Seasonal Variations in the Starch Properties of Sweet Potato Cultivars
Abstract
:1. Introduction
2. Materials and Methods
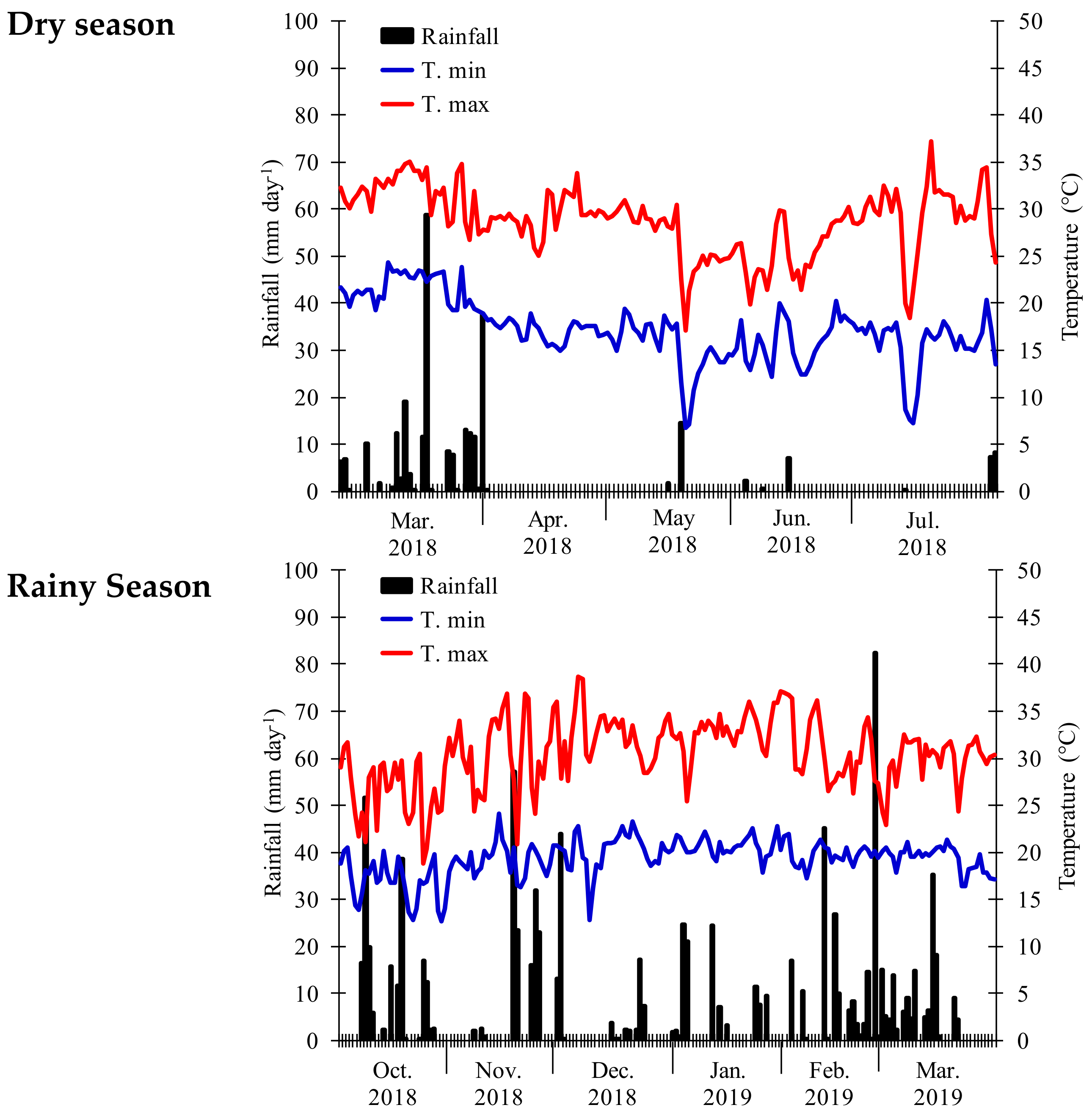
2.1. Cultivars, Experimental Area, and Treatments
2.2. Starch Isolation
2.3. Starch Analysis
2.3.1. Morphology and Granule Size
2.3.2. X-ray Diffraction Pattern (XRD) and Relative Crystallinity (RC)
2.3.3. Amylose and Resistant Starch
2.3.4. Swelling Power (SP) and Solubility (SS)
2.3.5. Pasting and Thermal Properties
2.4. Data Analysis
3. Results and Discussion
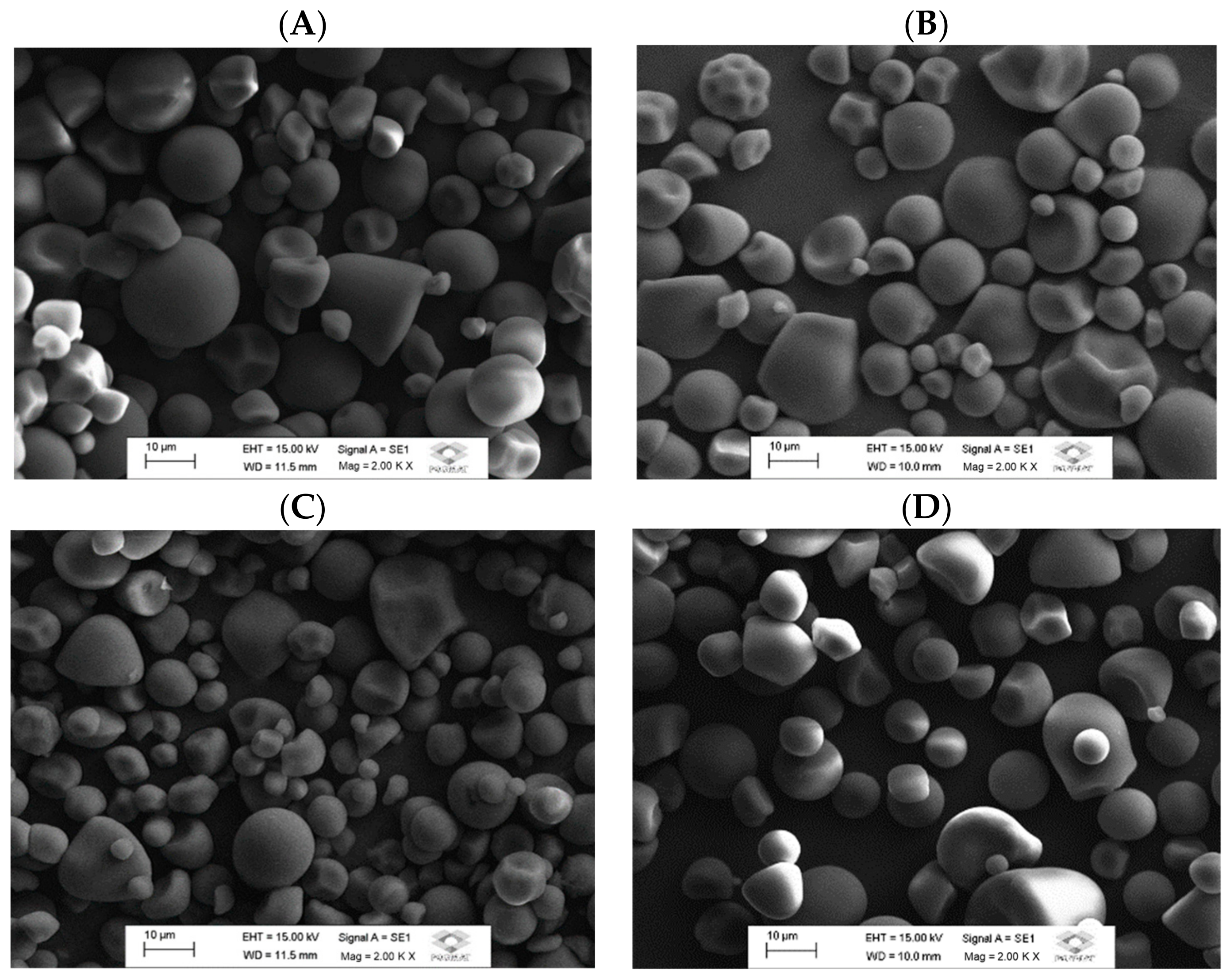
3.1. Morphology and Granule Size
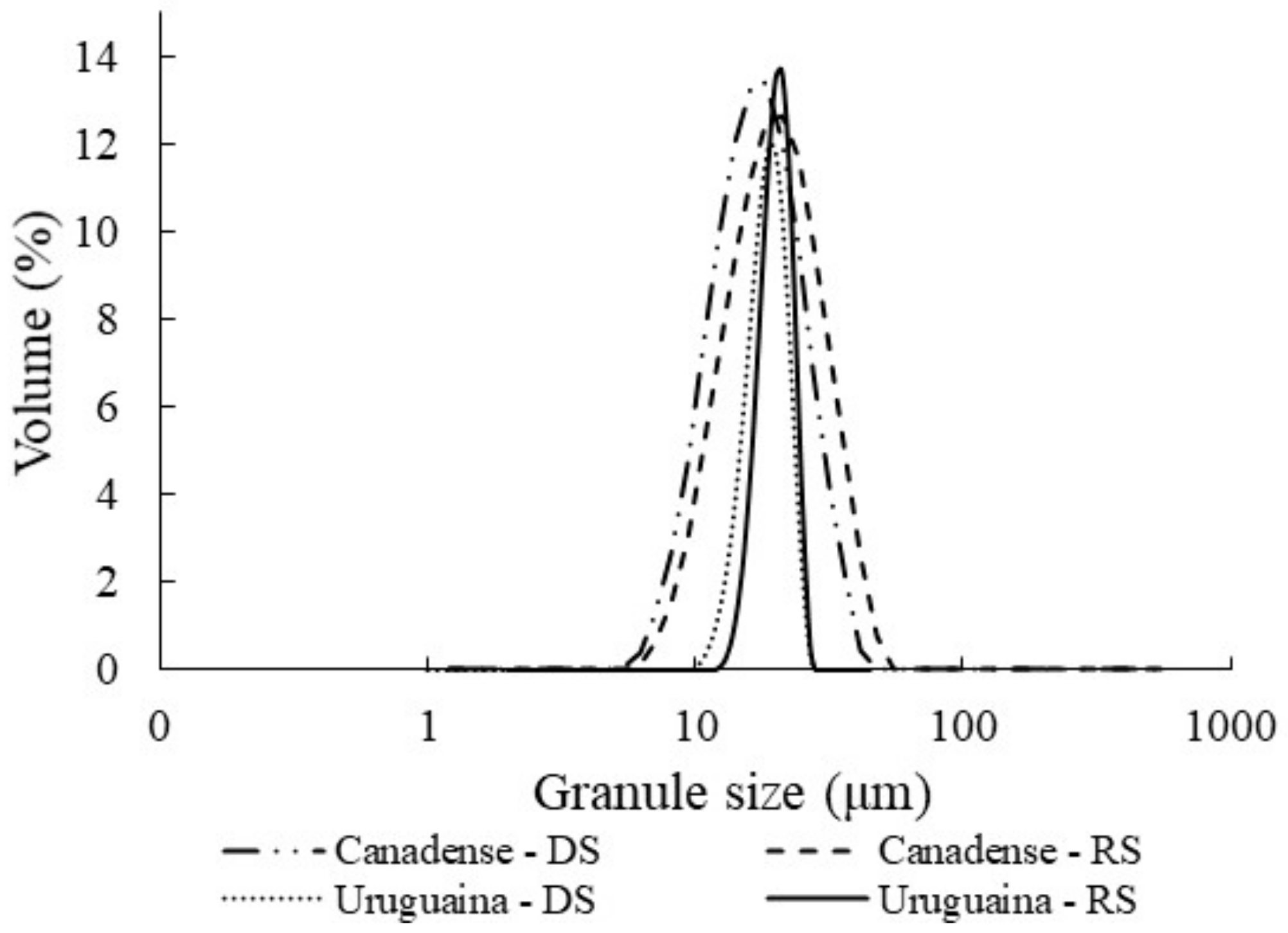
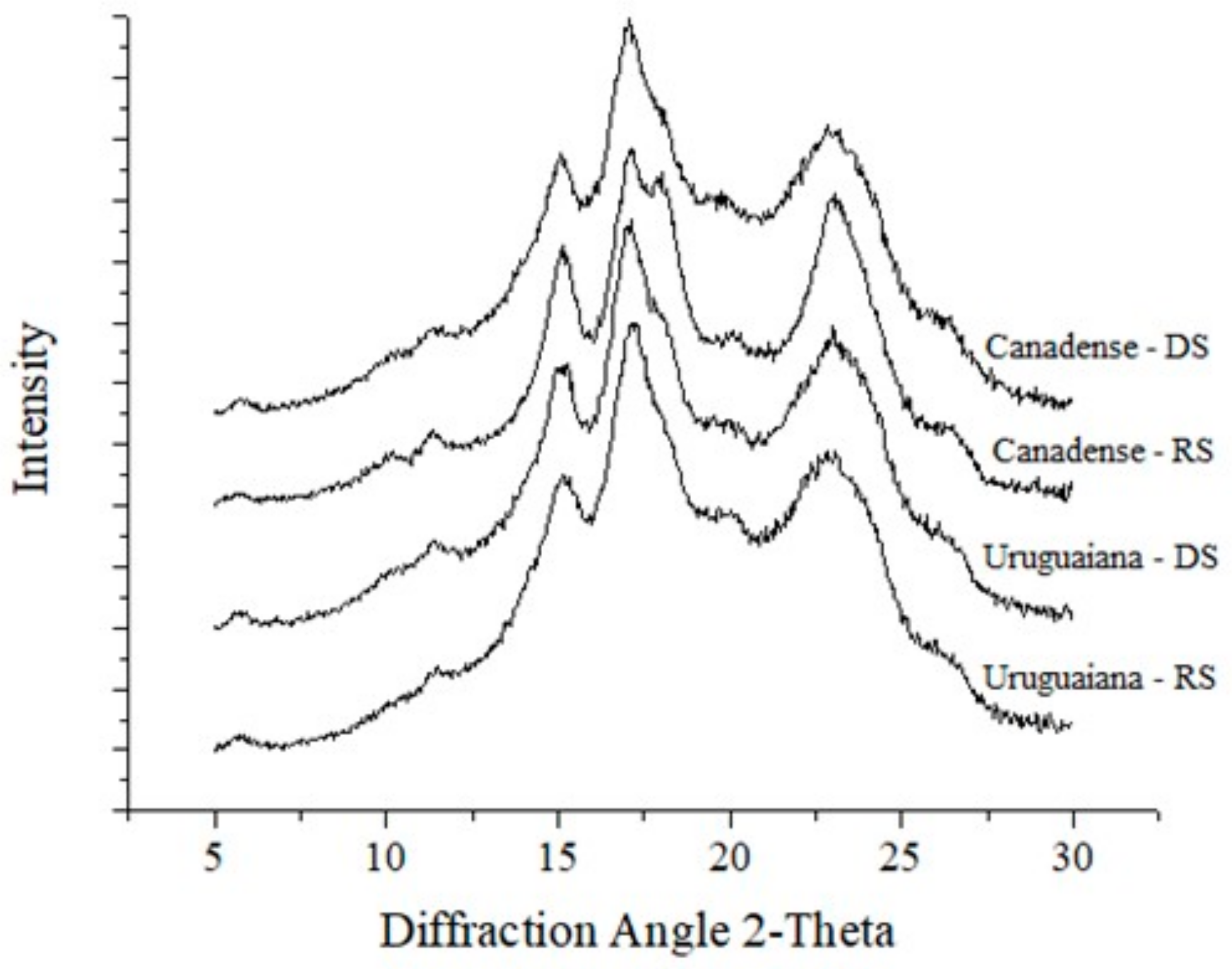
