Abstract
In vitro preservation of the “Kober 5BB” rootstock (Vitis berlandieri × Vitis riparia) was assessed with the encapsulation technique and slow growth storage. Shoot tips and nodal segments excised from in vitro cultures were encapsulated in calcium-alginate beads. A 30 min ion exchange time proved optimal for forming proper beads. The encapsulated and naked explants were stored at 4 °C in the dark or light. After 9 months of cold storage, the highest regrowth, 83.3%, was recorded for the encapsulated shoot tips maintained in darkness. The development of the encapsulated nodal segments was 55.6% under the same storage conditions. The encapsulated explants had a better regrowth capacity after storage than the naked explants.
1. Introduction
The encapsulation technique for creating synthetic seeds is an important application for in vitro culture. Shoot tips, axillary buds or nodal segments may be used to develop the synthetic seeds. Synthetic seeds have been defined as artificially encapsulated somatic embryos or non-embryogenic in vitro-derived propagules and are used for sowing under in vitro or ex vitro conditions [1,2,3]. Synthetic seed technology combines the advantages of clonal propagation with those of seed propagation (i.e., storability, easy to handle and transport, protection against diseases and pests). The most recent application foresees the use of synthetic seeds in medium and long-term storage. The encapsulation technique is used today in advanced procedures of cryopreservation such as encapsulation-dehydration and encapsulation-vitrification methods [4], obtaining very promising results for the long-term preservation of plant germplasm [5,6,7]. The encapsulation of shoot tips, axillary buds or nodal segments has been reported for various species [8,9,10,11,12,13], and slow growth storage has been applied as a method for medium-term conservation [14,15,16]. Slow growth storage involves limiting development by reducing temperature and/or light intensity, adding osmotic compounds such as mannitol or sucrose in the culture medium, and use of growth retardants [17]. Among these, the most commonly used are the reduction of temperature and light intensity. These two parameters have physiological consequences that result in a significant reduction in cell metabolism and, consequently, shoot growth. With in vitro slow growth storage, it is possible to extend the intervals between subcultures, thus reducing the cost of stock plant maintenance as well as the risk of contamination during subculturing [15,18].
There are a large number of grapevine cultivars and rootstocks, many of which are unique to small, remote environments, and are therefore important for preserving biodiversity and reducing genetic erosion. The aim of this study was to optimize an encapsulation and storage protocol for shoot tips and nodal segments of “Kober 5BB” grapevine rootstock (Vitis berlandieri × V. riparia) as an alternative to traditional conservation methods. The experiments evaluated the effect of storage on the regrowth ability of encapsulated shoot tips and nodal segments following different storage conditions. This report describes the initial results of an investigation aimed at the development of a protocol for encapsulation of “Kober 5BB” explants.
2. Materials and Methods
2.1. Culture Media and Conditions
In vitro stock cultures of grapevine rootstock “Kober 5BB” were proliferated on MS semisolid medium [19] with 30 g/L sucrose at pH 5.7 (Figure 1A). The proliferation medium contained 1.5 mg/L benzyladenine (BA) and 3 g/L Gelrite®. The salts, vitamins and gelling agent used for the culture media were purchased from Sigma-Aldrich (St. Louis, MI, USA). The medium was autoclaved for 20 min at 121 °C. The cultures were maintained at 23 ± 1 °C in a growth chamber under a 16 h photoperiod with light intensity 60 μmol·m−2·s−1 (standard culture conditions).
