Genome-Wide Analysis of the Related to ABI3/VP1 Family Genes in Chrysanthemum seticuspe Reveals Their Response Patterns to Exogenous Ethylene Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Ethephon Treatment
2.2. Total RNA Isolation and qRT-PCR Analysis
2.3. Identification and Physicochemical Property Analysis of CsRAV Family Genes in C. seticuspe
2.4. Phylogenetic Tree Construction and Multiple Sequence Alignment
2.5. Protein and Gene Structure Prediction of CsRAVs
2.6. Promoter Sequence Analysis and Tissue Expression Analysis of CsRAV Family Genes
2.7. Statistical Analysis of Data
3. Results
3.1. Identification and Physicochemical Properties of CsRAV Genes in C. seticuspe
3.2. Phylogenetic Tree and Multiple Sequence Alignment Analyses of CsRAV Family Genes
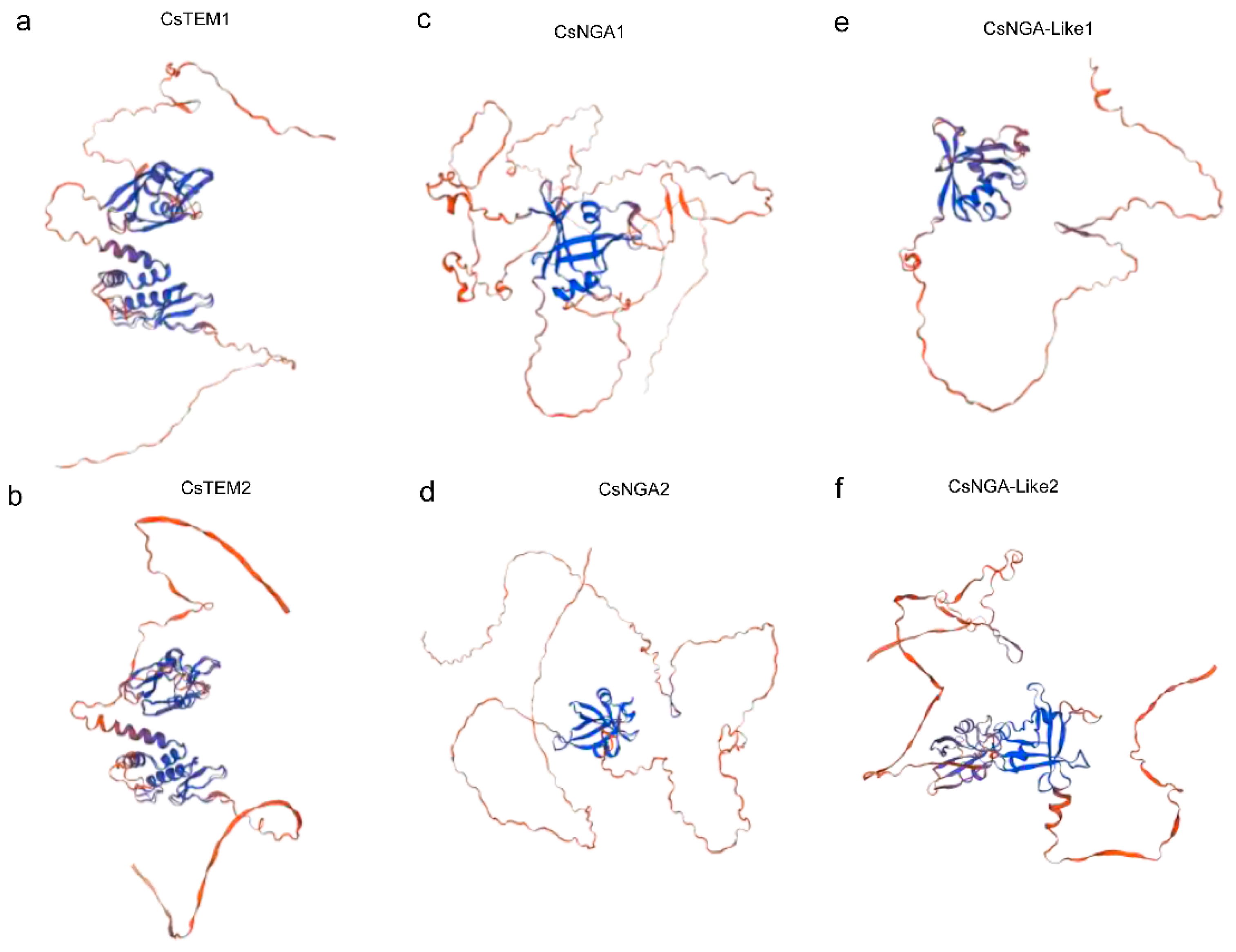
3.3. Predictions of Secondary and Tertiary Structural Characteristics of CsRAV Proteins
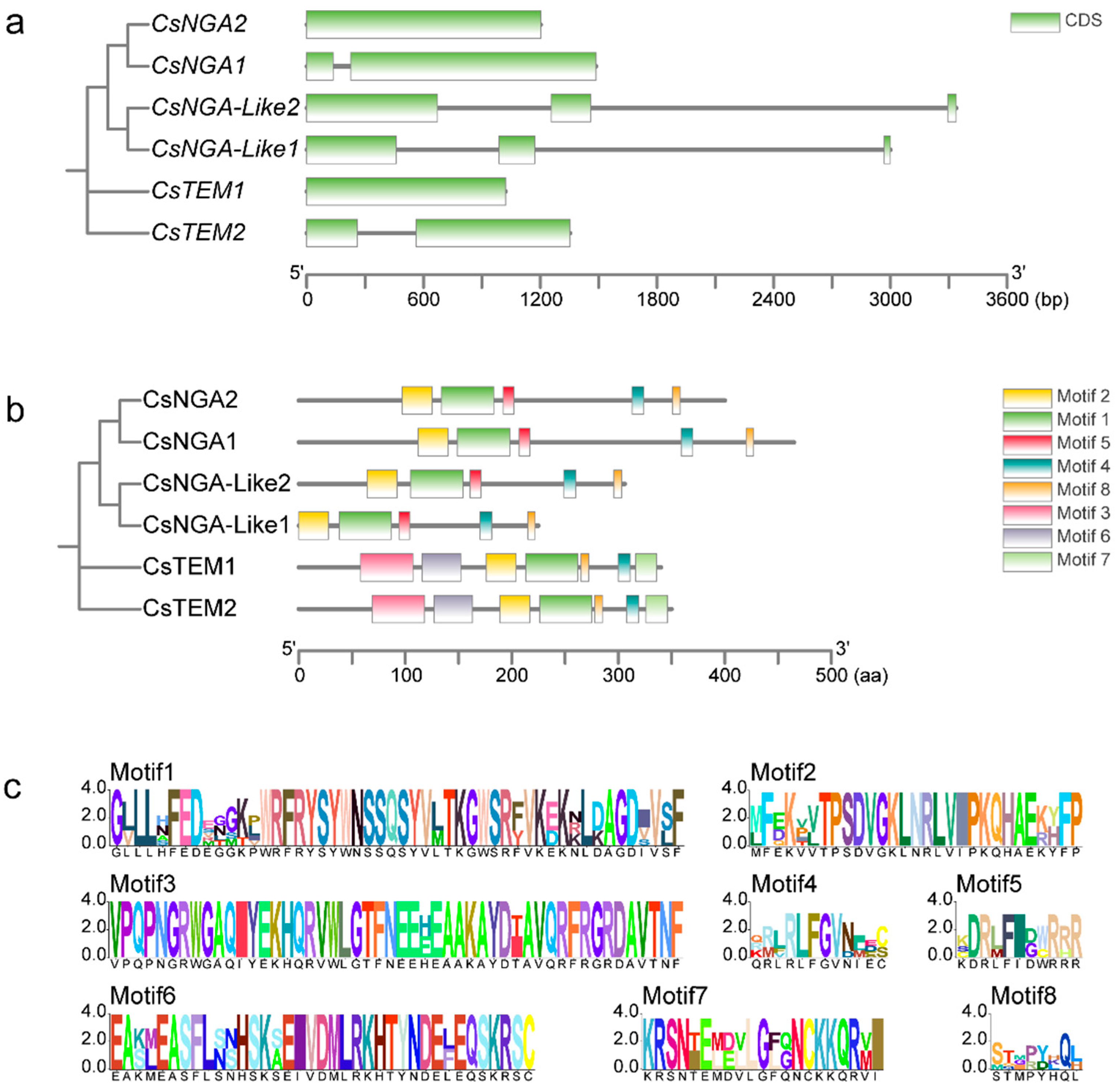
3.4. Analyses of Gene Structure of CsRAV Genes and Its Encoding Protein Conserved Motifs
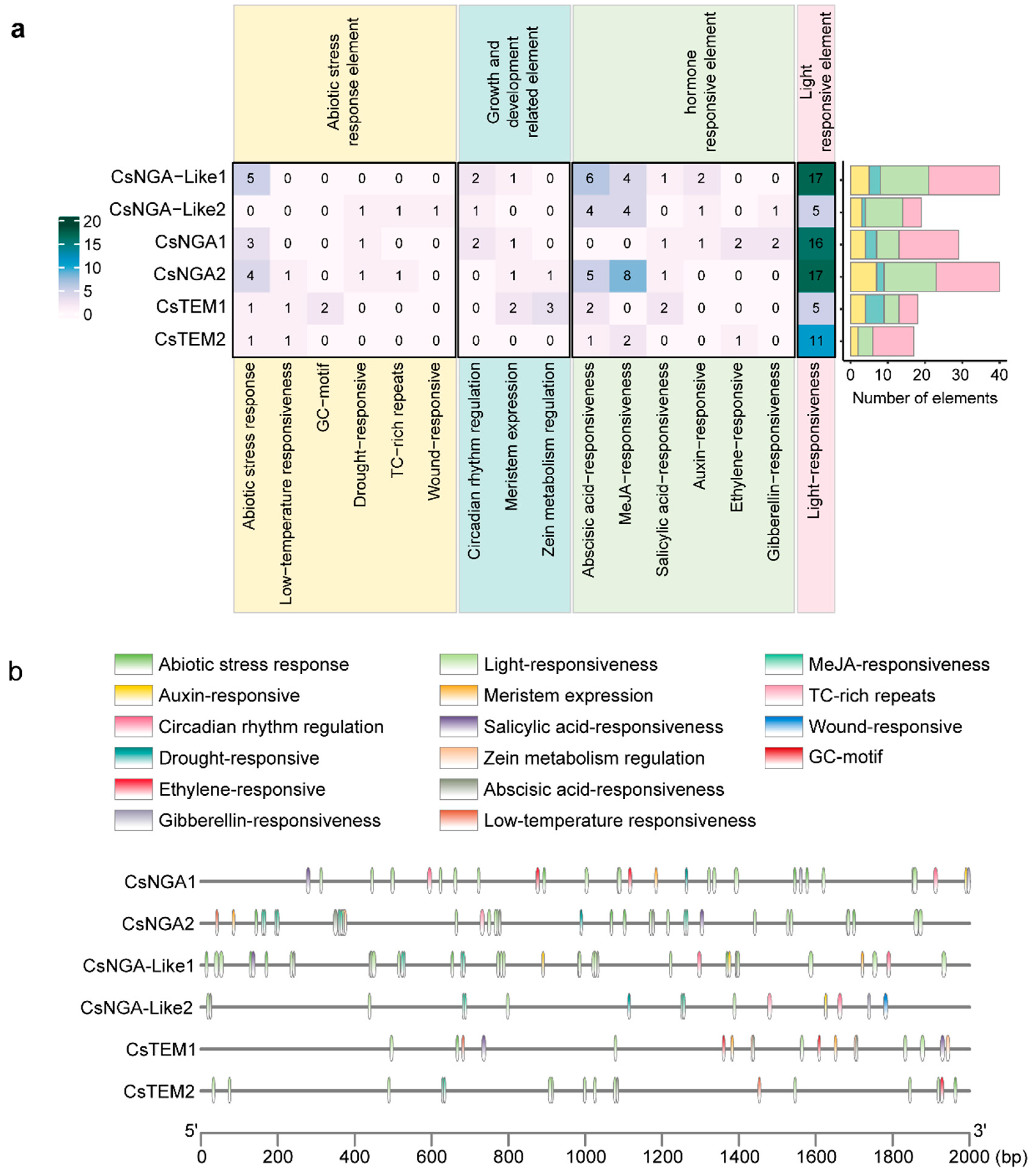
3.5. Analysis of Cis-Acting Elements in CsRAV Gene Family
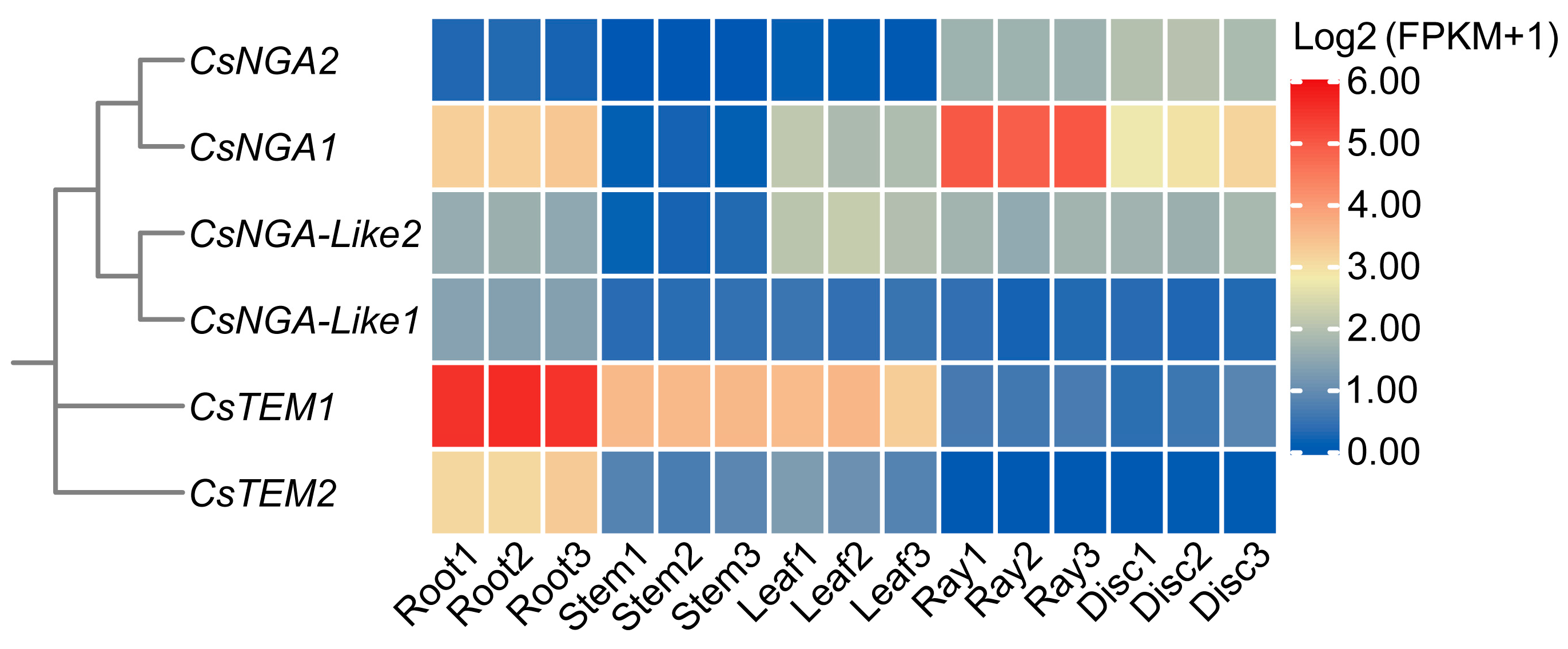
3.6. Expression Profiling of CsRAV Genes across Different Tissues
3.7. Expression Analysis of CsRAVs to Ethylene Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Bhatia, S. A comprehensive analysis of the B3 superfamily identifies tissue-specific and stress-responsive genes in chickpea (Cicer arietinum L.). 3 Biotech 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, K.; Peterson, K.; Jack, T. The plant B3 superfamily. Trends Plant Sci. 2008, 13, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Sgamma, T.; Jackson, A.; Muleo, R.; Thomas, B.; Massiah, A. TEMPRANILLO is a regulator of juvenility in plants. Sci. Rep. 2014, 4, 3704. [Google Scholar] [CrossRef] [PubMed]
