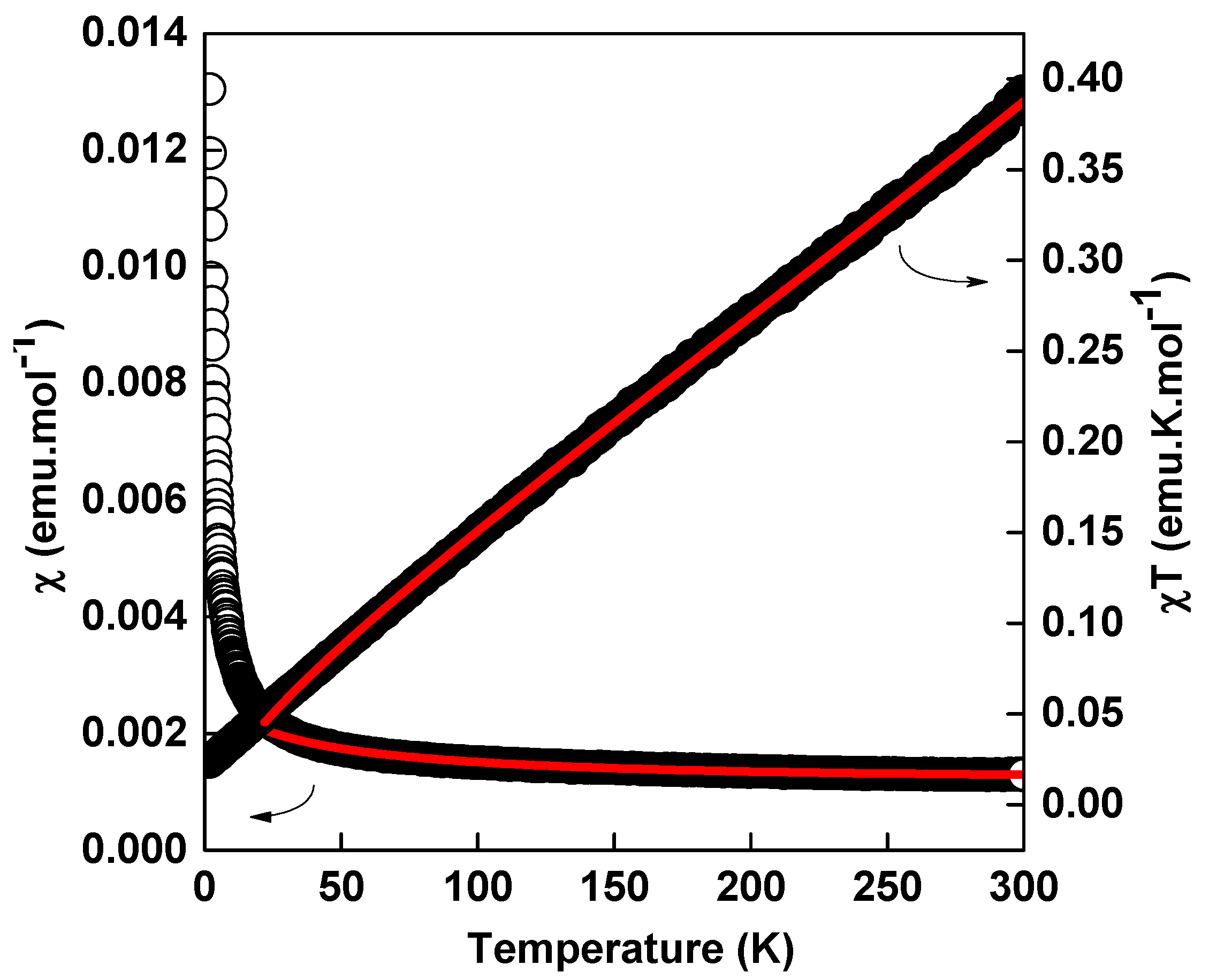
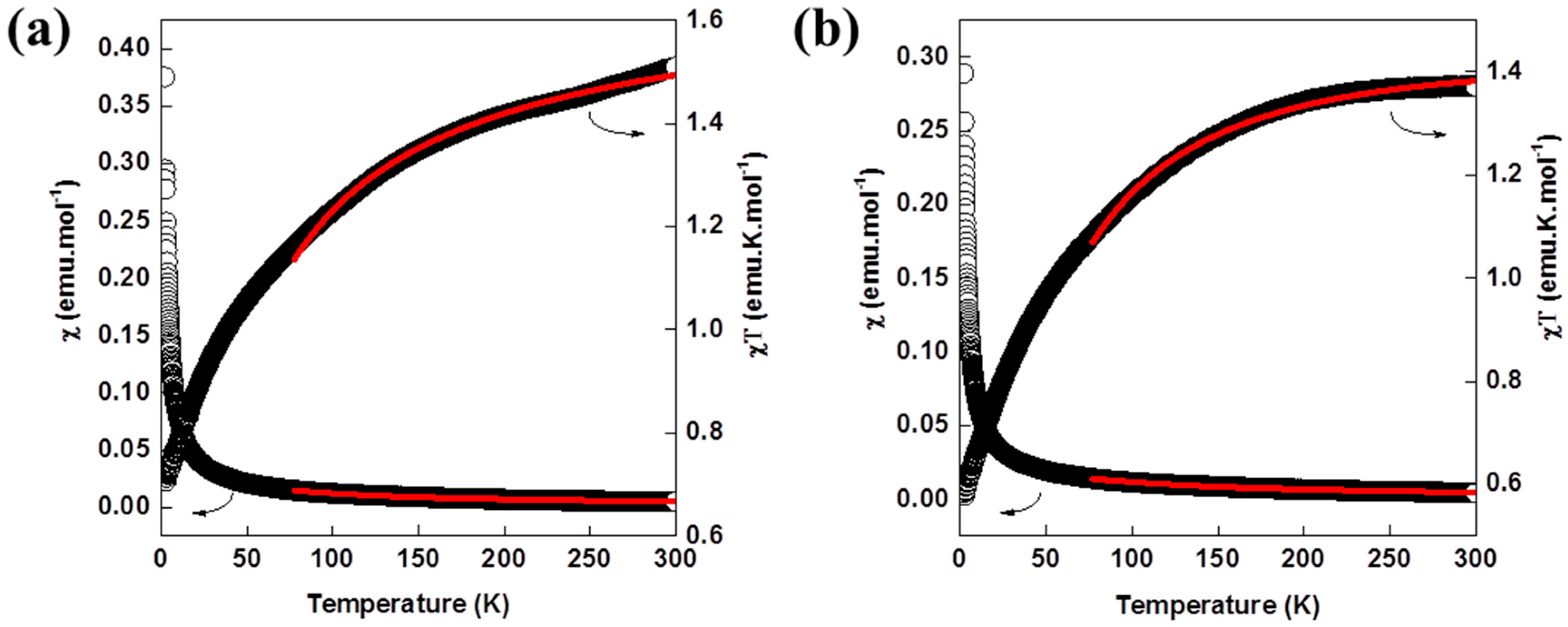
2.7. Magnetic Properties
2.8. Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis
3.2.1. Synthesis of 1,3-Bis(carboxymethyl)imidazolium Chloride [H2L][Cl]
3.2.2. Synthesis of 2-(1-(Carboxymethyl)-1H-imidazol-3-ium-3-yl)acetate [HL]
3.2.3. Synthesis of [Nd2(L)2(ox)(NO3)(H2O)3][NO3]
3.2.4. Synthesis of [Nd(L)(ox)(H2O)]·H2O
3.2.5. Synthesis of [Nd(L)(ox)0.5(H2O)][Cl]
3.2.6. Synthesis of [Sm(L)(ox)(H2O)]·H2O
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2781–2804. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regi, M.; Sebban, M.; Taulelle, F.; Ferey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.L.; Matsuda, R.; Kitagawa, S. Functional Hybrid Porous Coordination Polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Wang, Z.; Ananias, D.; Carné-Sánchez, A.; Brites, C.D.S.; Imaz, I.; Maspoch, D.; Rocha, J.; Carlos, L.D. Lanthanide–Organic Framework Nanothermometers Prepared by Spray-Drying. Adv. Funct. Mater. 2015, 25, 2824–2830. [Google Scholar] [CrossRef]
- Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Murray, K.S.; Cashion, J.D. Guest-Dependent Spin Crossover in a Nanoporous Molecular Framework Material. Science 2002, 298, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Mileo, P.G.M.; Devautour-Vinot, S.; Mouchaham, G.; Faucher, F.; Guillou, N.; Vimont, A.; Serre, C.; Maurin, G. Proton-Conducting Phenolate-Based Zr Metal–Organic Framework: A Joint Experimental–Modeling Investigation. J. Phys. Chem. C 2016, 120, 24503–24510. [Google Scholar] [CrossRef]
- Dhara, B.; Nagarkar, S.S.; Kumar, J.; Kumar, V.; Jha, P.K.; Ghosh, S.K.; Nair, S.; Ballav, N. Increase in Electrical Conductivity of MOF to Billion-Fold upon Filling the Nanochannels with Conducting Polymer. J. Phys. Chem. Lett. 2016, 7, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Cirera, J. Guest effect on spin-crossover frameworks. Rev. Inorg. Chem. 2014, 34, 199–216. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Liu, S.-Y.; Ye, J.-W.; Li, X.-Y.; Zhang, J.-P. Photoluminescent Metal–Organic Frameworks for Gas Sensing. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Gándara, F.; Andrés, A.D.; Gómez-Lor, B.; Gutiérrez-Puebla, E.; Iglesias, M.; Monge, M.A.; Proserpio, D.M.; Snejko, N. A Rare-Earth MOF Series: Fascinating Structure, Efficient Light Emitters, and Promising Catalysts. Cryst. Growth Des. 2008, 8, 378–380. [Google Scholar] [CrossRef]
