Improvement of Postharvest Quality of Asian Pear Fruits by Foliar Application of Boron and Calcium
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Materials
2.2. Physicochemical Quality Characteristics
2.3. The Total Phenol Content
2.4. Measuring the Activity of Polyphenol Oxidase Enzyme
2.5. Statistical Analysis
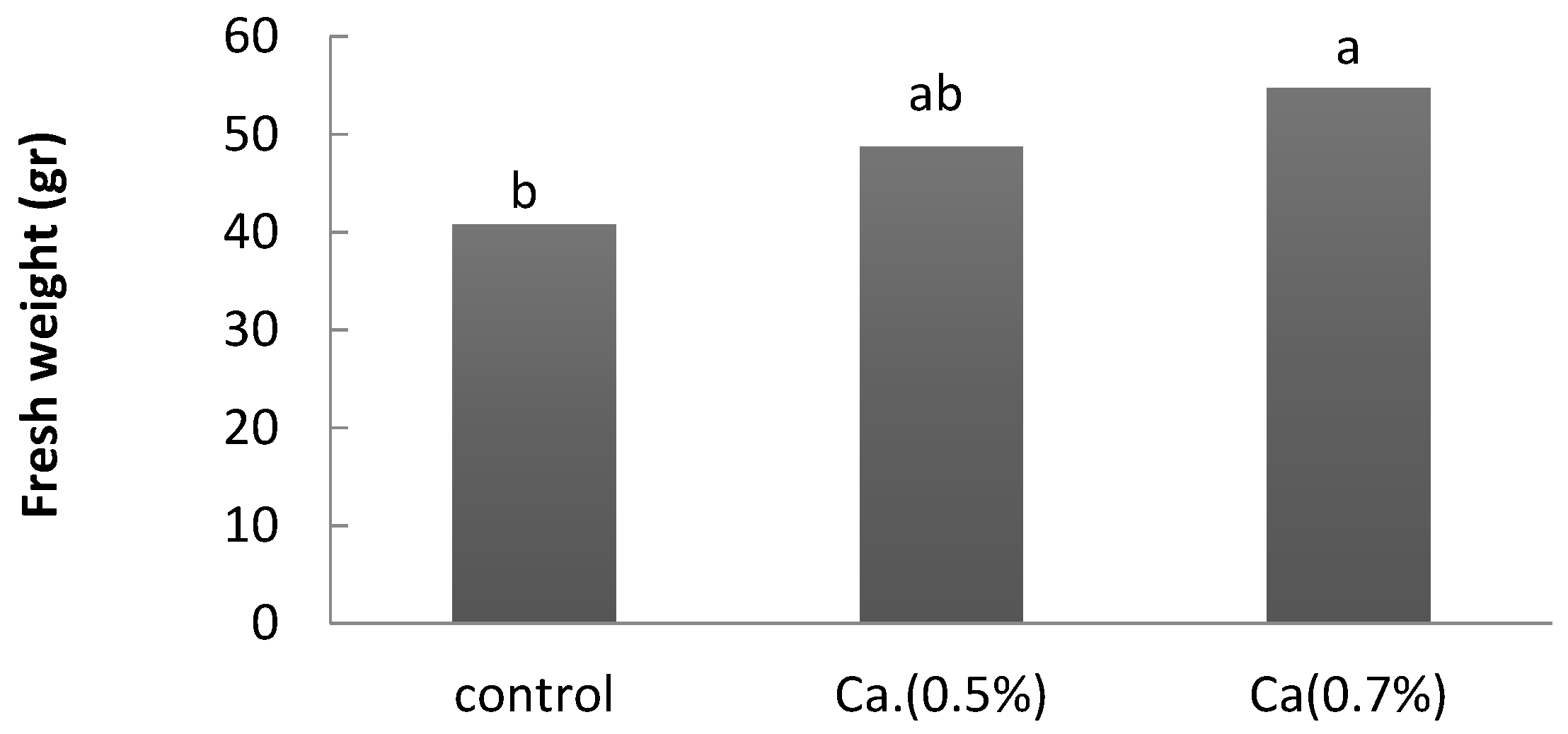
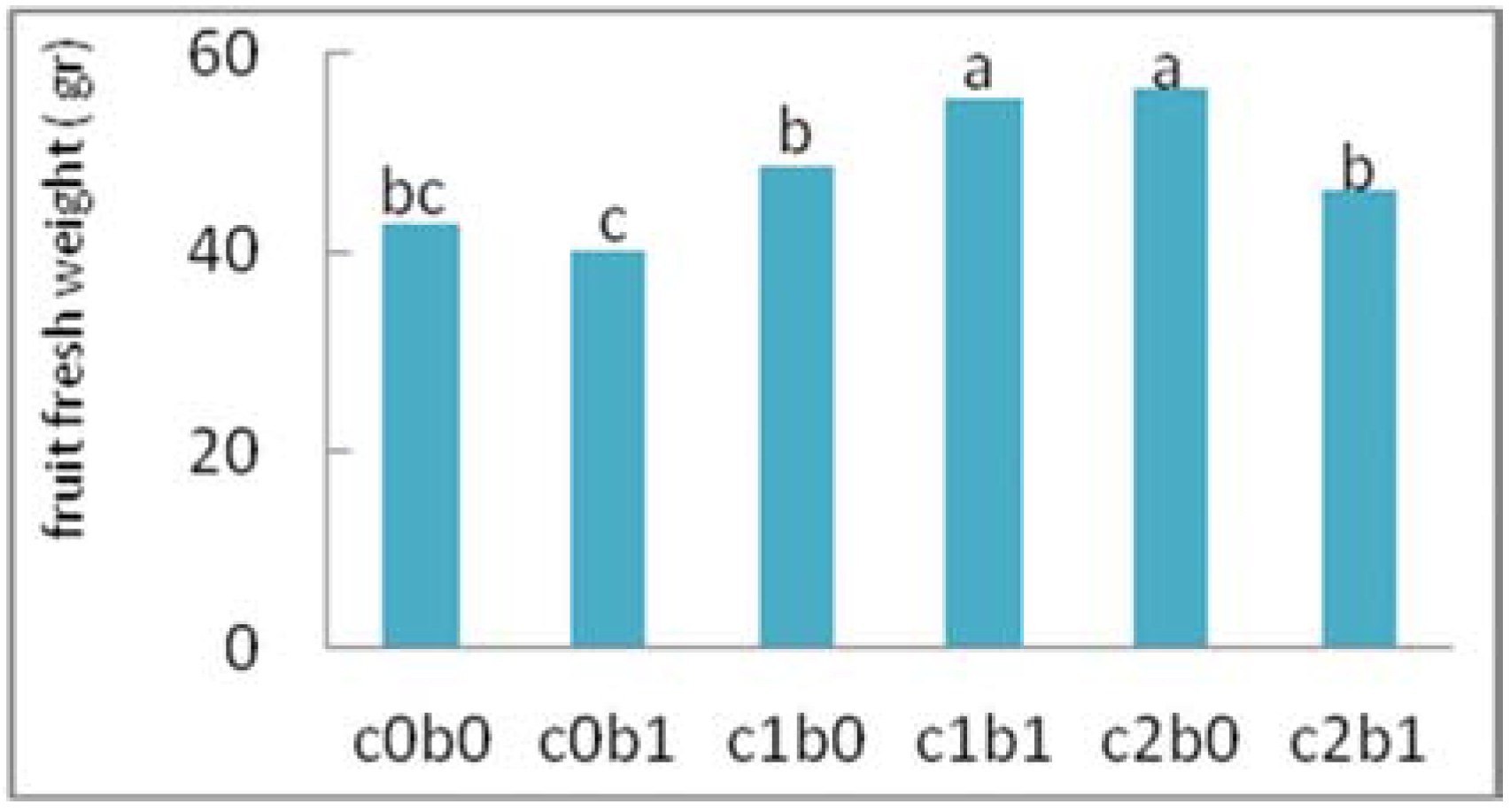
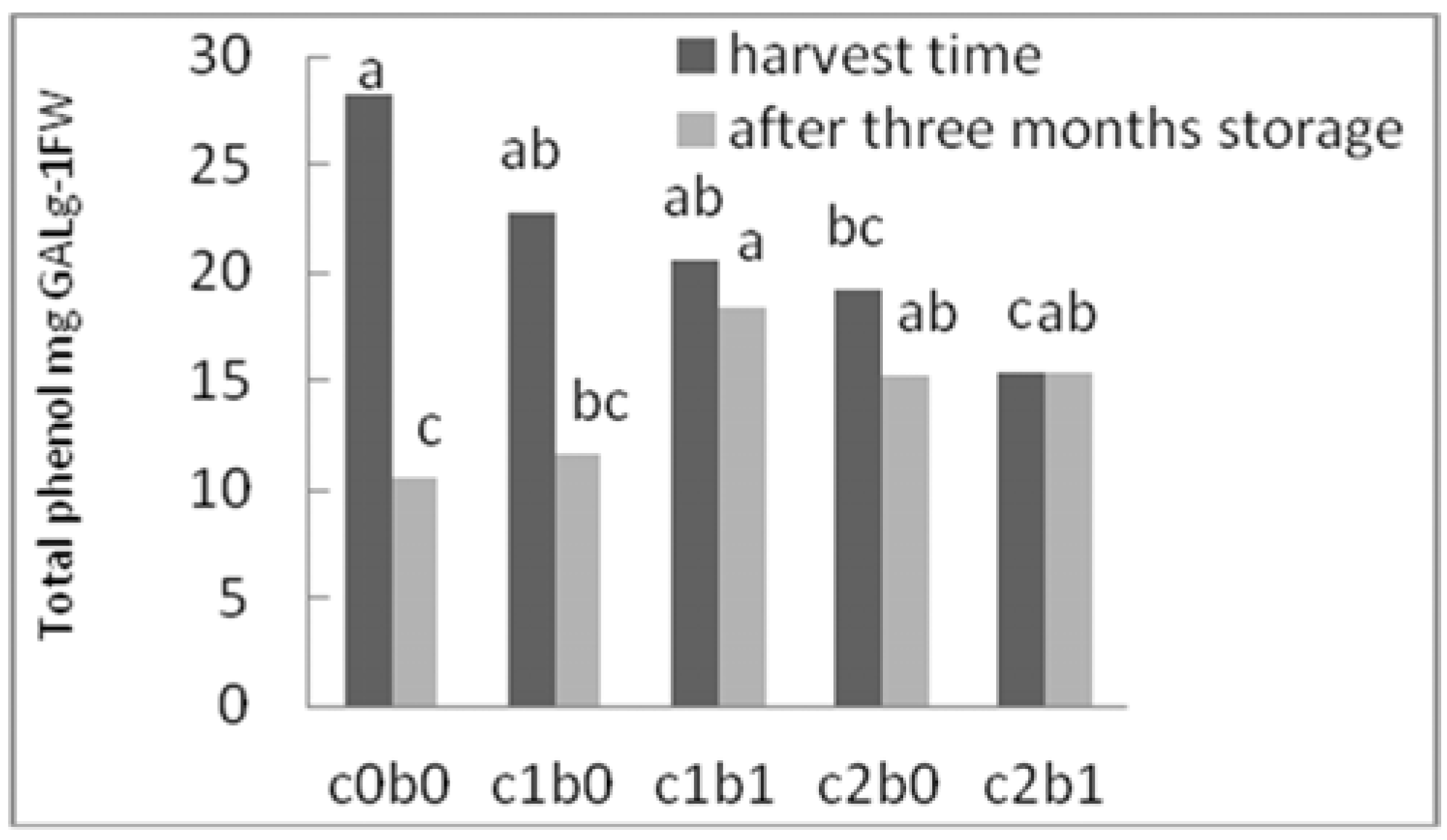
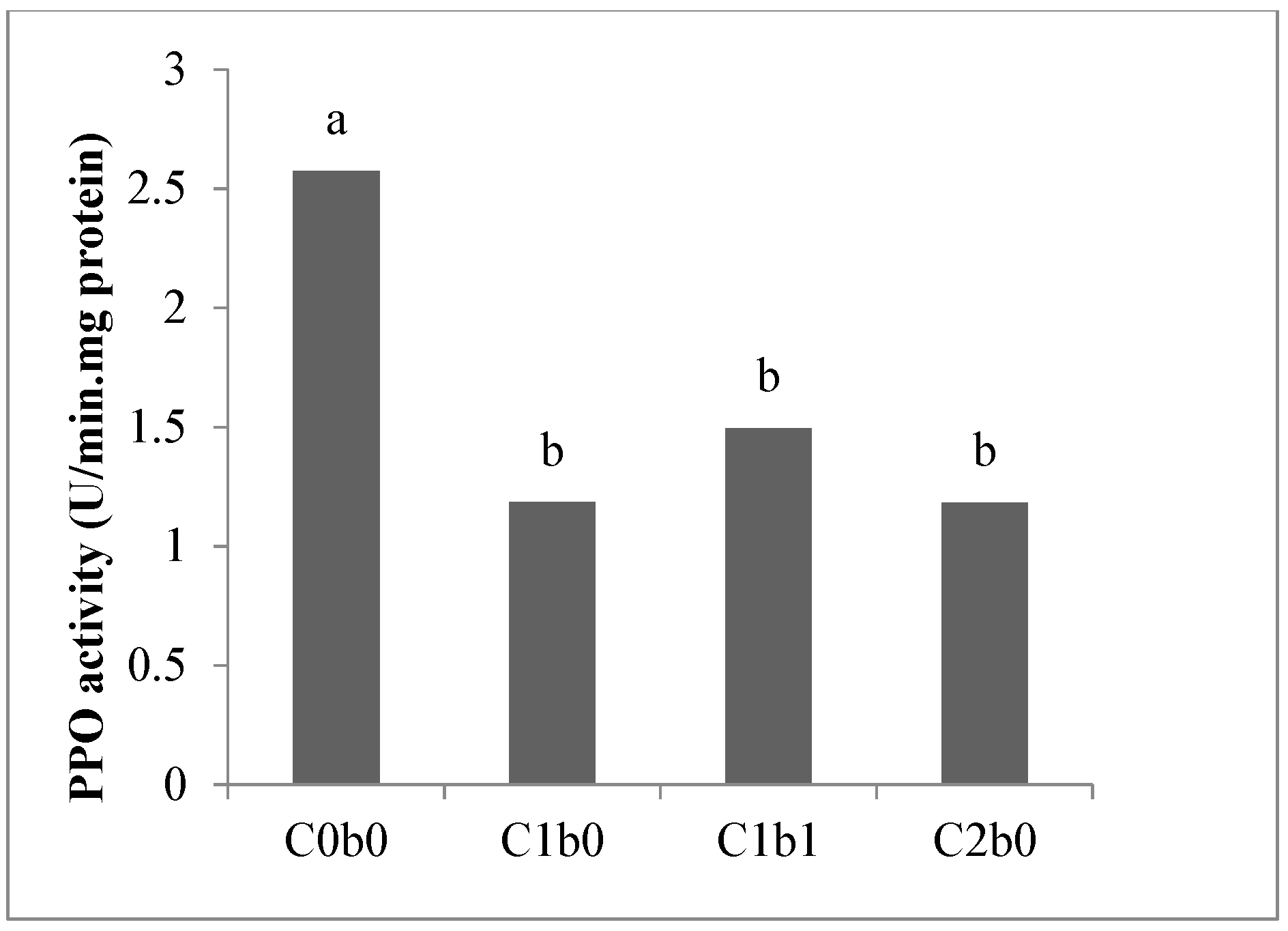
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.; Wang, Z.; Wu, J.; Wang, Q.; Hu, X. Chemical compositional characterization of eight pear cultivars. J. Food Chem. 2007, 104, 268–275. [Google Scholar] [CrossRef]
- Subburamu, K.; Singaravelu, M.; Nazar, A.; Irulappan, I. Pre-harvest spray of calcium in grapes (Vitis vinifera). South Indian Hort. 1990, 5, 268–269. [Google Scholar]
- Sharpels, R.O.; Johnson, D.S. The influence of calcium on senescence changes in apples. Ann. Appl. Biol. 1977, 85, 150–453. [Google Scholar]