- Matías-Hernández, L.; Aguilar-Jaramillo, A.E.; Marín-González, E.; Suárez-López, P.; Pelaz, S. RAV genes: Regulation of floral induction and beyond. Ann. Bot. 2014, 114, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Kang, H.K.; Son, S.H.; Kim, S.K.; Nam, K.H. A subset of Arabidopsis RAV transcription factors modulates drought and salt stress responses independent of ABA. Plant Cell Physiol. 2014, 55, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Salava, H.; Thula, S.; Sánchez, A.S.; Nodzyński, T.; Maghuly, F. Genome Wide Identification and Annotation of NGATHA Transcription Factor Family in Crop Plants. Int. J. Mol. Sci. 2022, 23, 7063. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, Y.; Ohmiya, K.; Hattori, T. RAV1, a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res. 1999, 27, 470–478. [Google Scholar] [CrossRef]
- Hu, Y.X.; Wang, Y.X.; Liu, X.F.; Li, J.Y. Arabidopsis RAV1 is down-regulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res. 2004, 14, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Luo, Q.; Yang, C.; Han, Y.; Li, W. A RAV-like transcription factor controls photosynthesis and senescence in soybean. Planta 2008, 227, 1389–1399. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Aguilar-Jaramillo, A.E.; Osnato, M.; Weinstain, R.; Shani, E.; Suárez-López, P.; Pelaz, S. TEMPRANILLO Reveals the Mesophyll as Crucial for Epidermal Trichome Formation. Plant Physiol. 2016, 170, 1624–1639. [Google Scholar] [CrossRef]
- Osnato, M.; Matias-Hernandez, L.; Aguilar-Jaramillo, A.E.; Kater, M.M.; Pelaz, S. Genes of the RAV Family Control Heading Date and Carpel Development in Rice. Plant Physiol. 2020, 183, 1663–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, C.; Sun, J.; Dong, L.; Li, M.; Liu, Y.; Wang, J.; Zhang, X.; Li, D.; Sun, J.; et al. GmRAV confers ecological adaptation through photoperiod control of flowering time and maturity in soybean. Plant Physiol. 2021, 187, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, T.; Wang, Z.; Kang, J.; Yang, Q.; Shen, Y.; Long, R. Expression of Three Related to ABI3/VP1 Genes in Medicago truncatula Caused Increased Stress Resistance and Branch Increase in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kwon, S.H.; Lee, S.J.; Park, S.K.; Song, J.T.; Lee, S.; Lee, M.M.; Hwang, Y.S.; Kim, J.H. The