3.2. X-ray Diffraction Pattern and Granule Size
3.3. Amylose and Resistant Starch
3.4. Swelling Power (SP) and Solubility (SS)
3.5. Pasting and Thermal Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. FAOSTAT: Production-Crops. 2022. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 5 January 2022).
- Galvao, A.C.; Nicoletto, C.; Zanin, G.; Vargas, P.F.; Sambo, P. Nutraceutical content and daily value contribution of sweet potato accessions for the European market. Horticulturae 2021, 7, 23. [Google Scholar] [CrossRef]
- Soto-Caro, A.; Luo, T.; Wu, F.; Guan, Z. The U.S. sweet potato market: Price response and impact of supply shocks. Horticulturae 2022, 8, 856. [Google Scholar] [CrossRef]
- Adu-Kwarteng, E.; Baafi, E.; Amoa-Owusu, A.; Okyere, F.; Carey, E. Expanding industrial uses of sweet potato for food security and poverty alleviation. Open Agric. 2021, 6, 382–391. [Google Scholar] [CrossRef]
- Somasundaram, K.; Santhosh Mithra, V.S. Madhuram: A simulation model for sweet potato growth. World J. Agric. Sci. 2008, 4, 241–254. [Google Scholar]
- Erpen, L.; Streck, N.A.; Uhlmann, L.O.; De Freitas, C.P.O.; Andriolo, J.A. Tuberization and yield of sweet potato as affected by planting date in a subtropical climate. Bragantia 2013, 72, 396–402. [Google Scholar] [CrossRef]
- Leonel, M.; Sarita Leonel, S.; Santos, T.P.R.; Souza, J.M.A.; Martins, R.C.; Silva, M.S.C. Agronomic yield and starch properties of banana cultivars. Pesq. Agropec. Bras. 2021, 56, e02491. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, S. Physicochemical properties, molecular structure, and uses of sweet potato starch. Trends Food Sci. Technol. 2014, 36, 68–78. [Google Scholar] [CrossRef]
- Marketwatch. Sweet Potato Starch Market Size 2020: Top Countries Data, Definition, SWOT Analysis, Applications, Trends and Forecast to 2024. Available online: https://www.marketwatch.com/press-release/sweet-potato-starch-market-size-2020-top-countries-data-defination-swot-analysis-applications-trends-and-forecast-to-2024-2020-02-14. (accessed on 15 May 2022).