- Dzondo-Gadet, M.; Mayap-Nzietchueng, R.; Hess, K.; Nabet, P.; Belleville, F.; Dousset, B. Action of boron at the molecular level: Effects on transcription and translation in an acellular system. Biol. Trace Elem. Res. 2002, 85, 23–33. [Google Scholar] [CrossRef]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ghanati, F.; Morita, A.; Yokota, H. Induction of suberin and increase of lignin content by excess boron in tobacco cell. Soil Sci. Plant Nutr. 2002, 48, 357–364. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Khoshgalb, H. The Effects of Calcium, Zinc and Boron on the Chemical Composition of Fruits, Postharvest Shelf Life and Reduce Symptoms of Internal Browning of Fruits of Two Varieties of Asian Pear (Pyrus serotina Rehd.) in Tehran Climate. Ph.D. Thesis, Faculty of Agriculture, Tarbiat Modarres University, Tehran, Iran.
- Akl, A.M.; Eid, A.F.M.; Hegab, M.Y. Effect of urea, some micronutrients and growth-regulators foliar spray on the yield, fruit quality, and some vegetative characteristics of “Washington Navel” orange trees. Hort. Sci. 1995, 30, 774–780. [Google Scholar]
- Veltman, R.H.; Sanders, M.G.; Persijn, S.T.; Peppelenbos, H.W.; Oosterhaven, J. Decreased ascorbic acid levels and brown core development in pears (Pyrus communis cv. “Conference”). Physiol. Plant. 1999, 107, 39–45. [Google Scholar] [CrossRef]