Arabidopsis thaliana NGATHA transcription factors negatively regulate cell proliferation of lateral organs. Plant Mol. Biol. 2015, 89, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, L.; Xu, R.; Cui, R.; Hao, J.; Sun, C.; Li, Y. Transcription factors SOD7/NGAL2 and DPA4/NGAL3 act redundantly to regulate seed size by directly repressing KLU expression in Arabidopsis thaliana. Plant Cell 2015, 27, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, D.; Jia, W.; Ma, X.; Yang, Y.; Xu, Z. Molecular characterization and systematic analysis of NtAP2/ERF in tobacco and functional determination of NtRAV-4 under drought stress. Plant Physiol. Biochem. 2020, 156, 420–435. [Google Scholar] [CrossRef]
- Zhao, S.P.; Xu, Z.S.; Zheng, W.J.; Zhao, W.; Wang, Y.X.; Yu, T.F.; Chen, M.; Zhou, Y.B.; Min, D.H.; Ma, Y.Z.; et al. Genome-Wide Analysis of the RAV Family in Soybean and Functional Identification of GmRAV-03 Involvement in Salt and Drought Stresses and Exogenous ABA Treatment. Front. Plant Sci. 2017, 8, 905. [Google Scholar] [CrossRef]
- Li, J.; Song, C.; Li, H.; Wang, S.; Hu, L.; Yin, Y.; Wang, Z.; He, W. Comprehensive analysis of cucumber RAV family genes and functional characterization of CsRAV1 in salt and ABA tolerance in cucumber. Front. Plant Sci. 2023, 14, 1115874. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lu, Y.; Bai, Y.; Wei, Y.; Liu, G.; Shi, H. MeRAV5 promotes drought stress resistance in cassava by modulating hydrogen peroxide and lignin accumulation. Plant J. 2021, 107, 847–860. [Google Scholar] [CrossRef]
- Zhuang, J.; Sun, C.C.; Zhou, X.R.; Xiong, A.S.; Zhang, J. Isolation and characterization of an AP2/ERF-RAV transcription factor BnaRAV-1-HY15 in Brassica napus L. HuYou15. Mol. Biol. Rep. 2011, 38, 3921–3928. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-Wide Insertional Mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Smet, D.; Van Der Straeten, D. Ethylene and Hormonal Cross Talk in Vegetative Growth and Development. Plant Physiol. 2015, 169, 61–72. [Google Scholar] [CrossRef]
