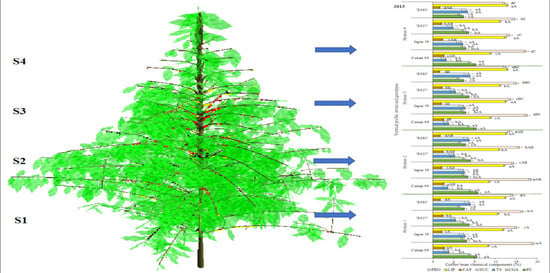
Variation in Yield, Berry Distribution and Chemical Attributes of Coffea arabica Beans among the Canopy Strata of Four Genotypes Cultivated under Contrasted Water Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Plant Coding, Computational Processing, Berry Harvests, and Yield
2.3. Chemical Attributes of the Coffee Beans
2.4. Statistical Analyses
3. Results
3.1. Environmental Conditions—Rainfall and Light Distribution along the Plant Canopy Strata
3.2. Plant Scale—Berry Production and Distribution, Leaf-to Fruit, and Branch-to Fruit Dependency on Water Regime and Genotype
3.3. Components of the Berry and Bean Yields at Plant Scale Dependent on Water Regime, Genotype, and Local Light Availability
3.4. Chemical Attributes of the Coffee Dependency on Water Regime, Genotype, and Irradiance Availability
3.5. Correlations among the Berry Distribution per Strata, Yield, and Chemical Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew. Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- Cassamo, C.T.; Mangueze, A.V.; Leitão, A.E.; Pais, I.P.; Moreira, R.; Campa, C.; Chiulele, R.; Reis, F.O.; Marques, I.; Scotti-Campos, P.; et al. Shade and altitude implications on the physical and chemical attributes of green coffee beans from Gorongosa Mountain, Mozambique. Agronomy 2022, 12, 2540. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/markets-and-trade/commodities/coffee/en/ (accessed on 18 October 2022).
- Coffee Industry Statistics. Available online: https://coffeeaffection.com/coffee-industry-statistics/ (accessed on 4 November 2022).
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests—An Architectural Analysis; Springer: Berlin, Germany, 1978; p. 441. [Google Scholar]
- Rakocevic, M.; Matsunaga, F.T.; Baroni, D.F.; Campostrini, E.; Costes, E. Multiscale analyses of growth and berry distributions along four branching orders and vertical profile of Coffea arabica L. cultivated under high-density planting systems. Sci. Hortic. 2021, 281, 109934. [Google Scholar] [CrossRef]
- Ferreira, T.; Shuler, J.; Guimarães, R.; Farah, A. Introduction to coffee plant and genetics. In Coffee: Production, Quality and Chemistry; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Rakocevic, M.; Androcioli-Filho, A. Morphophysiological characteristics of Coffea arabica L. in different arrangements: Lessons from a 3D virtual plant approach. Coffee Sci. 2010, 5, 54–166. [Google Scholar]
- Camargo, A.P.; Camargo, M.B.P. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 2001, 60, 65–68. [Google Scholar] [CrossRef]
- Quiñones, A.J.P.; Builes, V.H.R.; Robledo, A.J.; Sáenz, J.R.R.; Pulgarín, J.A. Effects of daylength and soil humidity on the flowering of coffee Coffea arabica L. in Colombia. Rev. Fac. Nac. Agron. Medellin 2011, 64, 5745–5754. [Google Scholar]
- Rena, A.B.; Barros, R.S. Aspectos críticos no estudo da floraçãao do café. In Efeitos da Irrigaçãao Sobre a Qualidade e Produtividade do Café; Zambolim, L., Ed.; Universidade Federal de Viçosa: Viçosa, Brazil, 2004; pp. 149–172. [Google Scholar]