- Conway, W.S.; Sams, C.E. Effect of postharvest calcium treatment on decay of Delicious apples. Plant Dis. 1982, 66, 402–403. [Google Scholar] [CrossRef]
- Dhatt, A.S.; Mahajan, B.V.C.; Bhatt, A.R. Effect of pre and post-harvest calcium treatments on the storage life of Asian pear. ISHS Acta Hortic. 2005, 696, 497–501. [Google Scholar] [CrossRef]
- Dolati Baneh, H.; Hacani, A.; Majidi, A.; Zomorrodi, S.; Hacani, G.; Malakoti, M.J. Effect of calcium chloride on foliar concentration and frequency characteristics of stiffness and a Lebanese Red apples stored in Urmia region. J. Agric. Sci. 2002, 12, 47–54. (In Persian) [Google Scholar]
- Betts, H.A.; Bramlag, W.J. Uptake of calcium by apples from postharvest dips in calcium chloride solutions. J. Am. Soc. Hortic. Sci. 1979, 102, 785–788. [Google Scholar]
- Ackerman, J.; Fischer, M.; Amado, R. Changes in sugars, acids, and amino acids during ripening and storage of apples (cv. Glockenapfel). J. Agric. Food Chem. 1992, 40, 1131–1134. [Google Scholar] [CrossRef]