- Wang, X.; Gomes, M.M.; Bailly, C.; Nambara, E.; Corbineau, F. Role of ethylene and proteolytic N-degron pathway in the regulation of Arabidopsis seed dormancy. J. Integr. Plant Biol. 2021, 63, 2110–2122. [Google Scholar] [CrossRef]
- Rashid, D.; Devani, R.S.; Rodriguez-Granados, N.Y.; Abou-Choucha, F.; Troadec, C.; Morin, H.; Tan, F.Q.; Marcel, F.; Huang, H.Y.; Hanique, M.; et al. Ethylene produced in carpel primordia controls CmHB40 expression to inhibit stamen development. Nat. Plants 2023, 9, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, S.; Yu, W.; Liao, Y.; Pan, C.; Zhang, M.; Tao, R.; Wei, J.; Gao, Y.; Wang, D.; et al. The ethylene-responsive transcription factor PpERF9 represses PpRAP2.4 and PpMYB114 via histone deacetylation to inhibit anthocyanin biosynthesis in pear. Plant Cell 2023, 35, 2271–2292. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Lei, M.; Fu, Y.; Zhao, J.; Ao, M.; Xu, L. Integrated DNA methylome and transcriptome analysis reveals the ethylene-induced flowering pathway genes in pineapple. Sci. Rep. 2017, 7, 17167. [Google Scholar] [CrossRef]
- Sumitomo, K.; Narumi, T.; Satoh, S.; Hisamatsu, T. Involvement of the ethylene response pathway in dormancy induction in chrysanthemum. J. Exp. Bot. 2008, 59, 4075–4082. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, J.H.; Kim, J.; Kim, J.; Lee, U.; Song, I.J.; Kim, J.H.; Lee, H.Y.; Nam, H.G.; Lim, P.O. The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis. J. Exp. Bot. 2010, 61, 3947–3957. [Google Scholar] [CrossRef]
- Chen, W.H.; Li, P.F.; Chen, M.K.; Lee, Y.I.; Yang, C.H. FOREVER YOUNG FLOWER Negatively Regulates Ethylene Response DNA-Binding Factors by Activating an Ethylene-Responsive Factor to Control Arabidopsis Floral Organ Senescence and Abscission. Plant Physiol. 2015, 168, 1666–1683. [Google Scholar] [CrossRef]
- Zhang, Y.; Zang, Y.; Chen, J.; Feng, S.; Zhang, Z.; Hu, Y.; Zhang, T. A truncated ETHYLENE INSENSITIVE3-like protein, GhLYI, regulates senescence in cotton. Plant Physiol. 2023, 193, 1177–1196. [Google Scholar] [CrossRef]