- Lewthwaite, S.L.; Triggs, C.M. Sweet potato cultivar response to prolonged drought. Agron. N. Z. 2012, 42, 1–10. [Google Scholar]
- Yooyongwech, S.; Theerawitaya, C.; Samphumphuang, T.; Cha-um, S. Water-deficit tolerant identification in sweet potato genotypes (Ipomoea batatas (L.) Lam.) in vegetative developmental stage using multivariate physiological indices. Sci. Hortic 2013, 162, 242–251. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J. The effects of environmental conditions on the structural features and physico-chemical properties of starches. Starch-Stärke 2001, 53, 513–519. [Google Scholar] [CrossRef]
- Noda, T.; Kobayashi, T.; Suda, I. Effect of soil temperature on starch properties of sweet potatoes. Carbohydr. Polym. 2001, 44, 239–246. [Google Scholar] [CrossRef]
- Beckles, D.M.; Thitisaksakul, M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch-Stärke 2014, 66, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, K.O.; Dias, S.N.P.; Augusta, I.M. A review ‘clean labeling’: Applications of natural ingredients in bakery products. J. Food Nutr. Res. 2018, 6, 285–294. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Varela, P.; Peschel, A.O. Consumers’ categorization of food ingredients: Do consumers perceive them as ‘clean label’ producers expect? An exploration with projective mapping. Food Qual. Prefer. 2019, 71, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Noguerol, A.T.; Pagán, M.J.; García-Segovia, P.; Varela, P. Green or clean? Perception of clean label plant-based products by omnivorous, vegan, vegetarian and flexitarian consumers. Food Res. Int. 2021, 149, 110652. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.-R. Clean label starch: Production, physicochemical characteristics, and industrial applications. Food Sci. Biotechnol. 2021, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Feltran, J.C.; Peressin, V.A.; Granja, N.P.; Silva Filho, H.M.; Lorenzi, J.O.; Fernandes, A.M.; Soratto, R.P.; Factor, T.L.; Rós, A.B.; Aguiar, E.B. Raízes e Tubérculos. In Boletim 100: Recomendações de Adubação e Calagem Para o Estado de São Paulo; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., Van Raij, B., Eds.; Instituto Agronômico (lAC): Campinas, Brasil, 2022; Volume 1, pp. 314–338. [Google Scholar]
- Santos, T.P.R.; Franco, C.M.L.; Leonel, M. Gelatinized sweet potato starches obtained at different preheating temperatures in a spray dryer. Int. J. Biol. Macromol. 2020, 149, 1339–1346. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch. Starch 1983, 35, 407–410. [Google Scholar] [CrossRef]