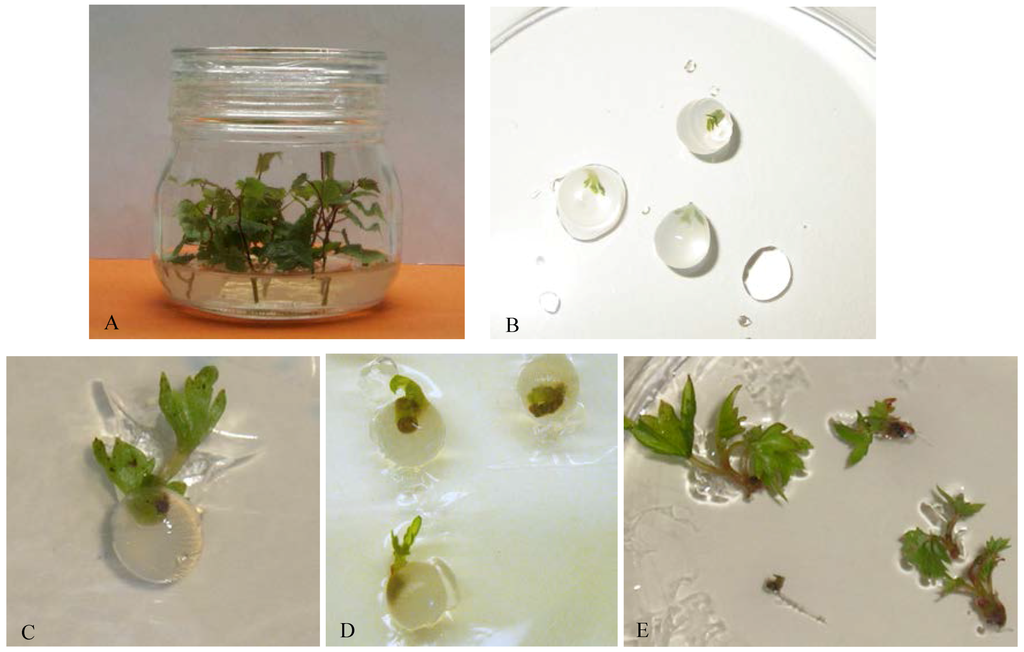
Figure 1.
(A) In vitro stock culture of “Kober 5BB” rootstock; (B) Encapsulated explants in 3% sodium alginate, 100 mM CaCl2·2H2O with ion exchange time of 30 min. Regrowth of explants under standard conditions (21 days) after 3 months of storage at 4 °C in darkness; (C) encapsulated shoot tip; (D) encapsulated nodal segments and (E) naked shoot tips.
2.2. Encapsulation Procedure and Storage
Shoot tips (2–3 mm) and nodal segments (5–6 mm) were excised from in vitro stock cultures of “Kober 5BB”. For encapsulation, the explants were plunged into a solution of 3% (w/v) sodium alginate (medium viscosity, Carlo Erba, Cornaredo, Italy) and MS liquid medium. Drops of alginate solution with shoot tip or nodal segment were sucked into a micropipette with sterile plastic tips and dropped into MS liquid medium supplemented with 100 mM calcium chloride (CaCl2·2H2O) for complexation. All operations were performed under sterile conditions. In order to achieve polymerization and prepare beads of an ideal shape and size with a uniform texture (Figure 1B), in the first experiment, different time periods (20, 30 or 40 min) for Na+/Ca2+ ion exchange in the calcium chloride solution were tested. After each incubation period in the complexing agent, the encapsulated explants were retrieved and rinsed three times in sterile distilled water in order to remove traces of calcium chloride. Regrowth of the explants was then assessed as described below.
The following experiment was carried out on both encapsulated, with 30 min of ion exchange time, and naked explants, which were placed in 90 cm diameter Petri dishes containing semisolid, hormone-free MS medium and held at 4 °C in a growth chamber in darkness or in light (30 μmol·m−2·s−1, 8 h photoperiod) for 3, 6 or 9 months of storage.
Regrowth of the explants (encapsulated and naked) was evaluated for bead incubation time and for each cold storage period after transferring them onto proliferation medium for 21 days, under standard conditions. Regrowth ability of encapsulated explants was determined as the time required for the shoot to appear and break through the gel [20]. Ten explants of each type were placed in a Petri dish, and dishes were replicated three times for each incubation time or treatment combination by storage period.
2.3. Data Analysis
The average regrowth time was calculated as follows: Σ (NxTx)/number of developed shoots; where Nx is the number of developed shoots within consecutive intervals of time, Tx is the number of days between the beginning of the test and the end of the specific time interval.
Data on regrowth were recorded and presented as means with standard error of the mean (SEM). Significant differences among means were analyzed following analysis of variance using Duncan’s Multiple Range Test at p < 0.05. Statistical analysis of percentages was carried out by a non-parametric Chi square test (p ≤ 0.05) for pairwise comparisons. All statistical tests were performed with Systat 13 (Systat Software, Inc., San Jose, CA, USA).