- Hou, Y.; Wong, D.C.J.; Li, Q.; Zhou, H.; Zhu, Z.; Gong, L.; Liang, J.; Ren, H.; Liang, Z.; Wang, Q.; et al. Dissecting the effect of ethylene in the transcriptional regulation of chilling treatment in grapevine leaves. Plant Physiol. Biochem. 2023, 196, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Ahmad, F.; Zang, Y.; Jin, S.; Ahmed, S.; Li, J.; Islam, F.; Ahmad, M.; Zhang, Y.; Hu, Y.; et al. HEAT-RESPONSIVE PROTEIN regulates heat stress via fine-tuning ethylene/auxin signaling pathways in cotton. Plant Physiol. 2023, 191, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hartman, S.; van Veen, H.; Zhang, H.; Leeggangers, H.; Martopawiro, S.; Bosman, F.; de Deugd, F.; Su, P.; Hummel, M.; et al. Ethylene augments root hypoxia tolerance via growth cessation and reactive oxygen species amelioration. Plant Physiol. 2022, 190, 1365–1383. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Moya, J.; Abreu, A.C.; Alonso, S.; Torres-García, M.T.; Martínez, C.; Fernández, I.; Jamilena, M. Physiological and metabolomic responses of the ethylene insensitive squash mutant etr2b to drought. Plant Sci. 2023, 336, 111853. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, J.; Shi, H.; Gu, J.; Dong, J.; Deng, X.W.; Huang, R. Salt Stress and Ethylene Antagonistically Regulate Nucleocytoplasmic Partitioning of COP1 to Control Seed Germination. Plant Physiol. 2016, 170, 2340–2350. [Google Scholar] [CrossRef]
- Lumba, S.; Tsuchiya, Y.; Delmas, F.; Hezky, J.; Provart, N.J.; Shi Lu, Q.; McCourt, P.; Gazzarrini, S. The embryonic leaf identity gene FUSCA3 regulates vegetative phase transitions by negatively modulating ethylene-regulated gene expression in Arabidopsis. BMC Biol. 2012, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Q.; Zhang, Z.; Cheng, P.; Song, A.; Zhou, L.; Wang, L.; Chen, S.; Chen, F.; Jiang, J. The RAV transcription factor TEMPRANILLO1 involved in ethylene-mediated delay of chrysanthemum flowering. Plant J. 2023. [Google Scholar] [CrossRef]