- Rao, X.; Huang, Q.; Yang, X.; Cui, Y.; Yang, Y.; Wu, C.; Chen, B.; Qian, G. Color tunable and white light emitting Tb3+ and Eu3+ doped lanthanide metal-organic framework materials. J. Mater. Chem. 2012, 22, 3210–3214. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Picot, A.; D’Aléo, A.; Baldeck, P.L.; Grichine, A.; Duperray, A.; Andraud, C.; Maury, O. Long-Lived Two-Photon Excited Luminescence of Water-Soluble Europium Complex: Applications in Biological Imaging Using Two-Photon Scanning Microscopy. J. Am. Chem. Soc. 2008, 130, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Richardson, F.S. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 1982, 82, 541–552. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Lannes, A.; Intissar, M.; Suffren, Y.; Reber, C.; Luneau, D. Terbium(III) and Yttrium(III) Complexes with Pyridine-Substituted Nitronyl Nitroxide Radical and Different β-Diketonate Ligands. Crystal Structures and Magnetic and Luminescence Properties. Inorg. Chem. 2014, 53, 9548–9560. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A Family of Rare-Earth-Based Single Chain Magnets: Playing with Anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Reichert, W.M.; Holbrey, J.D.; Vigour, K.B.; Morgan, T.D.; Broker, G.A.; Rogers, R.D. Approaches to crystallization from ionic liquids: Complex solvents-complex results, or, a strategy for controlled formation of new supramolecular architectures? Chem. Commun. 2006, 4767–4779. [Google Scholar] [CrossRef]
- Jin, K.; Huang, X.; Pang, L.; Li, J.; Appel, A.; Wherland, S. [Cu(I)(bpp)]BF4: The first extended coordination network prepared solvothermally in an ionic liquid solvent. Chem. Commun. 2002, 2872–2873. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal Synthesis of Zeolites, Metal–Organic Frameworks, and Inorganic–Organic Hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.-C.; Sun, Y.-Q.; Lei, R.; Chen, Y.-P.; Zhang, S.; Cao, Y.-N.; Zhang, H.-H. A Series of Lanthanide Frameworks with a Flexible Ligand, N,N′-Diacetic Acid Imidazolium, in Different Coordination Modes. Cryst. Growth Des. 2009, 10, 658–668. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Maynard-Casely, H.E.; Robson, R.; White, K.F. Copper(ii) coordination polymers of imdc− (H2imdc+ = the 1,3-bis(carboxymethyl)imidazolium cation): Unusual sheet interpenetration and an unexpected single crystal-to-single crystal transformation. CrystEngComm 2013, 15, 9729–9737. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Gao, S.; Huo, L.