- Rakocevic, M.; Braga, K.S.M.; Batista, E.R.; Maia, A.H.N.; Scholz, M.B.S.; Filizola, H.F. The vegetative growth assists to reproductive responses of Arabic coffee trees in a long-term FACE experiment. Plant Growth Regul. 2020, 91, 305–316. [Google Scholar] [CrossRef]
- Cardon, C.H.; de Oliveira, R.R.; Lesy, V.; Ribeiro, T.H.; Fust, C.; Pereira, L.P.; Colasanti, J.; Chalfun-Junior, A. Expression of coffee florigen CaFT1 reveals a sustained floral induction window associated with asynchronous flowering in tropical perennials. Plant Sci. 2022, 325, 111479. [Google Scholar] [CrossRef]
- Morais, H.; Caramori, P.H.; Koguishi, M.S.; Ribeiro, A.M.A. Detailed phenological scale of the reproductive phase of Coffea arabica. Bragantia 2008, 67, 257–260. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Pot, D.; Marraccini, P.; Pereira, L.F.P.; Scholz, M.B.S. Flavor precursors and sensory attributes of coffee submitted to different post-harvest processing. AIMS Agric. Food 2020, 5, 700–714. [Google Scholar] [CrossRef]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of total phenols and antioxidants in coffee samples of different origins and evaluation of the effect of degree of roasting on their levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef] [PubMed]
- Cannell, M.G. Physiology of the coffee crop. In Coffee—Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Crom Helm: London, UK, 1985; pp. 108–134. [Google Scholar]
- Rakocevic, M.; Batista, E.R.; Pazianotto, R.A.A.; Scholz, M.B.S.; Souza, G.A.R.; Campostrini, E.; Ramalho, J.C. Leaf gas exchange and bean quality fluctuations over the whole canopy vertical profile of Arabic coffee cultivated under elevated CO2. Funct. Plant Biol. 2021, 48, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Farah, A. Coffee constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.-F., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 21–58. [Google Scholar]
- Brige, F.A.A.; Celestino, S.M.C.; Amabile, R.F.; Fagioli, M.; Delvico, F.M.S.; Montalvão, A.P.L.; Sala, P.I.A.L. Genetic variability in conilon coffee related to grain attributes in an irrigated crop in the Cerrado. Pesqui. Agropecu Bras. 2019, 54, e00358. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.C.; Calado, V.; Franca, A.S.; Trugo, L.C. Food chemistry correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Kitzberger, C.S.G.; Durand, N.; Rakocevic, M. From the field to coffee cup: Impact of planting design on chlorogenic acid isomers and other compounds in coffee beans and sensory attributes of coffee beverage. Eur. Food. Res. Technol. 2018, 244, 793–1802. [Google Scholar] [CrossRef]
- Velásquez, S.; Banchón, C. Influence of pre-and post-harvest factors on the organoleptic and physicochemical quality of coffee: A short review. J. Food Sci. Technol. 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Lambot, C.; Herrera, J.C.; Bertrand, B.; Sadeghian, S.; Benavides, P.; Gaitán, A. Cultivating coffee quality—Terroir and agro-ecosystem. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 17–49. [Google Scholar] [CrossRef]