- Song, A.; Su, J.; Wang, H.; Zhang, Z.; Zhang, X.; Van de Peer, Y.; Chen, F.; Fang, W.; Guan, Z.; Zhang, F.; et al. Analyses of a chromosome-scale genome assembly reveal the origin and evolution of cultivated chrysanthemum. Nat. Commun. 2023, 14, 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Li, P.; Gao, Y.; Yu, Q.; Sun, D.; Zhang, L.; Wang, S.; Tian, J.; Wang, Z.; et al. Development of a Transformation System and Locus Identification Pipeline for T-DNA in Chrysanthemum seticuspe, A Model Species for Hexaploid Cultivated Chrysanthemum. Int. J. Mol. Sci. 2022, 23, 11426. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Zhang, H.; Ma, Q.; Wei, Z.; Chen, J.; Sun, Z. Genome-Wide Analysis of the RAV Transcription Factor Genes in Rice Reveals Their Response Patterns to Hormones and Virus Infection. Viruses 2021, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.X.; Chen, S.K.; Wang, P.D.; Peng, D.; Zhang, X.; Li, H.F.; Feng, C.Z. Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress. Int. J. Mol. Sci. 2022, 23, 8834. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ding, L.; Jiang, J.; Shentu, Y.; Zhao, W.; Zhao, K.; Zhang, X.; Song, A.; Chen, S.; Chen, F. Overexpression of the CmJAZ1-like gene delays flowering in Chrysanthemum morifolium. Hortic. Res. 2021, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Sumitomo, K.; Hisamatsu, T.; Nagano, S.; Shirasawa, K.; Higuchi, Y.; Kusaba, M.; Koshioka, M.; Nakano, Y.; Yagi, M.; et al. De novo whole-genome assembly in Chrysanthemum seticuspe, a model species of Chrysanthemums, and its application to genetic and gene discovery analysis. DNA Res. 2019, 26, 195–203. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, J.; He, J.; Geng, Z.; Li, S.; Zhang, J.; Li, P.; Zhang, L.; Wang, Z.; Wang, L.; et al. Whole-transcriptome profiles of Chrysanthemum seticuspe improve genome annotation and shed new light on mRNA-miRNA-lncRNA networks in ray florets and disc florets. BMC Plant Biol. 2022, 22, 515. [Google Scholar] [CrossRef]
- Shang, H.; Li, W.; Zou, C.; Yuan, Y. Analyses of the NAC transcription factor gene family in Gossypium raimondii Ulbr.: Chromosomal location, structure, phylogeny, and expression patterns. J. Integr. Plant Biol. 2013, 55, 663–676. [Google Scholar] [CrossRef]