- Williams, P.C.; Kuzina, F.D.; Hlynka, L. A rapid calorimetric procedure for estimating the amylose content of starches and flour. Cereal Chem. 1970, 47, 411–420. [Google Scholar]
- Goñi, I.; García-Diz, I.; Mañas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Schoch, T.J. Swelling power and solubility of granular starches. In Methods in Carbohydrate Chemistry, 4th ed.; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1964; pp. 106–109. [Google Scholar]
- Lossolli, N.A.B.; Leonel, M.; Leonel, S.; Izidoro, M.; Paula, G.V.d.; Santos, T.P.R.; Oliveira, L.A.d. Cultivars and fruit part as differentiating factors of physicochemical characteristics of mango starches. Horticulturae 2023, 9, 69. [Google Scholar] [CrossRef]
- Zhu, F.; Corke, H.; Bertoft, E. Amylopectin internal molecular structure in relation to physical properties of sweet potato starch. Carbohydr. Polym. 2011, 84, 907–918. [Google Scholar] [CrossRef]
- Santos, T.P.R.; Franco, C.M.L.; Mischan, M.M.; Leonel, M. Behavior of sweet potato starch after spray-drying under different pretreatment conditions. Starch-Stärke 2019, 71, 1800245. [Google Scholar] [CrossRef]
- Gou, M.; Wu, H.; Saleh, A.S.M.; Jing, L.; Liu, Y.; Zhao, K.; Su, C.; Zhang, B.; Jiang, H.; Li, W. Effects of repeated and continuous dry heat treatments on properties of sweet potato starch. Int. J. Biol. Macromol. 2019, 129, 869–877. [Google Scholar] [CrossRef]
- Teerawanichpan, P.; Lertpanyasampatha, M.; Netrphan, S.; Varavinit, S.; Boonseng, O.; Narangajavana, J. Influence of cassava storage root development and environmental conditions on starch granule size distribution. Starch-Stärke 2008, 60, 696–705. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweet potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, Y.T. Physicochemical and structural properties of different colored sweet potato starches. Starch-Stärke 2017, 69, 3–4. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Sun, J.; Fang, Y.; Liu, J.; Jin, C. Comparison of the structural characterization and physicochemical properties of starches from seven purple sweet potato varieties cultivated in China. Int. J. Biol. Macromol. 2018, 120, 1632–1638. [Google Scholar] [CrossRef]
- Genkina, N.K.; Wasserman, L.A.; Noda, T.; Tester, R.F.; Yuryev, V.P. Effects of annealing on the polymorphic structure of starches from sweet potatoes (Ayamurasaki and Sunnyred cultivars) grown at various soil temperatures. Carbohydr. Res. 2004, 339, 1093–1098. [Google Scholar] [CrossRef]