- Ginbo, T. Heterogeneous impacts of climate change on crop yields across altitudes in Ethiopia. Clim. Chang. 2022, 170, 12. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bertrand, B.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Léran, S.; Markussen, B.; et al. Shade effects on yield across different Coffea arabica cultivars-how much is too much? A meta-analysis. Agron. Sustain. Dev. 2022, 42, 55. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Rigal, C.; Vaast, P.; Ramalho, J.C.; et al. Shaded-coffee: A nature-based strategy for coffee production under climate change? A Review. Front. Sustain. Food Syst. 2022, 6, 877476. [Google Scholar] [CrossRef]
- Rakocevic, M.; Scholz, M.B.S.; Kitzberger, C.S.G. Berry distributions on coffee trees cultivated under high densities modulate the chemical composition of respective coffee beans during one biannual cycle. Int. J. Fruit Sci. 2018, 18, 117–137. [Google Scholar] [CrossRef]
- Reis, A.R.; Favarin, J.L.; Gallo, L.A.; Moraes, M.F.; Tezotto, T.; Lavres, J.J. Influence of nitrogen fertilization on nickel accumulation and chemical composition of coffee plants during fruit development. J. Plant Nutr. 2011, 34, 1853–1866. [Google Scholar] [CrossRef]
- Vinecky, F.; Davrieux, F.; Mera, A.C.; Alves, G.S.; Lavagnini, G.; Leroy, T.; Bonnot, F.; Rocha, O.C.; Bartholo, G.F.; Guerra, A.F.; et al. Controlled irrigation and nitrogen, phosphorous and potassium fertilization affect the biochemical composition and quality of Arabica coffee beans. J. Agric. Sci. 2017, 155, 902–918. [Google Scholar] [CrossRef]
- Marcheafave, G.G.; Tormena, C.D.; Terrile, A.E.; Salamanca-Neto, C.A.; Sartori, E.R.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S.; Pauli, E.D. Ecometabolic mixture design-fingerprints from exploratory multi-block data analysis in Coffea arabica beans from climate changes: Elevated carbon dioxide and reduced soil water availability. Food. Chem. 2021, 362, 129716. [Google Scholar] [CrossRef] [PubMed]
- Gokavi, N.; Mote, K.; Jayakumar, M.; Raghuramulu, Y.; Surendran, U. The effect of modified pruning and planting systems on growth, yield, labour use efficiency and economics of Arabica coffee. Sci. Hortic. 2021, 276, 109764. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Pais, I.P.; Leitão, A.E.; Guerra, M.; Reboredo, F.H.; Máguas, C.M.; Carvalho, M.L.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Lidon, F.J.; et al. Can elevated air [CO2] conditions mitigate the predicted warming impact on the quality of coffee bean? Front. Plant Sci. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Nogueira, F.D.; Guimarães, P.T.G.; Chagas, S.J.R.; Costa, L. Sources and doses of potassium on the yield and quality of green coffee. Pesq. Agropec. Bras. 1999, 34, 335–345. [Google Scholar] [CrossRef]
- Carr, M. The water relations and irrigation requirements of coffee. Exp. Agric. 2001, 37, 1–36. [Google Scholar] [CrossRef]
- Tesfaye, S.G.; Ismail, M.R.; Kausar, H.; Marziah, M.; Ramlan, M.F. Plant water relations, crop yield and quality in coffee (Coffea arabica L.) as influenced by partial root zone drying and deficit irrigation. AJCS 2013, 7, 1361–1368. [Google Scholar] [CrossRef]