3. Results
3.1. Shoot Regrowth from Beads
Based on regrowth, optimal ion exchange between 3% sodium alginate and 100 mM calcium chloride was observed at 30 min (Table 1). This proved to be the most effective time interval for producing reasonably smooth beads around the explants. With this time, more than 96% of “Kober 5BB” beads containing shoot tips and 85% containing nodal segments were regrown in the shortest average regrowth time, 15 and 25 days, respectively. Low percentages of regrowth ability were observed when the complexation period was shorter or longer than 30 min. In addition, at 20 min of complexation time, the beads were too soft and difficult to handle, while they showed a well-defined shape but regrowth was slower at 40 min. Following encapsulation, shoot tips developed more quickly (15 vs. 25 days) and at a higher percentage (96% vs. 85%) than nodal segments.

Table 1.
Effect of ion exchange time on regrowth ability and average regrowth time of encapsulated explants of “Kober 5BB” rootstock.
3.2. Storage in Slow-Growth Conditions
Irrespective of tissue type and storage condition, the explants exhibited some level of regrowth through 9 months of cold storage (Figure 2). No significant differences were observed in the regrowth ability of encapsulated versus naked shoot tip or nodal explants at 3 months, but encapsulated shoot tip and nodal explants exhibited significantly higher regrowth percentages than naked explants in both light and dark storage at 6 and 9 months (Figure 2B,C).
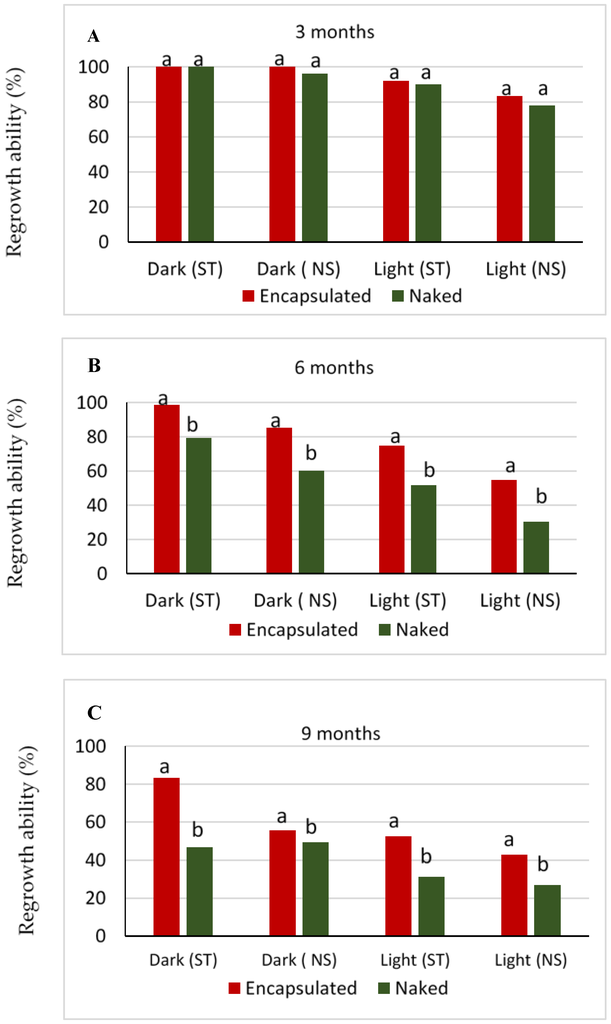
Figure 2.
Regrowth ability (%) of encapsulated and naked shoot tips (ST) or nodal segments (NS) of “Kober 5BB” rootstock stored at 4 °C in darkness and light conditions for each storage period. (A) three months; (B) six months; (C) nine months. Different letters indicate percentages of encapsulated versus naked means within tissue type and light versus dark conditions that significantly differed (χ2 test, p ≤ 0.05) at each storage period.
Regrowth percentages of all explants in both growing conditions decreased over storage time (Figure 2 and Figure 3), and the decrease was most evident between 3 and 6 months of storage. At 6 and 9 months, shoot tip explants showed higher regrowth ability than nodal explants (Figure 3). Furthermore, the highest regrowth ability at 6 and 9 months was recorded for the encapsulated shoot tips kept in the dark (Figure 2).