- Kitahara, K.; Fukunaga, S.; Katayama, K.; Takahata, Y.; Nakazawa, Y.; Yoshinaga, M.; Suganuma, T. Physicochemical properties of sweet potato starches with different gelatinization temperatures. Starch-Stärke 2005, 57, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Aina, A.J.; Falade, K.O.; Akingbala, J.O.; Titus, P. Physicochemical properties of caribbean sweet potato (Ipomoea batatas (L) Lam) starches. Food Bioprocess Technol. 2012, 5, 576–583. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Bian, X.; Guo, K.; Zhou, L.; Wei, C. Characterization and comparative study of starches from seven purple sweet potatoes. Food Hydrocoll. 2018, 80, 168–176. [Google Scholar] [CrossRef]
- Zhao, X.; Andersson, M.; Andersson, R. Resistant starch and other dietary fiber components in tubers from a high-amylose potato. Food Chem. 2018, 251, 58–63. [Google Scholar] [CrossRef]
- Future Market Information. Resistant Starch Market Outlook (2022–2032). Available online: https://www.futuremarketinsights.com/reports/resistant-starch-market. (accessed on 12 October 2022).
- Peroni, F.H.G.; Rocha, T.S.; Franco, C.M.L. Some structural and physicochemical characteristics of tuber and root starches. Food Sci. Technol. Int. 2006, 12, 505–513. [Google Scholar] [CrossRef]
- Abegunde, O.K.; Mu, T.; Chen, J.; Deng, F. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Tsakama, M.; Mwangwela, A.M.; Manani, T.A.; Mahungu, N.M. Physicochemical and pasting properties of starch extracted from eleven sweet potato varieties. Afr. J. Food Sci. 2010, 1, 90–98. [Google Scholar]
- Almeida, V.O.; Batista, K.A.; Di-Medeirosa, M.C.B.; Moraes, M.G.; Fernandes, K.F. Effect of drought stress on the morphological and physicochemical properties of starches from Trimezia juncifolia. Carbohydr. Polym. 2019, 212, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kaur, A.; Shevkani, K.; Ezekiel, R.; Kaur, P.; Isono, N.; Noda, T. Structural, morphological, thermal, and pasting properties of starches from diverse Indian potato cultivars. Starch-Stärke 2018, 70, 1700130. [Google Scholar] [CrossRef]
- Campanha, R.B.; Franco, C.M.L. Gelatinization properties of native starches and their Näegeli dextrins. J. Therm. Anal. Calorim. 2011, 106, 799–804. [Google Scholar] [CrossRef]


| Canadense | Uruguaiana | ANOVA Source of Variation | |||||
|---|---|---|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | C | GS | CxGS | |
| D[4,3] (µm) | 19.29 ± 0.12 bA | 22.61 ± 0.02 aA | 14.68 ± 0.24 bB | 17.14 ± 0.22 aB | *** | *** | ** |
| D[3,2] (µm) | 16.72 ± 0.14 bA | 19.28 ± 0.02 aA | 12.23 ± 0.11 bB | 14.93 ± 0.19 aB | *** | *** | ns |
| D(0.5) (µm) | 18.11 ± 0.12 bA | 21.13 ± 0.02 aA | 13.49 ± 0.16 bB | 16.12 ± 0.20 aB | *** | *** | * |
| Relative crystalinity (%) | 25.67 ± 0.30 bB | 29.92 ± 0.77 aB | 26.96 ± 0.32 bA | 31.13 ± 0.40 aA | ** | *** | ns |
| Amylose (%) | 25.49 ± 5.6 aB | 25.35 ± 1.1 aB | 26.29 ± 2.0 aA | 25.61 ± 1.3 bA | * | ns | * |