- Liu, X.; Qi, Y.; Li, F.; Yang, Q.; Yu, L. Impacts of regulated deficit irrigation on yield, quality and water use efficiency of Arabica coffee under different shading levels in dry and hot regions of southwest China. Agric. Water Manag. 2018, 204, 292–300. [Google Scholar] [CrossRef]
- Silva, E.A.; Mazzafera, P.; Brunini, O.; Sakai, E.; Arruda, F.B.; Mattoso, L.H.C.; Carvalho, C.R.L.; Pires, R.C.M. The influence of water management and environmental conditions on the chemical composition and beverage quality of coffee beans. Braz. J. Plant Physiol. 2005, 17, 229–238. [Google Scholar] [CrossRef]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate change and coffee quality: Systematic review on the effects of environmental and management variation on secondary metabolites and sensory attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.; Ramalho, J.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Anthony, F.; Quiros, O.; Topart, P.; Bertrand, B.; Lashermes, P. Detection by simple sequence repeat markers of introgression from Coffea canephora in Coffea arabica cultivars. Plant Breed. 2002, 121, 542–544. [Google Scholar] [CrossRef]
- Pérez-Molina, J.P.; de Toledo Picoli, E.A.; Oliveira, L.A.; Silva, B.T.; de Souza, G.A.; dos Santos Rufino, J.L.; Pereira, A.A.; de Freitas Ribeiro, M.; Malvicini, G.L.; Turello, L.; et al. Treasured exceptions: Association of morphoanatomical leaf traits with cup quality of Coffea arabica L. cv. “Catuaí”. Food Res. Int. 2021, 141, 110118. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.L.; Cortez, G.L.; Zaro, G.C.; Zorzenoni, T.O.; Melo, T.R.; Figueiredo, A.; Aquino, G.S.; Medina, C.D.; Ralisch, R.; Caramori, P.H.; et al. Soil morphostructural characterization and coffee root distribution under agroforestry system with Hevea Brasiliensis. Sci. Agric. 2021, 78, e20190150. [Google Scholar] [CrossRef]
- Meireles, E.J.; Camargo, M.B.; Pezzopane, J.R.; Thomaziello, R.A.; Fahl, J.I.; Bardin, L.; Santos, J.C.; Japiassú, L.B.; Garcia, A.W.; Miguel, A.E.; et al. Fenologia do Cafeeiro: Condições Agrometeorológicas e Balanço Hídrico do ano Agrícola 2004–2005; Embrapa Informação Tecnológica: Brasíla, Brazil, 2009; p. 128. [Google Scholar]
- Cesanelli, A.; Guarracino, L. Numerical modeling of actual evapotranspiration of a coffee crop. Sci. Agric. 2011, 68, 395–399. [Google Scholar] [CrossRef]
- Godin, C.; Caraglio, Y. A multiscale model of plant topological structures. J. Theor. Biol. 1998, 191, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, F.T.; Tosti, J.B.; Androcioli-Filho, A.; Brancher, J.D.; Costes, E.; Rakocevic, M. Strategies to reconstruct 3D Coffea arabica L. plant structure. SpringerPlus 2016, 5, 2075. [Google Scholar] [CrossRef]
- Pradal, C.; Boudon, F.; Nouguier, C.; Chopard, J.; Godin, C. PlantGL: A Python-based geometric library for 3D plant modelling at different scales. Graph. Models 2009, 71, 1–21. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Kitzberger, C.S.G.; Pereira, L.F.P.; Davrieux, F.; Pot, D.; Charmetant, P.; Leroy, T. Application of near infrared spectroscopy for green coffee biochemical phenotyping. J. Near Infrared Spectrosc. 2014, 22, 411–421. Available online: https://opg.optica.org/jnirs/abstract.cfm?URI=jnirs-22-6-411 (accessed on 15 December 2022). [CrossRef]