-H.; Zhao, H. A two-dimensional cobalt(II) coordination polymer: Poly[chloro(μ-imidazole-1,3-diyldiacetato-κ4O:O′:O′′:O′′′)cobalt(II)]. Acta Crystallogr. Sect. E 2006, 62, m3359–m3361. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Gao, S.; Huo, L.-H.; Zhao, H. Poly[[chloromanganese(II)]-μ4-imidazole-1,3-diyldiacetato]. Acta Crystallogr. Sect. E 2006, 62, m3365–m3367. [Google Scholar] [CrossRef]
- Fei, Z.; Geldbach, T.J.; Zhao, D.; Scopelliti, R.; Dyson, P.J. A Nearly Planar Water Sheet Sandwiched between Strontium-Imidazolium Carboxylate Coordination Polymers. Inorg. Chem. 2005, 44, 5200–5202. [Google Scholar] [CrossRef] [PubMed]
- Farger, P.; Guillot, R.; Leroux, F.; Parizel, N.; Gallart, M.; Gilliot, P.; Rogez, G.; Delahaye, E.; Rabu, P. Imidazolium Dicarboxylate Based Metal–Organic Frameworks Obtained by Solvo-Ionothermal Reaction. Eur. J. Inorg. Chem. 2015, 2015, 5342–5350. [Google Scholar] [CrossRef]
- Martin, N.P.; Falaise, C.; Volkringer, C.; Henry, N.; Farger, P.; Falk, C.; Delahaye, E.; Rabu, P.; Loiseau, T. Hydrothermal Crystallization of Uranyl Coordination Polymers Involving an Imidazolium Dicarboxylate Ligand: Effect of pH on the Nuclearity of Uranyl-Centered Subunits. Inorg. Chem. 2016, 55, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ling, X.-L.; Liu, L.; Song, H.-L.; Wang, L.-Y.; Ng, S.-W.; Su, B.Y. A series of 3D lanthanide frameworks constructed from aromatic multi-carboxylate ligand: Structural diversity, luminescence and magnetic properties. Dalton Trans. 2013, 42, 10292–10303. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Cen, Z.-M.; Ni, Q.-L.; Jiang, X.-F.; Lian, H.-C.; Gui, L.-C.; Zuo, H.-H.; Wang, Z.-Y. Synthesis Structures, and Properties of Functional 2-D Lanthanide Coordination Polymers [Ln2(dpa)2(C2O4)2(H2O)2]n (dpa = 2,2′-(2-methylbenzimidazolium-1,3-diyl)diacetate, C2O42− = oxalate, Ln = Nd, Eu, Gd, Tb). Cryst. Growth Design 2010, 10, 2960–2968. [Google Scholar] [CrossRef]
- Thuery, P. Neodymium(iii) d(−)-citramalate: A chiral three-dimensional framework with water-filled channels. CrystEngComm 2007, 9, 460–462. [Google Scholar] [CrossRef]
- Zucchi, G.; Maury, O.; Thuéry, P.; Ephritikhine, M. Structural Diversity in Neodymium Bipyrimidine Compounds with Near Infrared Luminescence: From Mono- and Binuclear Complexes to Metal-Organic Frameworks. Inorg. Chem. 2008, 47, 10398–10406. [Google Scholar] [CrossRef] [PubMed]