- Chen, J.L.; Yan, S.J.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of “YaLi” pear (Pyrus bertschneideri Rehd) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Coseteng, M.Y.; Lee, C.Y. Changes in apple polyphenoloxidase and polyphenol concentrations in relation to degree of browning. J. Food Sci. 1987, 52, 985–989. [Google Scholar] [CrossRef]
- Malakoti, M.J.; Tehrani, M.M. The Role of Micronutrients in Increasing Yield and Improving the Quality of Agricultural Products, 3rd ed.; Tarbiat Modares University: Tehran, Iran, 2001. [Google Scholar]
- Dunn, J.L.; Able, A.J. Pre-harvest calcium effects on sensory quality and calcium mobility in strawberry fruit. Acta Hortic. 2006, 708, 307–312. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil, M.I.; Castaner, M.; Artes, F.; Saltveit, M.E. Effect of selected browning inhibitors on phenolic metabolism in stem tissue of harvested lettuce. J. Agric. Food Chem. 1997, 45, 583–589. [Google Scholar] [CrossRef]
- Tohidloo, G.; Souri, M.K. Uptake and translocation of boron in two different tomato genotypes. Hortic. Environ. Biotechnol. 2009, 50, 487–491. [Google Scholar]



| Treatment | Calcium Chloride (%) | Boric Acid (%) |
|---|---|---|
| c0b0 | 0 | 0 |