- R Core Team. Available online: https://wwwr-projectorg/ (accessed on 18 December 2022).
- Covre, A.M.; Oliveira, M.G.; Martins, L.D.; Bonomo, R.; Rodrigues, W.N.; Tomaz, M.A.; Duarte, H.; de Sá Paye, H.; Partelli, F.L. How is the fruit development of Coffea canephora trees modulated by the water supply? An analysis of growth curves for irrigated and rainfed systems. Semin. Ciênc. Agrár. Londrina 2022, 43, 2359–2374. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the impact of drought in Coffea genotypes: Transcriptomic analysis supports a common high resilience to moderate water deficit but a genotype dependent sensitivity to severe water deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- Ghosh, P.; Venkatachalapathy, N. Processing and drying of coffee—A review. Int. J. Eng. Res. Technol. 2014, 3, 784–794. [Google Scholar]
- Pérez, V.O.; Pérez, L.G.M.; Fernandez-Alduenda, M.R.; Barreto, C.I.A.; Agudelo, C.P.G.; Restrepo, E.C.M. Chemical composition and sensory quality of coffee fruits at different stages of maturity. Agronomy 2023, 13, 341. [Google Scholar] [CrossRef]
- Soares, A.R. Irrigação Fertirrigação Fisiologia e Produção do Cafeeiro Adulto na Região da Zona da Mata de Minas Gerais. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2001; p. 84. [Google Scholar]
- Baïram, E.; leMorvan, C.; Delaire, M.; Buck-Sorlin, G. Fruit and leaf response to different source–sink ratios in apple, at the scale of the fruit-bearing branch. Front. Plant Sci. 2019, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.C.V.; Araújo, W.L.; Fernie, A.; Moraes, G.A.B.K. In field-grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol. 2008, 178, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Yeager, S.E.; Batali, M.E.; Guinard, J.-X.; Ristenpart, W.D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2021, 23, 1–27. [Google Scholar] [CrossRef]
- González, A.R.; Hernández, C.Y.F.; Rios, O.G.; Quiroz, M.L.S.; Amaro, R.M.G.; Estrada, Z.J.H.; Duarte, P.R. Coffee chlorogenic acids incorporation for bioactivity enhancement of foods: A review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Vaast, P.; Bertrand, B.; Perriot, J.-J.; Guyot, B.; Génard, M. Fruit thinning and shade improve bean characteristics and beverage quality of coffee (Coffea arabica L.) under optimal conditions. J. Sci. Food Agric. 2006, 86, 197–204. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Silveira, S.R.; Ruas, P.M.; Ruas, C.F.; Sera, T.; Carvalho, V.P.; Coelho, A.S.G. Assessment of genetic variability within and among coffee progenies and cultivars using RAPD markers. Genet. Mol. Biol. 2003, 26, 329–336. [Google Scholar] [CrossRef]
- Silva, F.B.; Tormena, C.D.; Pauli, E.D.; de Almeida, A.G.; Berg, A.B.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S.; Marcheafave, G.G. Time dependent berry maturation for planting density levels in Coffea arabica L. beans: Mixture design-fingerprinting using near-infrared transmittance spectroscopy. J. Food Compos. Anal. 2021, 97, 103795. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. Chemistry of Coffee: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Cambridge, MA, USA, 2013; pp. 1–28. [Google Scholar] [CrossRef]
