Production of Konjac Glucomannan Antimicrobial Film for Extending Shelf Life of Fresh-Cut Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Emulsion Preparation and Antimicrobial Assessment
2.2.2. Film Preparation and Antimicrobial Assessment
2.2.3. Evaluation of Mechanical Property of Antimicrobial Film
2.2.4. Antimicrobial Assessment of Fresh-Cut Vegetables during Storage
2.2.5. Statistical Analyses
3. Results and Discussion
3.1. Antimicrobial Assessment of KGM/SB Emulsion and Film
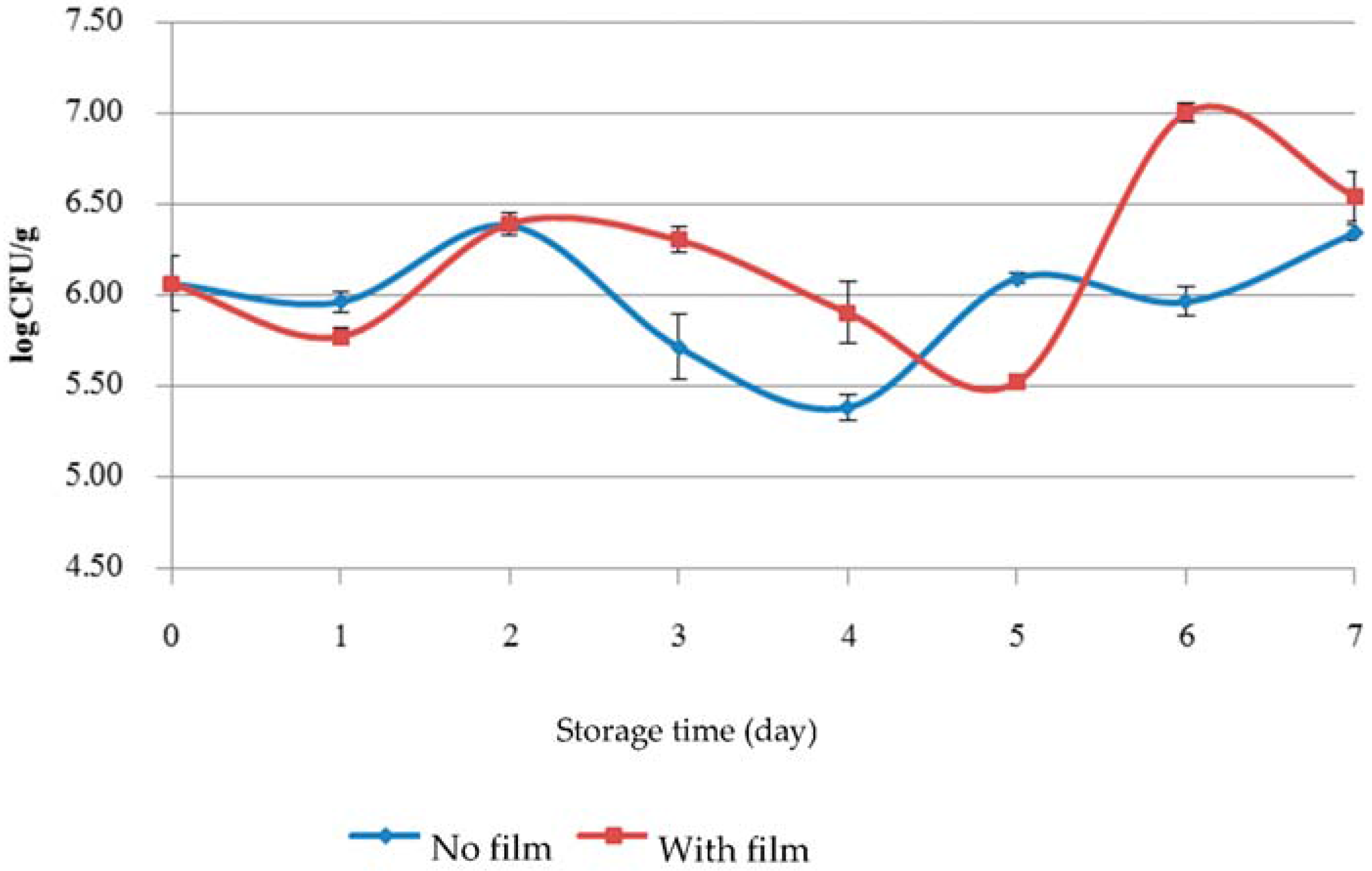
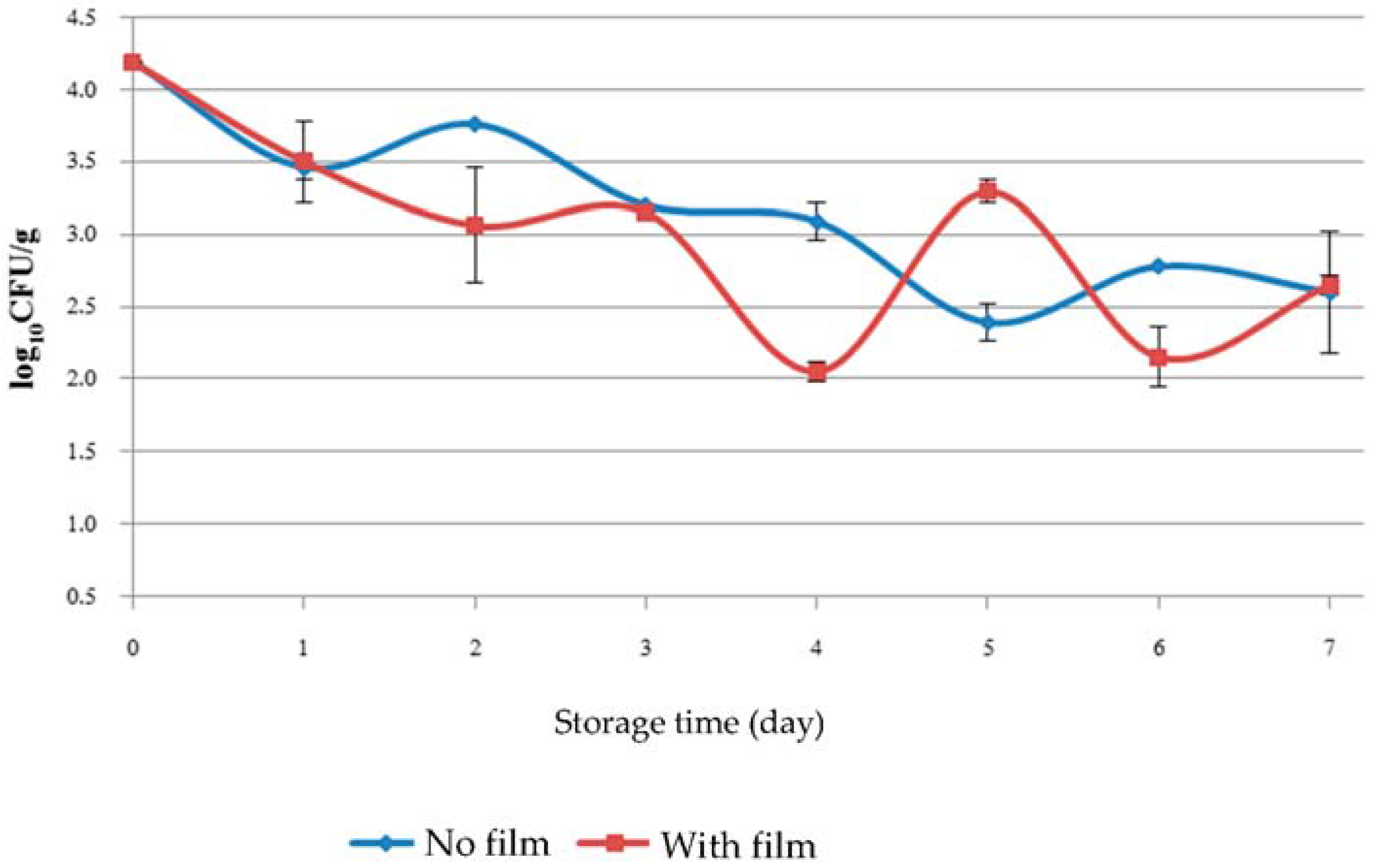
3.2. Microbial Assessment of Fresh Cut Vegetable during Storage
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Standard for Testing and Materials. Standard Test Method for Water Vapor Transmission of Material; ASTM E95-96; Annual Book of ASTM Standard; American Society for Testing and Materials Publishing: Philadelphia, PA, USA, 1995. [Google Scholar]
- American Standard for Testing and Materials. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM D882; Annual Book of ASTM Standard; American Society for Testing and Materials Publishing: Philadelphia, PA, USA, 2001. [Google Scholar]
- Davé, V.; McCarthy, S.P. Review of konjac glucomannan. J. Environ. Polym. Degrad. 1997, 5, 237–241. [Google Scholar]
- Dave, V.; Sheth, M.; McCarthy, S.P.; Ratto, J.A.; Kaplan, D.L. Liquid crystalline, rheological and thermal properties of konjac glucomannan. Polymer 1998, 39, 1139–1148. [Google Scholar] [CrossRef]