- Hu, W.; Wei, Y.; Xia, Z.; Yan, Y.; Hou, X.; Zou, M.; Lu, C.; Wang, W.; Peng, M. Genome-Wide Identification and Expression Analysis of the NAC Transcription Factor Family in Cassava. PLoS ONE 2015, 10, e0136993. [Google Scholar] [CrossRef]
- Wan, S.; Li, W.; Zhu, Y.; Liu, Z.; Huang, W.; Zhan, J. Genome-wide identification, characterization and expression analysis of the auxin response factor gene family in Vitis vinifera. Plant Cell Rep. 2014, 33, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ohme-Takagi, M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 2009, 50, 970–975. [Google Scholar] [CrossRef]
- Rose, A.B. Intron-mediated regulation of gene expression. Curr. Top. Microbiol. Immunol. 2008, 326, 277–290. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Marín-González, E.; Matías-Hernández, L.; Aguilar-Jaramillo, A.E.; Lee, J.H.; Ahn, J.H.; Suárez-López, P.; Pelaz, S. SHORT VEGETATIVE PHASE Up-Regulates TEMPRANILLO2 Floral Repressor at Low Ambient Temperatures. Plant Physiol. 2015, 169, 1214–1224. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, L.; Li, D.; Hao, D.; Zhan, Y.; Li, W. A GmRAV ortholog is involved in photoperiod and sucrose control of flowering time in soybean. PLoS ONE 2014, 9, e89145. [Google Scholar] [CrossRef]
- Shin, H.Y.; Nam, K.H. RAV1 Negatively Regulates Seed Development by Directly Repressing MINI3 and IKU2 in Arabidopsis. Mol. Cells 2018, 41, 1072–1080. [Google Scholar] [CrossRef]
- Kabir, N.; Lin, H.; Kong, X.; Liu, L.; Qanmber, G.; Wang, Y.; Zhang, L.; Sun, Z.; Yang, Z.; Yu, Y.; et al. Identification, evolutionary analysis and functional diversification of RAV gene family in cotton (G. hirsutum L.). Planta 2021, 255, 14. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Castillejo, C.; Matías-Hernández, L.; Pelaz, S. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat. Commun. 2012, 3, 808. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lu, X.