- Toci, A.T.; Neto, V.J.; Torres, A.G.; Farah, A. Changes in triacylglycerols and free fatty acids composition during storage of roasted coffee. Food Sci. Technol. 2013, 50, 581–590. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; Kochko, A.; Dussert, S. Influence of environmental factors, wet processing, and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- Worku, M.; Meulenaer, B.; Duchateau, L.; Boeckx, P. Effect of altitude on biochemical composition and quality of green arabica coffee beans can be affected by shade and postharvest processing method. Food Res. Int. 2018, 105, 278–285. [Google Scholar] [CrossRef]
- Santos, C.A.F.; Leitão, A.E.; Pais, I.P.; Lidon, F.C.; Ramalho, J.C. Perspectives on the potential impacts of climate changes on coffee plant and bean quality. Emir. J. Food Agric. 2015, 27, 152–163. [Google Scholar] [CrossRef]
- Barbosa, M.S.G.; Scholz, M.B.S.; Kitzberger, C.S.G.; Benassi, M.T. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Borém, F.M.; Cirillo, M.A.; Malta, M.R.; Alvarenga, A.A.; Alves, H.M.R. Coffee quality and its interactions with environmental factors in Minas Gerais. Brazil. J. Agric. Sci. 2012, 4, 181–190. [Google Scholar] [CrossRef]
- Mazzafera, P.; Robinson, S.P. Characterization of polyphenol oxidase in coffee. Phytochemistry 2000, 55, 285–296. [Google Scholar] [CrossRef]
- Mintesnot, A.; Dechassa, N. Effect of altitude, shade, and processing methods on the quality and biochemical composition of green coffee beans in Ethiopia. East Afr. J. Sci. 2018, 12, 87–100. [Google Scholar]
- Upadhyay, R.; Rao, L.J.M. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Marcheafave, G.G.; Pauli, E.D.; Tormena, C.D.; Ortiz, M.C.; de Almeida, A.G.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. Factorial design fingerprint discrimination of Coffea arabica beans under elevated carbon dioxide and limited water conditions. Talanta 2020, 209, 120591. [Google Scholar] [CrossRef] [PubMed]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef] [PubMed]
- Geromel, C.; Ferreira, L.P.; Davrieux, F.; Guyot, B.; Ribeyre, F.; dos Santos Scholz, M.B.; Pereira, L.F.; Vaast, P.; Pot, D.; Leroy, T.; et al. Effects of shade on the development and sugar metabolism of coffee (Coffea arabica L.) fruits. Plant Physiol. Biochem. 2008, 46, 569–579. [Google Scholar] [CrossRef] [PubMed]






| Harvest Year | Water Regime | Genotype | FM | DM | BM | Initial Berry Moisture | DM Performance | BM Performance |
|---|---|---|---|---|---|---|---|---|
| 2012 | IRR | ‘E083’ | 573.1 aA | 240.8 aA | 117.4 aA | 54.8 bA | 47.4 aA | 20.6 aA |
| Iapar 59 | 701.0 aA | 254.8 aA | 118.5aA | 64.3 abA | 48.1 aA | 17.6 abA | ||
| Catuaí 99 | 469.1 aA | 168.3 aA | 75.3 aA | 66.8 aA | 43.6 aA | 15.0 bA | ||
| NI | ‘E083’ | 271.4 aB | 117.0 aB | 52.8 aB | 55.3 bA | 40.6 aB | 17.7 aB | |
| Iapar 59 | 398.9 aB | 131.0 aB | 53.9 aB | 63.8 abA | 41.4 aB | 14.6 abB | ||
| Catuaí 99 | 166.9 aB | 44.6 aB | 44.6 aB | 66.3 aA | 36.8 aB | 12.1 bB | ||
| p-value | Genotype | 0.2535 | 0.1609 | 0.0876 | 0.0465 | 0.1085 | 0.0024 | |
| Water regime | 0.0242 | 0.0088 | 0.0031 | 0.8881 | 0.0033 | 0.0161 | ||