| Resistant Starch (%) | 59.16 ± 9.7 bA | 64.34 ± 6.2 aA | 54.88 ± 9.8 bB | 69.22 ± 2.2 aB | *** | *** | ns |
| Swelling Power (g g−1) | 40.09 ± 1.26 aA | 27.18 ± 0.15 bB | 39.07 ± 1.31 aA | 30.56 ± 0.13 bA | * | *** | ** |
| Solubility (%) | 33.38 ± 0.30 aA | 18.48 ± 0.05 bB | 32.15 ± 1.37 aA | 20.39 ± 0.33 bA | ** | *** | ns |
| Canadense | Uruguaiana | ANOVA Source of Variation | |||||
|---|---|---|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | C | GS | CxGS | |
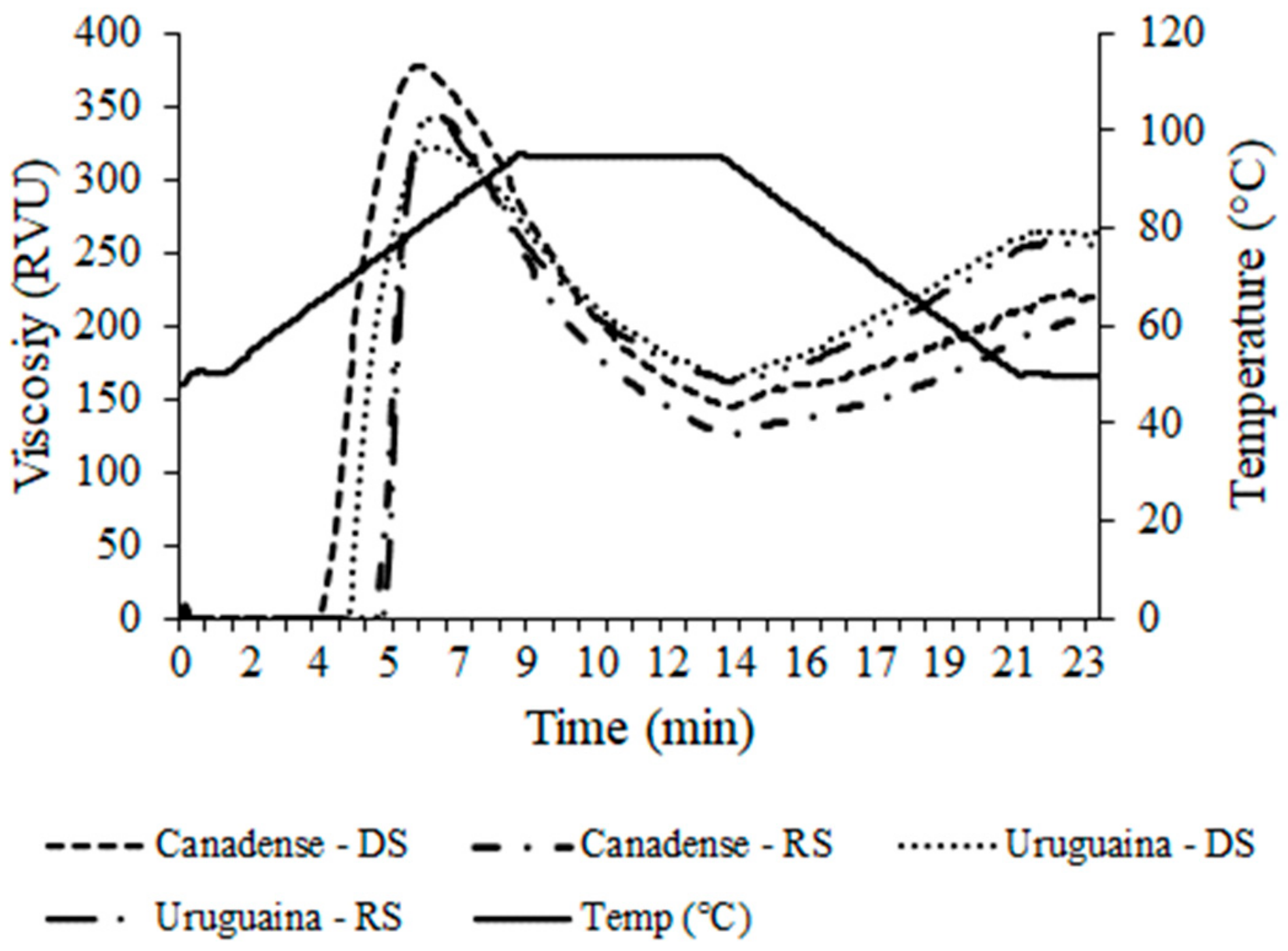
| Peak Viscosity (RVU) | 382.08 ± 13.92 aA | 315.42 ± 0.34 bB | 338.47 ± 5.10 aB | 324.25 ± 2.22 bA | ** | ** | *** |
| Breakdown (RVU) | 234.22 ± 10.24 aA | 193.63 ± 1.54 bA | 177.55 ± 3.30 aB | 156.97 ± 5.17 bB | *** | ** | *** |
| Final Viscosity (RVU) | 222.39 ± 9.94 aB | 198.71 ± 2.13 bB | 251.08 ± 4.63 bA | 271.92 ± 8.81 aA | *** | ** | ns |
| Setback (RVU) | 74.53 ± 1.08 bB | 76.92 ± 0.25 aB | 90.17 ± 1.28 bA | 107.75 ± 0.82 aA | *** | * | ** |
| Pasting Temperature (°C) | 66.23 ± 1.18 bB | 75.63 ± 0.03 aA | 69.57 ± 0.45 bA | 75.38 ± 0.42 aA | * | *** | ** |
| Canadense | Uruguaiana | ANOVA Source of Variation | |||||
|---|---|---|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | C | GS | CxGS | |
| Tonset (°C) | 57.47 ± 0.19 bB | 70.32 ± 0.20 aB | 58.10 ± 0.17 bA | 71.72 ± 0.05 aA | *** | *** | ** |
| Tpeak (°C) | 62.07 ± 0.07 bB | 74.69 ± 0.20 aB | 63.30 ± 0.19 bA | 75.31 ± 0.14 aA | *** | *** | ** |
| Tconclusion (°C) | 68.23 ± 0.09 bB | 79.28 ± 0.10 aA | 70.50 ± 0.41 bA | 79.34 ± 0.23 aA | *** | *** | *** |
| Tconclusion-Tonset (°C) | 10.77 ± 0.27 aB | 8.96 ± 0.15 bA | 12.40 ± 0.41 aA | 7.62 ± 0.19 bB | *** | *** | *** |
| ΔH (J g−1) | 12.51 ± 0.29 bB | 14.84 ± 0.19 aA | 13.94 ± 0.26 bA | 14.85 ± 0.30 aA | * | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, T.P.R.; Leonel, M.; de Oliveira, L.A.; Fernandes, A.M.; Leonel, S.; da Silva Nunes, J.G. Seasonal Variations in the Starch Properties of Sweet Potato Cultivars. Horticulturae 2023, 9, 303. https://doi.org/10.3390/horticulturae9030303
dos Santos TPR, Leonel M, de Oliveira LA, Fernandes AM, Leonel S, da Silva Nunes JG. Seasonal Variations in the Starch Properties of Sweet Potato Cultivars. Horticulturae. 2023; 9(3):303. https://doi.org/10.3390/horticulturae9030303
Chicago/Turabian Styledos Santos, Thaís Paes Rodrigues, Magali Leonel, Luciana Alves de Oliveira, Adalton Mazetti Fernandes, Sarita Leonel, and Jason Geter da Silva Nunes. 2023. "Seasonal Variations in the Starch Properties of Sweet Potato Cultivars" Horticulturae 9, no. 3: 303. https://doi.org/10.3390/horticulturae9030303