- Nakano, M.; Takikawa, K.; Arita, T. Release characteristics of dibucaine dispersed in konjac gels. J. Biomed. Mater. Res. 1979, 13, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Mekkerdchoo, O.; Patipasena, P.; Borompichaichartkul, C. Liposome encapsulation of antimicrobial extracts in pectin film for inhibition of food spoilage microorganisms. Asian J. Food Agro-Ind. 2009, 2, 817–838. [Google Scholar]
- Ponce, A.; Fritz, R.; del Valle, C.; Roura, S. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. Lebensm. Wiss. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Arunwatcharin, P. Pathogens and spoilage microorganism’s inhibition ability of Thai herb essential oils. In Proceeding of the 45th Conference on Agriculture and Home Economics, Agro-Industry, Kasetsart University, Bangkok, Thailand, 30 January–2 February 2007; pp. 508–515. (In Thai)
- Chacon, P.A.; Buffo, R.A.; Holley, R.A. Inhibitory effects of microencapsulated allyl isothiocyanate (AIT) against Escherichia coli O157:H7 in refrigerated, nitrogen packed, finely chopped beef. Int. J. Food Microbiol. 2006, 107, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kennedy, J.F.; Peng, J.L.; Yie, X.; Xie, B.J. Preparation and performance evaluation of glucomannan-chitosan-nisin ternary antimicrobial blend film. Carbohydr. Polym. 2006, 65, 488–494. [Google Scholar] [CrossRef]
- Suppakul, P.; Sonneveld, K.; Bigger, S.W.; Miltz, J. Efficacy of polyethylene-based antimicrobial films containing principal constituents of basil. LWT-Food Sci. Technol. 2008, 41, 779–788. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Gustaw, W.; Świeca, M.; Baraniak, B. A study on the mechanical properties of pea protein isolate films. J. Food Process. Preserv. 