| Gen × Water | - | - | - | - | - | - | ||
| 2013 | IRR | ‘E083’ | 3943 aA | 1287 aA | 599 aA | 65.3 aA | 38.6 bB | 17.4 cB |
| ‘E027’ | 1464 bA | 503 bA | 255 bA | 66.9 aA | 33.3 dA | 15.9 dA | ||
| Iapar 59 | 3596 aA | 1300 aA | 637 aA | 65.9 aA | 37.6 cA | 18.9 bA | ||
| Catuaí 99 | 1816 bA | 724 bA | 354 bA | 57.9 bA | 40.8 aB | 20.4 aB | ||
| NI | ‘E083’ | 645 cB | 248 bB | 109 bB | 61.3 aA | 40.1 bA | 20.7 bA | |
| ‘E027’ | 640 cB | 230 bB | 107 bB | 62.8 aA | 31.3 dB | 14.9 dB | ||
| Iapar 59 | 1830 aB | 654 aB | 311 aB | 61.9 aA | 32.9 cB | 16.3 cB | ||
| Catuaí 99 | 1212 bB | 622 aB | 318 bB | 53.8 bA | 49.1 aA | 24.3 aA | ||
| p-value | Genotype | 0.0422 | 0.0036 | 0.0049 | 0.0454 | <0.0001 | <0.0001 | |
| Water regime | <0.0001 | 0.0001 | 0.0001 | 0.0990 | <0.0001 | <0.0001 | ||
| Gen × Water | 0.0248 | 0.0186 | 0.0216 | - | <0.0001 | <0.0001 |
| Harvest Year | Stratum | Genotype | FM | DM | BM | Initial Berry Moisture | DM Performance | BM Performance |
|---|---|---|---|---|---|---|---|---|
| 2012 | S3 | ‘E083’ | 178.2 aA | 85.2 aA | 44.2 aA | 57.1 aA | 47.4 aA | 20.5 aA |
| Iapar 59 | 303.0 aA | 108.4 aA | 52.3 aA | 62.2 aA | 45.8 aA | 18.8 aA | ||
| Catuaí 99 | 187.1 aA | 65.2 aA | 30.5 aA | 65.0 aA | 42.3 bA | 15.4 bA | ||
| S2 | ‘E083’ | 220.4 aA | 98.1 aA | 46.3 aA | 53.1 aA | 45.2 aA | 20.9 aA | |
| Iapar 59 | 336.1 aA | 121.3 aA | 54.4 aA | 58.3 aA | 48.1 aA | 19.2 aA | ||
| Catuaí 99 | 211.4 aA | 78.1 aA | 32.6 aA | 61.0 aA | 40.1 bA | 15.8 bA | ||
| S1 | ‘E083’ | 151.2 aA | 70.0 aA | 35.1 aA | 55.3 aA | 46.0 aA | 20.5 aA | |
| Iapar 59 | 268.1 aA | 93.2 aA | 43.2 aA | 60.4 aA | 46.6 aA | 18.7 aA | ||
| Catuaí 99 | 142.3 aA | 49.9 aA | 21.4 aA | 63.2 aA | 40.8 bA | 15.3 bA | ||
| p-value | Genotype | 0.3217 | 0.4274 | 0.3739 | 0.2044 | 0.0298 | 0.0245 | |
| Stratum | 0.7031 | 0.5813 | 0.6561 | 0.6534 | 0.7322 | 0.9427 | ||
| Gen × Stratum | - | - | - | - | - | - | ||
| 2013 | S4 | ‘E083’ | 488.7 abA | 152.1 abAB | 65.3 abAB | 61.4 abA | 43.2 bA | 18.6 bA |
| ‘E027’ | 131.6 bA | 37.1 bAB | 14.4 bAB | 64.8 aA | 50.5 bAB | 19.4 bA | ||
| Iapar 59 | 752.1 aA | 263.7 aAB | 124.4 aAB | 63.7 aA | 44.5 bA | 18.3 bA | ||
| Catuaí 99 | 283.0 abA | 143.3 abAB | 68.0 abAB | 55.7 bA | 51.4 aA | 24.8 aA | ||
| S3 | ‘E083’ | 799.0 abA | 290.7 abA | 135.9 abA | 60.8 abA | 42.5 bA | 17.3 bA | |
| ‘E027’ | 441.9 bA | 175.6 bA | 85.1 bA | 64.2 aA | 49.6 aAB | 18.1 bA | ||
| Iapar 59 | 1062.4 aA | 402.2 aA | 195.0 aA | 63.1 aA | 46.7 abA | 17.0 bA | ||
| Catuaí 99 | 593.3 abA | 281.9 abA | 138.6 abA | 55.1 bA | 48.7 aB | 23.5 aA | ||
| S2 | ‘E083’ | 777.9 abA | 252.2 abAB | 116.2 abAB | 60.8 abA | 43.3 bA | 17.2 bA | |
| ‘E027’ | 420.8 bA | 137.1 bAB | 65.3 bAB | 64.2 aA | 51.3 aA | 18.0 bA | ||
| Iapar 59 | 1041.3 aA | 363.8 aAB | 175.3 aAB | 63.1 aA | 48.9 aA | 16.9 bA | ||
| Catuaí 99 | 572.2 abA | 243.4 abAB | 118.9 abAB | 55.1 bA | 46.5 abB | 23.4 aA | ||
| S1 | ‘E083’ | 408.9 abA | 122.7 abB | 52.2 abB | 62.2 abA | 44.8 abA | 16.1 bA | |
| ‘E027’ | 51.8 bA | 7.7 bB | 1.33 bB | 65.6 aA | 44.6 abB | 16.9 bA | ||
| Iapar 59 | 672.2 aA | 234.3 aB | 111.3 aB | 64.4 aA | 49.3 aA | 15.8 bA | ||