- Polido Legaria, E.; Topel, S.D.; Kessler, V.G.; Seisenbaeva, G.A. Molecular insights into the selective action of a magnetically removable complexone-grafted adsorbent. Dalton Trans. 2015, 44, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Han, L.; Cai, T.-J.; Zheng, Y.-Q.; Chen, J.-Z.; Deng, Q. A Novel Chiral Doubly Folded Interpenetrating 3D Metal-Organic Framework Based on the Flexible Zwitterionic Ligand. Cryst. Growth Des. 2007, 7, 1027–1030. [Google Scholar] [CrossRef]
- Gabrielyan, V.T.; Kaminskii, A.A.; Li, L. Absorption and luminescence spectra and energy levels of Nd3+ and Er3+ ions in LiNbO3 crystals. Phys. Status Solidi A 1970, 3, K37–K42. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, Y.; Liu, T.; Zhang, J.Z.; Dang, S.; Feng, J.; Wang, Z.; Zhang, H.; Shi, L. Near Infrared and Visible Luminescence from Xerogels Covalently Grafted with Lanthanide [Sm3+, Yb3+, Nd3+, Er3+, Pr3+, Ho3+] β-Diketonate Derivatives Using Visible Light Excitation. ACS Appl. Mater. Interfaces 2013, 5, 9585–9593. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Chang, Y.-H.; Lin, Y.-F.; Chang, Y.-S.; Lin, Y.-J. Synthesis and luminescent properties of Ln3+ (Eu3+, Sm3+, Dy3+)-doped lanthanum aluminum germanate LaAlGe2O7 phosphors. J. Alloys Compd. 2007, 439, 367–375. [Google Scholar] [CrossRef]
- D’Vries, R.F.; Gomez, G.E.; Hodak, J.H.; Soler-Illia, G.J.A.A.; Ellena, J. Tuning the structure, dimensionality and luminescent properties of lanthanide metal-organic frameworks under ancillary ligand influence. Dalton Trans. 2016, 45, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.-F.; Li, H.-X.; Li, W.-Z.; Yan, P.-F.; Su, X.-H.; Li, G.-M. Two Series of Luminescent Flexible Polycarboxylate Lanthanide Coordination Complexes with Double Layer and Rectangle Metallomacrocycle Structures. Cryst. Growth Des. 2013, 13, 3374–3380. [Google Scholar] [CrossRef]
- Cepeda, J.; Balda, R.; Beobide, G.; Castillo, O.; Fernández, J.; Luque, A.; Pérez-Yáñez, S.; Román, P. Synthetic Control to Achieve Lanthanide(III)/Pyrimidine-4,6-dicarboxylate Compounds by Preventing Oxalate Formation: Structural, Magnetic, and Luminescent Properties. Inorg. Chem. 2012, 51, 7875–7888. [Google Scholar] [CrossRef] [PubMed]
- Andruh, M.; Bakalbassis, E.; Kahn, O.; Trombe, J.C.; Porcher, P. Structure, spectroscopic and magnetic properties of rare earth metal(III) derivatives with the 2-formyl-4-methyl-6-(N-(2-pyridylethyl)formimidoyl)phenol ligand. Inorg. Chem. 1993, 32, 1616–1622. [Google Scholar] [CrossRef]