2014, 38, 1726–1736. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Physical and antimicrobial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Manikantan, M.R.; Varadharaju, N. Preparation and properties of linear low density polyethylene based nanocomposite films for food packaging. Indian J. Eng. Mater. Sci. 2012, 19, 54–66. [Google Scholar]
- Hyun, K.; Chong, W.; Koo, M.; Chung, I.J. Physical properties of polyethylene/silicate nanocomposite blown films. J. Appl. Polym. Sci. 2003, 89, 2131–2136. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Wang, L.; Scullen, O.J.; Sommers, C.H. Antimicrobial films and coatings for inactivation of Listeria innocua on ready-to-eat deli turkey meat. Food Control 2014, 40, 64–70. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and antimicrobial properties of starch-PVA blend films as affected by the incorporation of natural antimicrobial agents. Foods 2015. [Google Scholar] [CrossRef]
- Ponce, A.G.; Roura, S.I.; del Valle, C.E.; Moreira, M.R. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biol. Technol. 2008, 49, 294–300. [Google Scholar] [CrossRef]
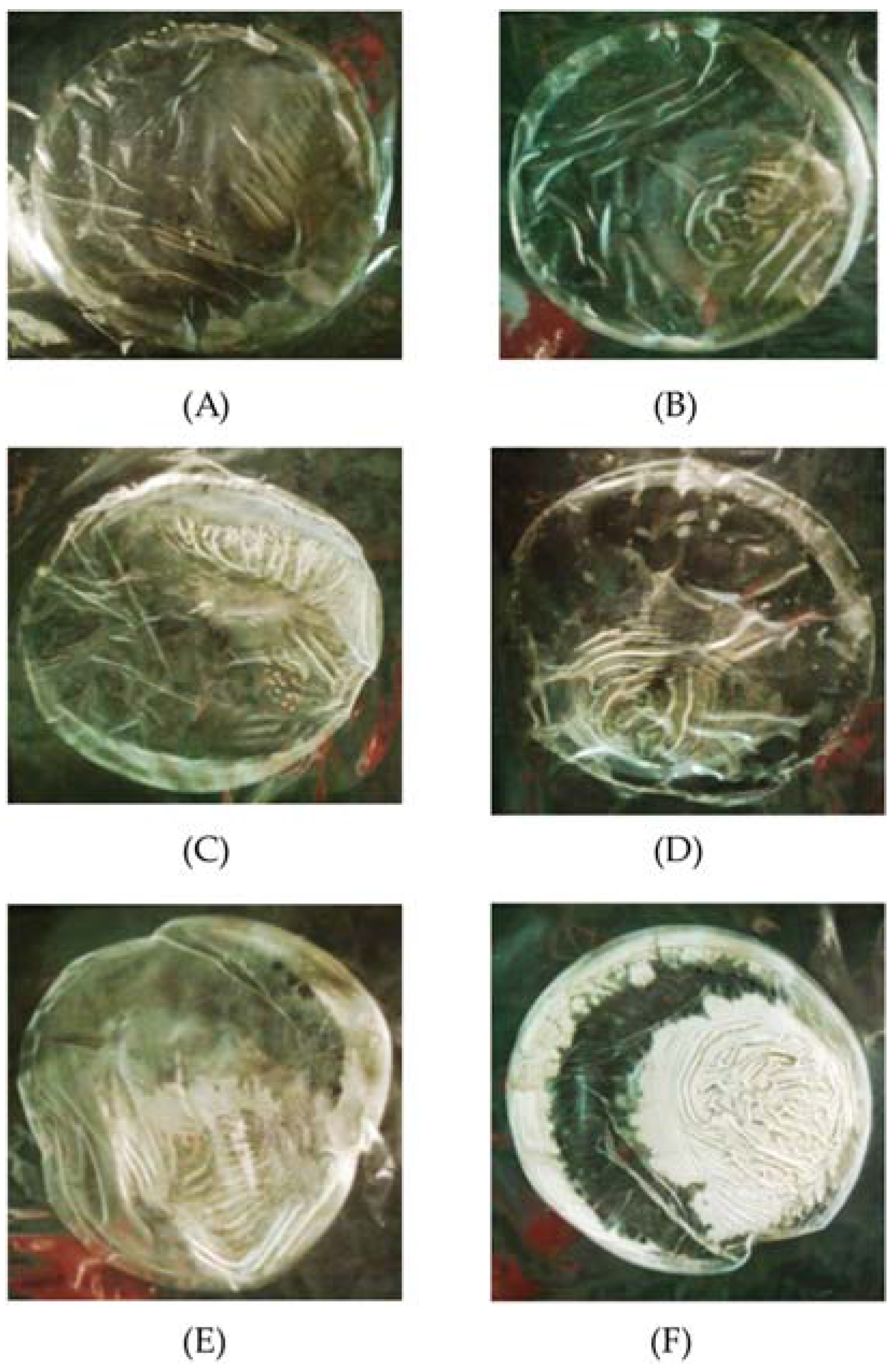
| KGM/SB Emulsion (%, v/v) | Diameter of Clear Zone (mm) |
|---|---|
| Control | No clear zone |
| 1 | 7.1 ± 0.5 f |
| 2 | 7.6 ± 0.6 d,e |
| 3 | 7.5 ± 0.6 d,e,f |
| 4 | 7.8 ± 1.1 c,d |
| 5 | 7.3 ± 1.1 e,f |
| 6 | 8.2 ± 1.2 c |
| 7 | 7.6 ± 1.0 d,e |
| 8 | 7.7 ± 0.6 d,e |
| Sweet basil oil 100 | 13.2 ± 0.8 b |
| Tetracycline | 23.7 ± 1.6 a |