| Catuaí 99 | 203.1 abA | 114.0 abB | 54.9 abB | 56.5 bA | 41.0 bB | 22.3 aA | ||
| p-value | Genotype | 0.0358 | <0.0001 | 0.0353 | 0.0213 | <0.0001 | <0.0001 | |
| Stratum | 0.1581 | 0.0429 | 0.0473 | 0.9569 | 0.0428 | 0.2244 | ||
| Gen × Stratum | - | - | - | - | 0.0005 | - |
| Harvest Year | Water Regime | Genotype | PRO | LIP | CAF | SUC | TS | CGA | PC |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | IRR | ‘E083’ | 15.0 bB | 15.4 aB | 1.32 bA | 7.41 aA | 7.82 aA | 5.31 bA | 6.80 aA |
| Iapar 59 | 16.3 aB | 14.0 bB | 1.58 aA | 7.01 aA | 7.43 aA | 5.75 aA | 7.28 aA | ||
| Catuaí 99 | 16.4 aB | 13.5 bB | 1.56 aA | 6.43 aA | 6.75 aA | 5.10 bA | 7.60 aA | ||
| NI | ‘E083’ | 15.8 bA | 16.2 aA | 1.25 bA | 6.47 aA | 6.94 aA | 5.22 bA | 7.27 aA | |
| Iapar 59 | 17.1 aA | 14.8 bA | 1.51 aA | 6.07 aA | 6.55 aA | 5.66 aA | 7.76 aA | ||
| Catuaí 99 | 17.2 aA | 14.3 bA | 1.49 aA | 5.49 aA | 5.87 aA | 5.01 bA | 8.08 aA | ||
| p-value | Genotype | <0.0001 | 0.0004 | 0.0004 | 0.1401 | 0.1053 | 0.0036 | 0.0855 | |
| Water regime | 0.0003 | 0.0258 | 0.1776 | 0.0703 | 0.0848 | 0.5799 | 0.1759 | ||
| Gen × Water | - | - | - | - | - | - | - | ||
| 2013 | IRR | ‘E083’ | 14.6 cA | 14.2 aA | 1.34 dA | 6.74 aA | 6.99 aA | 4.96 cA | 5.88 bA |
| ‘E027’ | 16.3 bA | 12.3 cB | 1.80 bA | 4.47 bA | 4.59 bA | 5.65 bA | 6.57 bA | ||
| Iapar 59 | 15.4 cA | 13.2 bB | 1.61 cA | 6.13 aA | 6.37 aA | 5.52 bA | 6.08 bA | ||
| Catuaí 99 | 18.3 aA | 10.9 dA | 2.03 aA | 3.28 bA | 3.48 cA | 6.84 aA | 7.93 aA | ||
| NI | ‘E083’ | 14.2 cA | 14.0 aA | 1.30 dA | 6.87 aA | 7.19 aA | 5.12 cA | 6.23 bA | |
| ‘E027’ | 15.9 bA | 13.1 bA | 1.76 bA | 4.60 bA | 4.79 bA | 5.81 bA | 6.93 bA | ||
| Iapar 59 | 15.0 cA | 14.4 aA | 1.57 cA | 6.26 aA | 6.57 aA | 5.67 bA | 6.43 bA | ||
| Catuaí 99 | 18.0 aA | 10.9 cA | 1.98 aA | 3.42 bA | 3.59 cA | 7.00 aA | 8.28 aA | ||
| p-value | Genotype | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Water regime | 0.1634 | 0.0019 | 0.2509 | 0.6737 | 0.5294 | 0.2812 | 0.1308 | ||
| Gen × Water | - | 0.0001 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakocevic, M.; dos Santos Scholz, M.B.; Pazianotto, R.A.A.; Matsunaga, F.T.; Ramalho, J.C. Variation in Yield, Berry Distribution and Chemical Attributes of Coffea arabica Beans among the Canopy Strata of Four Genotypes Cultivated under Contrasted Water Regimes. Horticulturae 2023, 9, 215. https://doi.org/10.3390/horticulturae9020215
Rakocevic M, dos Santos Scholz MB, Pazianotto RAA, Matsunaga FT, Ramalho JC. Variation in Yield, Berry Distribution and Chemical Attributes of Coffea arabica Beans among the Canopy Strata of Four Genotypes Cultivated under Contrasted Water Regimes. Horticulturae. 2023; 9(2):215. https://doi.org/10.3390/horticulturae9020215
Chicago/Turabian StyleRakocevic, Miroslava, Maria Brigida dos Santos Scholz, Ricardo Antônio Almeida Pazianotto, Fabio Takeshi Matsunaga, and José Cochicho Ramalho. 2023. "Variation in Yield, Berry Distribution and Chemical Attributes of Coffea arabica Beans among the Canopy Strata of Four Genotypes Cultivated under Contrasted Water Regimes" Horticulturae 9, no. 2: 215. https://doi.org/10.3390/horticulturae9020215