- Boča, R. Theoretical Foundations of Molecular Magnetism; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Lhoste, J.; Perez-Campos, A.; Henry, N.; Loiseau, T.; Rabu, P.; Abraham, F. Chain-like and dinuclear coordination polymers in lanthanide (Nd, Eu) oxochloride complexes with 2,2[prime or minute]:6[prime or minute],2[prime or minute][prime or minute]-terpyridine: Synthesis, XRD structure and magnetic properties. Dalton Trans. 2011, 40, 9136–9144. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-R.; Tang, X.-L.; Ju, Z.-H.; Zhang, K.-M.; Jiang, H.-E.; Liu, W.-S. Lanthanide metal-organic frameworks constructed by asymmetric 2-nitrobiphenyl-4,4[prime or minute]-dicarboxylate ligand: Syntheses, structures, luminescence and magnetic investigations. CrystEngComm 2013, 15, 9020–9031. [Google Scholar] [CrossRef]
- Manna, S.C.; Zangrando, E.; Bencini, A.; Benelli, C.; Chaudhuri, N.R. Syntheses, Crystal Structures, and Magnetic Properties of [LnIII2(Succinate)3(H2O)2]·0.5H2O [Ln = Pr, Nd, Sm, Eu, Gd, and Dy] Polymeric Networks: Unusual Ferromagnetic Coupling in Gd Derivative. Inorg. Chem. 2006, 45, 9114–9122. [Google Scholar] [CrossRef] [PubMed]
- Kahwa, I.A.; Selbin, J.; O’Connor, C.J.; Foise, J.W.; McPherson, G.L. Magnetic and luminescence characteristics of dinuclear complexes of lanthanides and a phenolic schiff base macrocyclic ligand. Inorg. Chim. Acta 1988, 148, 265–272. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Hudson, T.A.; Robson, R. Coordination networks incorporating the in situ generated ligands [OC(CO2)3]4− and [OCH(CO2)2]3−. J. Mol. Struct. 2006, 796, 2–8. [Google Scholar] [CrossRef]
- Knope, K.E.; Kimura, H.; Yasaka, Y.; Nakahara, M.; Andrews, M.B.; Cahill, C.L. Investigation of in Situ Oxalate Formation from 2,3-Pyrazinedicarboxylate under Hydrothermal Conditions Using Nuclear Magnetic Resonance Spectroscopy. Inorg. Chem. 2012, 51, 3883–3890. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Vayasmudri, S.; Mostafa, G.; Maji, T.K. Lanthanide (LaIII/HoIII)-oxalate open framework materials formed by in situ ligand synthesis. J. Mol. Struct. 2009, 932, 123–128. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Synthesis of Zinc Oxalate Coordination Polymers via Unprecedented Oxidative Coupling of Methanol to Oxalic Acid. Cryst. Growth Des. 2001, 1, 9–11. [Google Scholar] [CrossRef]