| Concentration (%, v/v) | Volume per Area (mL·cm−2) | Diameter of Clear Zone (mm) |
|---|---|---|
| 6 | 0.325 | 9.6 ± 0.32 b |
| 6 | 0.455 | 10.1 ± 0.41 a |
| 4 | 0.325 | 10.1 ± 0.34 a |
| 4 | 0.455 | 9.8 ± 0.44 a,b |
| 4 | 0.585 | 9.5 ±0.49 b |
| Mechanical Properties | Values |
|---|---|
| Thickness (mm) | 0.074 ± 0.018 |
| Tensile Strength (MPa) | 68.08 ± 7.54 |
| Elongation (%) | 33.56 ± 7.38 |
| WVTR (g·cm−2·h−1) | 4.44 × 10−3 ± 1.38 × 10−4 |
| Storage Time (Day) | Treatment | Total Plate Count (log10 CFU/g) | Coliform (log10 CFU/g) |
|---|---|---|---|
| 0 | Fresh cut vegetable | 5.79 ± 0.15 b | 4.92 ± 0.12 b |
| 1 | No film | 9.05 ± 0.95 a | 8.16 ± 0.41 a |
| 1 | Film placed inside the package | 8.69 ± 1.29 a | 7.85 ± 0.21 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeheng, P.; Eamsakulrat, P.; Mekkerdchoo, O.; Borompichaichartkul, C. Production of Konjac Glucomannan Antimicrobial Film for Extending Shelf Life of Fresh-Cut Vegetables. Horticulturae 2017, 3, 17. https://doi.org/10.3390/horticulturae3010017
Saeheng P, Eamsakulrat P, Mekkerdchoo O, Borompichaichartkul C. Production of Konjac Glucomannan Antimicrobial Film for Extending Shelf Life of Fresh-Cut Vegetables. Horticulturae. 2017; 3(1):17. https://doi.org/10.3390/horticulturae3010017
Chicago/Turabian StyleSaeheng, Panya, Panuwat Eamsakulrat, Orachorn Mekkerdchoo, and Chaleeda Borompichaichartkul. 2017. "Production of Konjac Glucomannan Antimicrobial Film for Extending Shelf Life of Fresh-Cut Vegetables" Horticulturae 3, no. 1: 17. https://doi.org/10.3390/horticulturae3010017