| c0b1 | 0 | 0.5 |
| c1b0 | 0.5 | 0 |
| c1b1 | 0.5 | 0.5 |
| c2b0 | 0.7 | 0 |
| c2b1 | 0.7 | 0.5 |
| Source of Variation | df | Fruit Length | Fruit Diameter | Fruit Fresh Weight | Fruit Dry Weight | Fruit Firmness | Total Soluble Solids | Titratable Acidity |
|---|---|---|---|---|---|---|---|---|
| Block | 2 | 9.25 ns z | 7.68 ns | 49.14 ns | 0.06 ns | 0.06 ns | 0.09 ns | 729.5 ns |
| Calcium | 2 | 22.31 ns | 30.09 ns | 293.9 * | 1.47 ns | 4.5 ** | 7.72 ** | 909.6 ns |
| Boron | 1 | 1.91 ns | 9.7 ns | 54.21 ns | 0.02 ns | 0.88 ** | 2.72 ** | 122.3 ns |
| Calcium × Boron | 2 | 1.48 ns | 2.8 ns | 22.43 ns | 2.99 * | 0.1 ns | 4.05 ** | 1029.1 ns |
| Error | 10 | 9.02 | 11.55 | 67.9 | 0.4 | 0.06 | 0.14 | 399.6 |
| CV(%) z | - | 7.97 | 7.56 | 17.14 | 3.84 | 5.08 | 2.83 | 20.72 |
| Source of Variation | df | Fruit Length | Fruit Diameter | Fruit Fresh Weight | Fruit Firmness | TSS | TA |
|---|---|---|---|---|---|---|---|
| Block | 2 | 0.66 ns | 0.99 ns | 5.07 ns | 0.07 ns | 1.39 * | 1.93 ns |
| Calcium | 2 | 16.61 ns | 7.52 ns | 216.64 ** | 1.39 ** | 31.35 ** | 630.396 ns |
| Boron | 1 | 3.78 ns | 0.01 ns | 21.15 ns | 0.02 ns | 6.12 ** | 360.08 ns |