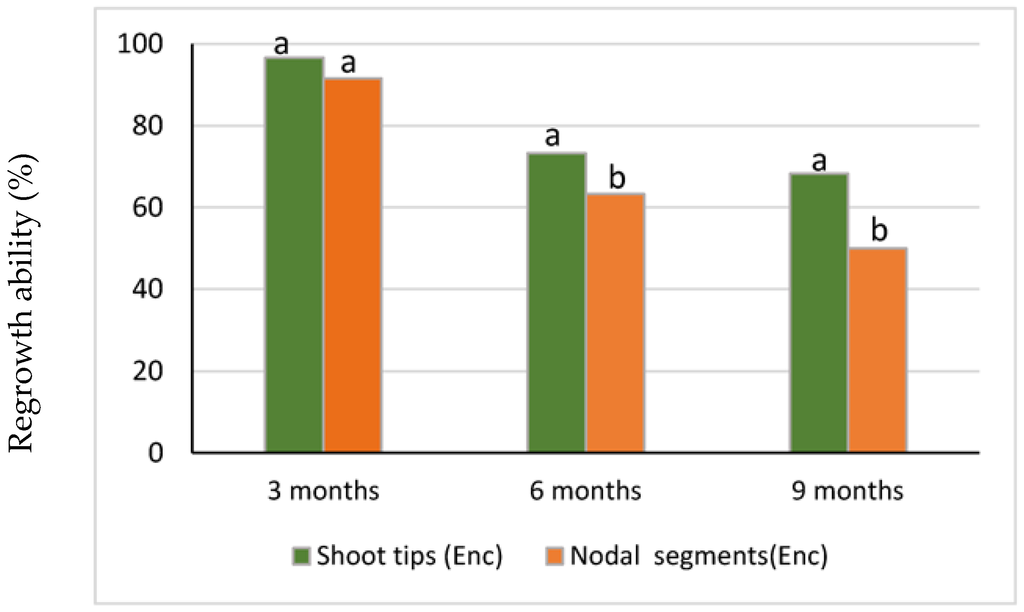
Figure 3.
Regrowth ability of encapsulated shoot tips versus nodal segments after 3, 6, and 9 months of 4 °C storage. Different letters indicate percentages of shoot tip versus nodal segments that significantly differed (χ2 test, p ≤ 0.05) at each storage period.
Overall, darkness had a positive influence on the regrowth of the explants at each storage period (Figure 4). The preservation conditions assessed proved to be more suitable for the encapsulated shoot tips than for the encapsulated nodal segments. No morphological changes were observed for the encapsulated explants that developed into shoots.
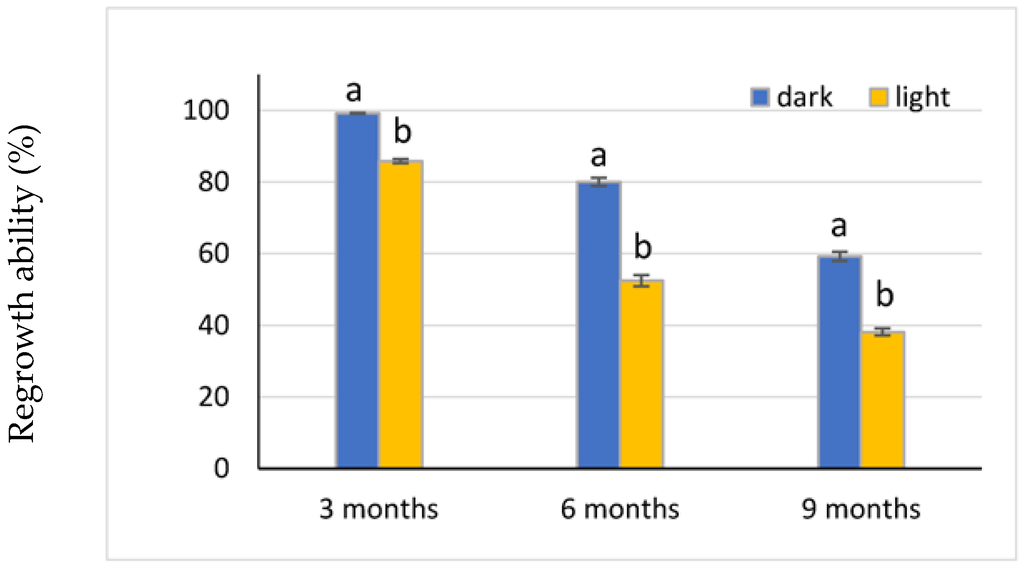
Figure 4.