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene signaling in rice and Arabidopsis: Conserved and diverged aspects. Mol. Plant 2015, 8, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Oluwasanya, D.; Esan, O.; Hyde, P.T.; Kulakow, P.; Setter, T.L. Flower Development in Cassava Is Feminized by Cytokinin, While Proliferation Is Stimulated by Anti-Ethylene and Pruning: Transcriptome Responses. Front. Plant Sci. 2021, 12, 666266. [Google Scholar] [CrossRef] [PubMed]



| Cse_ID | Cse_Name | Number of Amino Acids | Theoretical pI | Molecular Weight | Instability Index | Grand Average of Hydropathicity | Predicted Location |
|---|---|---|---|---|---|---|---|
| CsG_LG1.g11139.1 | CsNGA2 | 400 aa | 8.81 | 45.22 kDa | 67.19 | −0.755 | Nucleus |
| CsG_LG2.g22046.1 | CsNGA-Like2 | 306 aa | 6.34 | 35.13 kDa | 54.52 | −0.992 | Nucleus |
| CsG_LG3.g53814.1 | CsNGA1 | 465 aa | 6.28 | 51.70 kDa | 59.07 | −0.898 | Nucleus |
| CsG_LG8.g07956.1 | CsNGA-Like1 | 225 aa | 6.63 | 25.60 kDa | 49.42 | −0.669 | Nucleus |
| CsG_LG8.g32031.1 | CsTEM1 | 340 aa | 9.12 | 37.98 kDa | 39.72 | −0.555 | Nucleus |
| CsG_LG9.g58287.i1 | CsTEM2 | 350 aa | 9.52 | 39.31 kDa | 52.27 | −0.667 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Yang, Y.; Li, J.; Chen, S.; Chen, F.; Jiang, J. Genome-Wide Analysis of the Related to ABI3/VP1 Family Genes in Chrysanthemum seticuspe Reveals Their Response Patterns to Exogenous Ethylene Treatment. Horticulturae 2023, 9, 1316. https://doi.org/10.3390/horticulturae9121316
Cheng H, Yang Y, Li J, Chen S, Chen F, Jiang J. Genome-Wide Analysis of the Related to ABI3/VP1 Family Genes in Chrysanthemum seticuspe Reveals Their Response Patterns to Exogenous Ethylene Treatment. Horticulturae. 2023; 9(12):1316. https://doi.org/10.3390/horticulturae9121316
Chicago/Turabian StyleCheng, Hua, Yiman Yang, Jiayu Li, Sumei Chen, Fadi Chen, and Jiafu Jiang. 2023. "Genome-Wide Analysis of the Related to ABI3/VP1 Family Genes in Chrysanthemum seticuspe Reveals Their Response Patterns to Exogenous Ethylene Treatment" Horticulturae 9, no. 12: 1316. https://doi.org/10.3390/horticulturae9121316