- Oliveira, C.K.; de Menezes Vicenti, J.R.; Burrow, R.A.; Alves, S., Jr.; Longo, R.L.; Malvestiti, I. Exploring the mechanism of in situ formation of oxalic acid for producing mixed fumarato-oxalato lanthanide (Eu, Tb and Gd) frameworks. Inorg. Chem. Commun. 2012, 22, 54–59. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Crystallogr. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Edgington, P.R.; McCabe, P.; Macrae, C.F.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Brønsted Acidic Ionic Liquids and Their Zwitterions: Synthesis, Characterization and pKa Determination. Chem. A Eur. J. 2004, 10, 4886–4893. [Google Scholar] [CrossRef] [PubMed]
- VelÍŠEk, J.; DavÍDek, T.; DavÍEk, J.; TrŠKa, P.; KvasniČKa, F.; VelcovÁ, K. New Imidazoles Formed in Nonenzymatic Browning Reactions. J. Food Sci. 1989, 54, 1544–1546. [Google Scholar] [CrossRef]
- Kühl, O.; Palm, G. Imidazolium salts from amino acids—A new route to chiral zwitterionic carbene precursors? Tetrahedron Asymmetry 2010, 21, 393–397. [Google Scholar] [CrossRef]





| Compound | [Sm(L)(ox)(H2O)]·H2O | [Nd(L)(ox)(H2O)]·H2O | [Nd2(L)2(ox)(NO3)(H2O)3][NO3] | [Nd(L)(ox)0.5(H2O)2][Cl] |
|---|---|---|---|---|
| Formula | C9H7N2O10Sm | C9H7N2O10Nd | C16H14N6O21Nd2 | C8H7N2O8Cl1Nd |
| Crystal size (mm3) | 0.156 × 0.108 × 0.094 | 0.131 × 0.056 × 0.052 | 0.084 × 0.048 × 0.047 | 0.132 × 0.082 × 0.054 |
| Formula weight (g·mol−1) | 453.52 | 447.41 | 914.81 | 451.68 |
| Temperature (K) | 293(2) | 293(2) | 293(2) | 293(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 | P-1 |
| Unit cell dimension | ||||
| a (Å) | 7.9948(9) | 8.010(3) | 8.076(3) | 7.9870(10) |
| b (Å) | 9.2408(15) | 9.203(3) | 12.545(4) | 8.534(3) |
| c (Å) | 9.434(2) | 9.5230(19) | 15.713(3) | 11.259(3) |
| α (°) | 80.411(13) | 79.91(2) | 71.896(18) | 71.961(17) |
| β (°) | 71.829(11) | 72.043(16) | 82.14(2) | 84.27(2) |
| γ (°) | 89.793(10) | 89.27(2) | 75.62(3) | 68.045(18) |
| V (Å3) | 652.1(2) | 656.8(3) | 1462.7(8) | 676.7(3) |
| Z | 2 | 2 | 2 | 2 |
| Dcalc (g·cm−3) | 2.310 | 2.262 | 2.077 | 2.154 |
| Absorption coefficient (mm−1) | 4.345 | 3.981 | 3.585 | 4.040 |
| F (0 0 0) | 434 | 430 | 880 | 420 |
| Index range | −9 < h < 10 | −10 < h < 6 | −7 < h < 10 | −10 < h < 10 |
| −11 < k < 12 | −11 < k < 11 | −13 < k < 16 | −11 < k < 9 | |
| −12 < l < 11 | −12 < l < 11 | −17 < l < 20 | −14 < l < 14 | |
| Collected reflections | 6075 | 6785 | 15481 | 6346 |
| Independent reflections (Rint) | 2983 (0.0382) | 3005 (0.0518) | 6677 (0.0840) | 3091 (0.1740) |
| Observed reflections (I > 2σ(I)) | 2662 | 2725 | 3882 | 2334 |
| Refinement method | Full matrix least square on F2 | Full matrix least square on F2 | Full matrix least square on F2 | Full matrix least square on F2 |