Effect of dark versus light on explant regrowth after 3, 6, and 9 months of 4 °C storage. Different letters indicate percentages for dark versus light that significantly differed (χ2 test, p ≤ 0.05) at each storage period.
4. Discussion
Encapsulation of explants is affected by sodium alginate and calcium chloride concentrations, and by ion exchange time, which together can give beads their optimum characteristics [11]. Sodium alginate and calcium salt have proven to be the best combination for encapsulation since they are low cost, non-damaging, easy to use and give high propagule-to-plant conversion [21]. The hardness of beads mainly depends on the number of sodium ions exchanged with calcium ions, and complexing time should be optimized in order to form uniformly spherical and firm beads. The exchange time generally has ranged between 20–40 min [22]. For “Kober 5BB” beads, the best results were obtained with the 30 min hardening process; this ion exchange time showed the best regrowth for shoot tips (96.7%) and nodal segments (85.3%). Most studies on fruit, ornamental and vegetable species suggest that a 30 min exchange time is best for obtaining a uniform spherical shape and a good level of sprouting from the beads [9,23,24,25,26,27,28,29]. The shortest average regrowth time of encapsulated “Kober 5BB” shoot tips was 15 d when the encapsulated explants were hardened for 30 min, while it was 25–26 days for nodal segments, for the ion exchange time tested. Regrowth time has varied with species; for example, when applying the same protocol for encapsulation used in this study, regrowth in Photinia fraserii occurred in 10 days [30], while for Nerium oleander and Kalanchoe spp., it took 18 and 19 days, respectively [8].
The percentage of encapsulated and naked explants that formed shoots depended on the duration and conditions of storage. Under slow growth in vitro culture, the encapsulated explants of “Kober 5BB” were well suited for the storage conditions. Their better regrowth compared to naked explants could be attributed to their inclusion in the gel matrix which acts as an “artificial endosperm” for the explants. The present results confirmed the effectiveness of the protective coating. This agreed with previous literature which reported that the sodium alginate matrix provided protection and supplied nutrients to explants resulting in a higher regrowth percentage of encapsulated nodal segments than naked nodal segments [31].
The encapsulation technology could represent a useful technique for plant material conservation when used for medium or long-term preservation. In cryopreservation, this technique has been used for different species [32,33,34]. Moreover, various studies have described encapsulation for short and medium conservation (from 1 month to 1 year) without losing explant viability [35,36,37,38] and for increasing the interval between subcultures by reducing growth.
5. Conclusions
Implementation of the encapsulation technique may be used for clonal propagation and for effective storage at low temperatures. This study has shown that in vitro explant encapsulation could be used for storing “Kober 5BB” rootstock tissues up to 9 months at 4 °C without subcultures. The results indicated that the method could have great potential for preserving desirable elite genotypes of grapevine for medium periods, and were a useful prerequisite for further studies extending the storage period in this species.
In some plant species, poor regrowth of encapsulated propagules into plants has been a limitation for commercial application of the encapsulation technique, so further evaluation of the technique is needed. In addition, plant nurseries may be interested in achieving the development and growth of plants from synthetic seeds in soil without the aseptic conditions required for in vitro culture.
Conflicts of Interest
The author declares no conflict of interest.