| Calcium × Boron | 2 | 3.59 ns | 0.65 ns | 112.80 ** | 0.02 ns | 12.12 ** | 1109.26 * |
| Error | 10 | 5.15 | 7.98 | 10.04 | 0.03 | 0.29 | 164.43 |
| CV(%) z | - | 6.80 | 6.99 | 6.57 | 5.73 | 3.36 | 13.99 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaj, K.; Ahmadi, N.; Souri, M.K. Improvement of Postharvest Quality of Asian Pear Fruits by Foliar Application of Boron and Calcium. Horticulturae 2017, 3, 15. https://doi.org/10.3390/horticulturae3010015
Khalaj K, Ahmadi N, Souri MK. Improvement of Postharvest Quality of Asian Pear Fruits by Foliar Application of Boron and Calcium. Horticulturae. 2017; 3(1):15. https://doi.org/10.3390/horticulturae3010015
Chicago/Turabian StyleKhalaj, Kobra, Nima Ahmadi, and Mohammad Kazem Souri. 2017. "Improvement of Postharvest Quality of Asian Pear Fruits by Foliar Application of Boron and Calcium" Horticulturae 3, no. 1: 15. https://doi.org/10.3390/horticulturae3010015
APA StyleKhalaj, K., Ahmadi, N., & Souri, M. K. (2017). Improvement of Postharvest Quality of Asian Pear Fruits by Foliar Application of Boron and Calcium. Horticulturae, 3(1), 15. https://doi.org/10.3390/horticulturae3010015