| Final R indices (I > 2σ(I)) | R1 = 0.0297, wR2 = 0.0632 | R1 = 0.0289, wR2 = 0.0667 | R1 = 0.0699, wR2 = 0.1366 | R1 = 0.0802, wR2 = 0.1828 |
| Final R indices (all data) | R1 = 0.0387, wR2 = 0.0674 | R1 = 0.0352, wR2 = 0.0697 | R1 = 0.1517, wR2 = 0.1665 | R1 = 0.1134, wR2 = 0.2089 |
| S | 1.087 | 1.074 | 1.051 | 1.076 |
| (Dr)max, min (e·Å−3) | 1.436, −1.546 | 0.985, −1.643 | 4.237, −1.229 | 2.860, −3.995 |
| Band Number | [Nd2(L)(ox)(NO3)(H2O)3][NO3] and [Nd(L)(ox)(H2O)]·H2O | [Sm(L)(ox)(H2O)]·H2O |
|---|---|---|
| 1 | 356 nm | 318 nm |
| 4I9/2→2D1/2 | 6H5/2→4F11/2 | |
| 2 | 462 nm | 344 nm |
| 4I9/2→4G11/2 + 2K15/2 + 2P3/2+2D3/2 | 6H5/2→3H7/2 | |
| 3 | 524 nm | 362 nm |
| 4I9/2→2G9/2 | 6H5/2→4F9/2 | |
| 4 | 580 nm | 376 nm |
| 4I9/2→4G7/2 + 2G7/2 | 6H5/2→4D5/2 | |
| 5 | 626 nm | 404 nm |
| 4I9/2→2H11/2 | 6H5/2→4K11/2 | |
| 6 | 680 nm | 418 nm |
| 4I9/2→4F9/2 | 6H5/2→6P5/2 + 4M19/2 | |
| 7 | 744 nm | 440 nm |
| 4I9/2→4F7/2, 4S3/2 | 6H5/2→4G9/2 + 4I15/2 | |
| 8 | 798 nm | 464 nm |
| 4I9/2→4F5/2, 2H9/2 | 6H5/2→4F5/2 + 4I13/2 | |
| 9 | 870 nm | 478 nm |
| 4I9/2→4F3/2 | 6H5/2→4I11/2 + 4M15/2 | |
| 10 | 1624 nm | 950 nm |
| 4I9/2→4I15/2 | 6H5/2→6F11/2 | |
| 11 | - | 1088 nm |
| 6H5/2→6F9/2 | ||
| 12 | - | 1240 nm |
| 6H5/2→6F7/2 | ||
| 13 | - | 1390 nm |
| 6H5/2→6F5/2 | ||
| 14 | - | 1496 nm |
| 6H5/2→6H15/2 | ||
| 15 | - | 1562 nm |
| 6H5/2→6F3/2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farger, P.; Leuvrey, C.; Gallart, M.; Gilliot, P.; Rogez, G.; Rabu, P.; Delahaye, E. Elaboration of Luminescent and Magnetic Hybrid Networks Based on Lanthanide Ions and Imidazolium Dicarboxylate Salts: Influence of the Synthesis Conditions. Magnetochemistry 2017, 3, 1. https://doi.org/10.3390/magnetochemistry3010001
Farger P, Leuvrey C, Gallart M, Gilliot P, Rogez G, Rabu P, Delahaye E. Elaboration of Luminescent and Magnetic Hybrid Networks Based on Lanthanide Ions and Imidazolium Dicarboxylate Salts: Influence of the Synthesis Conditions. Magnetochemistry. 2017; 3(1):1. https://doi.org/10.3390/magnetochemistry3010001
Chicago/Turabian StyleFarger, Pierre, Cédric Leuvrey, Mathieu Gallart, Pierre Gilliot, Guillaume Rogez, Pierre Rabu, and Emilie Delahaye. 2017. "Elaboration of Luminescent and Magnetic Hybrid Networks Based on Lanthanide Ions and Imidazolium Dicarboxylate Salts: Influence of the Synthesis Conditions" Magnetochemistry 3, no. 1: 1. https://doi.org/10.3390/magnetochemistry3010001