References
- Murashige, T. Plant cell and organ cultures as horticultural practices. Acta Hortic. 1977, 78, 17–30. [Google Scholar]
- Aitkens-Christie, J.; Kozai, T.; Takayama, S. Automation in plant tissue culture: General introduction and overview. In Automation and Environmental Control in Plant Tissue Culture; Aitken-Christie, J., Kozai, T., Smith, M.A.L., Eds.; Kluwer Academic Publication: Dordrecht, The Netherlands, 1995; pp. 1–18. [Google Scholar]
- Standardi, A.; Piccioni, E. Recent perspectives on the synthetic seed technology using non-embryogenic vitro-derived explants. Int. J. Plant Sci. 1998, 159, 968–978. [Google Scholar]
- Dereuddre, J.; Blandin, S.; Hassen, N. Resistance of alginate-coated somatic embryos of carrot (Daucus carota L.) to desiccation and freezing in liquid nitrogen: 1. Effects of preculture. CryoLetters 1991, 12, 125–134. [Google Scholar]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar] [PubMed]
- Fabbri, A.; Ganino, T.; Lambardi, M.; Nisi, R. Crioconservazione di gemme di portinnesto ‘Kober’ 5BB (Vitis berlandieri × Vitis riparia): Aspetti anatomici. Ital. Hortus 2007, 14, 82–86. [Google Scholar]
- Benelli, C.; de Carlo, A.; Engelmann, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [PubMed]
- Lambardi, M.; Benelli, C.; Ozudogru, E.A.; Ozden-Tokatli, Y. Synthetic seed technology in ornamental plants. Floric. Ornam. Plant Biotechnol. Adv. Top. Issues 2006, 2, 347–354. [Google Scholar]
- Piccioni, E.; Standardi, A. Encapsulation of micropropagated buds of six woody species. Plant Cell Tissue Org. Cult. 1995, 42, 221–226. [Google Scholar] [CrossRef]
- Micheli, M.; Hafiz, I.A.; Standardi, A. Encapsulation of in vitro-derived explants of olive (Olea europaea L. cv. Moraiolo) II. Effects of storage on capsule and derived shoots performance. Sci. Hortic. 2007, 113, 286–292. [Google Scholar] [CrossRef]
- Rai, M.K.; Asthana, P.; Singh, S.K.; Jaiswal, V.S.; Jaiswal, U. The encapsulation technology in fruit plants—A review. Biotechnol. Adv. 2009, 27, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.M.; Murthy, K.S.R.; Pullaiah, T. Synthetic seeds: A review in agriculture and forestry. Afr. J. Biotechnol. 2012, 11, 14254–14275. [Google Scholar]
- Kulus, D.; Zalewska, M. In vitro plant recovery from alginate-encapsulated Chrysanthemum × grandiflorum (Ramat.) Kitam shoot tips. Propag. Ornam. Plants 2014, 14, 3–12. [Google Scholar]
- Nower, A.A.; Ali, E.A.; Rizkalla, A. Synthetic seeds of pear (Pyrus communis L.) rootstock storage in vitro. Aust. J. Basic Appl. Sci. 2007, 1, 262–270. [Google Scholar]
- Benelli, C.; Ozudogru, E.A.; Lambardi, M.; Dradi, G. In vitro conservation ornamental plants by slow growth storage. Acta Hortic. 2012, 961, 89–93. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; Henriques, A.B.; Sato, A. In vitro conservation of Mandevilla moricandiana (Apocynaceae): Short-term storage and encapsulation–dehydration of nodal segments. In Vitro Cell Dev. Biol. Plant 2014, 50, 326–336. [Google Scholar] [CrossRef]
- Grout, B.W.W. In vitro conservation of germplasm. In Plant Tissue Culture: Application and Limitations; Bhojwani, S.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 394–411. [Google Scholar]
- Ikhlaq, M.M.; Hafiz, I.A.; Micheli, M.; Ahmad, T.; Abbasi, N.A.; Standardi, A. In vitro storage of synthetic seeds: Effect of different storage conditions and intervals on their conversion ability. Afr. J. Biotechnol. 2010, 9, 5712–5721. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Standardi, A.; Micheli, M. Encapsulation of in vitro-derived explants: An innovative tool for nurseries. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: New York, NY, USA, 2013; pp. 397–418. [Google Scholar]
- Sharma, S.; Shahzad, A.; Teixeira da Silva, J.A. Synseed technology—A complete synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef] [PubMed]
- Standardi, A. Encapsulation: Promising technology for nurseries and plant tissue laboratories. AgroLife Sci. J. 2012, 1, 48–54. [Google Scholar]
- Sicurani, M.; Piccioni, E.; Standardi, A. Micropropagation and preparation of synthetic seed in M.26 apple rootstock I: Attempts towards saving labor in the production of adventitious shoot tips suitable for encapsulation. Plant Cell Tissue Org. Cult. 2001, 66, 207–216. [Google Scholar]
- Lisek, A.; Orlikowska, T. In vitro storage of strawberry and raspberry in calcium-alginate beads at 4 °C. Plant Cell Tissue Org. Cult. 2004, 78, 167–172. [Google Scholar] [CrossRef]
- Naik, S.K.; Chand, P.K. Nutrient-alginate encapsulation of in vitro nodal segments of pomegranate (Punica granatum L.) for germplasm distribution and exchange. Sci. Hortic. 2006, 108, 247–252. [Google Scholar] [CrossRef]
- Germanà, M.A.; Micheli, M.; Pulcini, L.; Standardi, A. Perspective of the encapsulation technology in the nursery activity of Citrus. Caryologia 2007, 60, 192–195. [Google Scholar]
- Daud, N.; Taha, R.M.; Hasbullah, N.A. Artificial seed production from encapsulated micro shoots of Saintpaulia ionantha Wendl. (African Violet). J. Appl. Sci. 2008, 8, 4662–4667. [Google Scholar]
- Sundararaj, S.G.; Agrawal, A.; Tyagi, R.K. Encapsulation for in vitro short-term storage and exchange of ginger (Zingiber officinale Rosc.) germplasm. Sci. Hortic. 2010, 125, 761–766. [Google Scholar]
- Nor Asmah, H.; Nor Hasnida, H.; Nashatul Zaimah, N.A.; Noraliza, A.; Nadiah Salmi, N. Synthetic seed technology for encapsulation and regrowth of in vitro-derived Acacia hybrid shoot and axillary buds. Afr. J. Biotechnol. 2011, 10, 7820–7824. [Google Scholar]
- Ozden-Tokatli, Y.; de Carlo, A.; Gumusel, F.; Pignattelli, S.; Lambardi, M. Development of encapsulation techniques for the production and conservation of synthetic seeds in ornamental plants. Propag. Ornam. Plants 2008, 8, 17–22. [Google Scholar]
- Singh, S.K.; Rai, M.K.; Asthana, P.; Sahoo, L. Alginate-encapsulation of nodal segments for propagation, short-term conservation and germplasm exchange and distribution of Eclipta alba (L.). Acta Physiol. Plant 2010, 32, 607–610. [Google Scholar] [CrossRef]
- Gonzalez-Arnao, M.T.; Engelmann, F. Cryopreservation of plant germplasm using the encapsulation-dehydration technique: Review and case study on sugarcane. CryoLetters 2006, 27, 155–168. [Google Scholar] [PubMed]
- Engelmann, F.; Arnao, M.T.G.; Wu, Y.; Escobar, R. Development of encapsulation dehydration. In Plant Cryopreservation: A Practical Guide; Reed, B., Ed.; Springer: New York, NY, USA, 2008; pp. 59–75. [Google Scholar]
- Padrò, M.D.A.; Frattarelli, A.; Sgueglia, A.; Condello, E.; Damiano, C.; Caboni, E. Cryopreservation of white mulberry (Morus alba L.) by encapsulation-dehydration and vitrification. Plant Cell Tissue Org. Cult. 2012, 108, 167–172. [Google Scholar]
- Hung, C.D.; Trueman, S.J. Encapsulation technology for short-term preservation and germplasm distribution of the African mahogany Khaya senegalensis. Plant Cell Tissue Org. Cult. 2011, 107, 397–405. [Google Scholar] [CrossRef]
- Hung, C.D.; Trueman, S.J. Alginate encapsulation of shoot tips and nodal segments for short-term storage and distribution of the eucalypt Corymbia torelliana × C. citriodora. Acta Physiol. Plant 2012, 34, 117–128. [Google Scholar]
- Sakhanokho, H.F.; Pounders, C.T.; Blythe, E.K. Alginate encapsulation of Begonia microshoots for short-term storage and distribution. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Srivastava, V.; Khan, S.A.; Banerjee, S. An evaluation of genetic fidelity of encapsulated microshoots of the medicinal plant: Cineraria maritima following six months of storage. Plant Cell Tissue Org. Cult. 2009, 99, 193–198. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).