Sustainable Characterization of Some Extracts of Origanum vulgare L. and Biosafety Evaluation Using Allium cepa Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oregano Extract Preparation
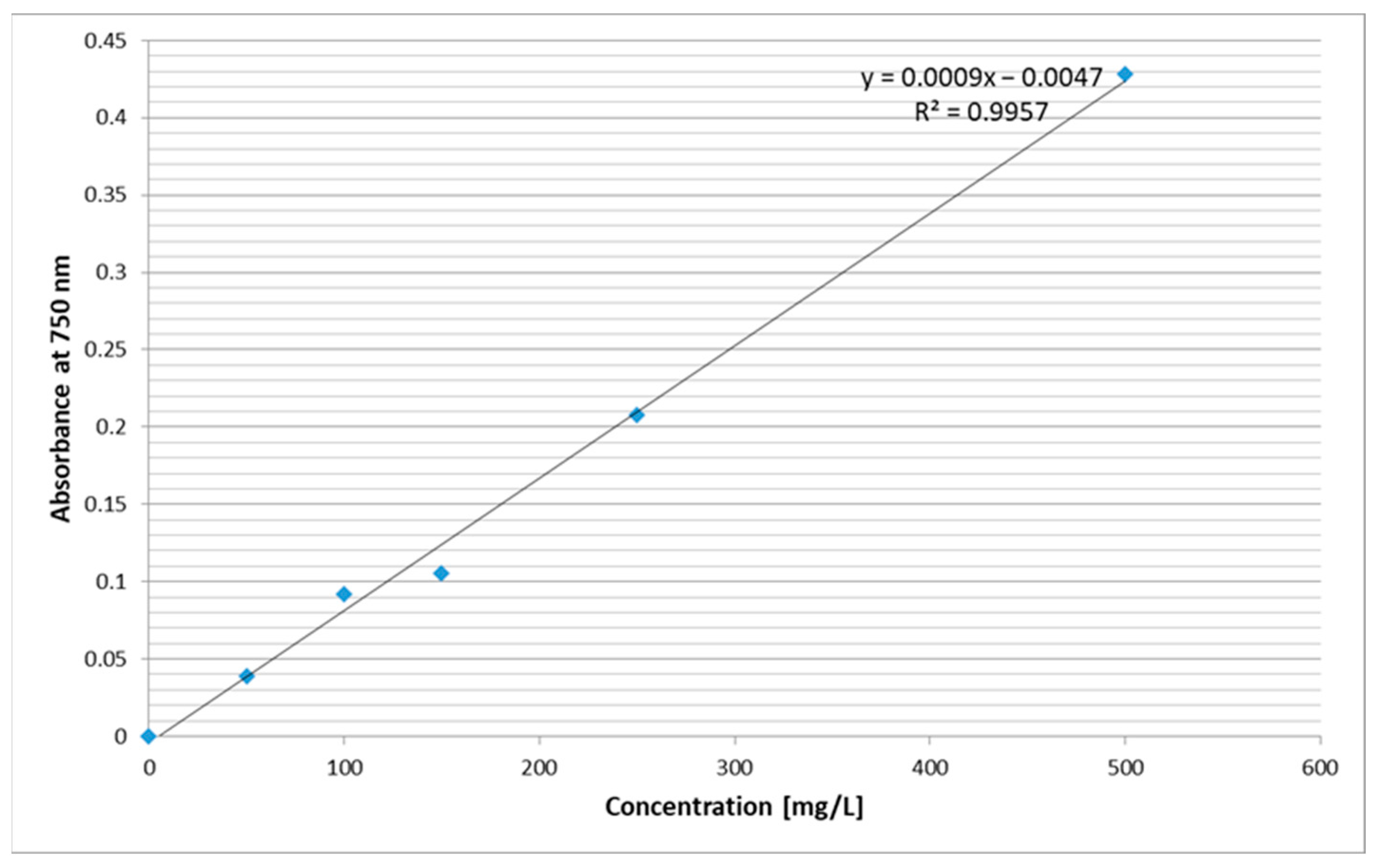
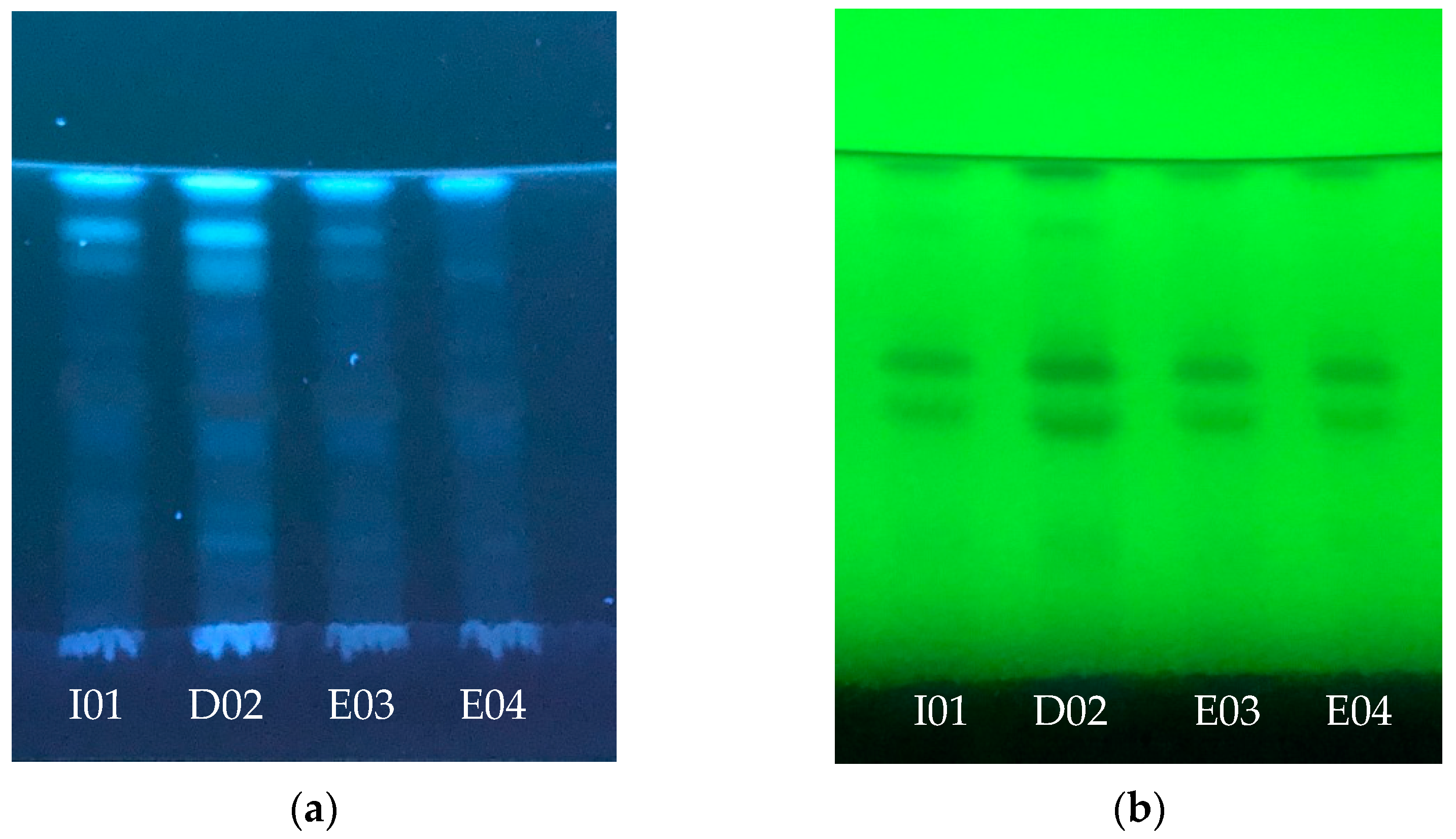
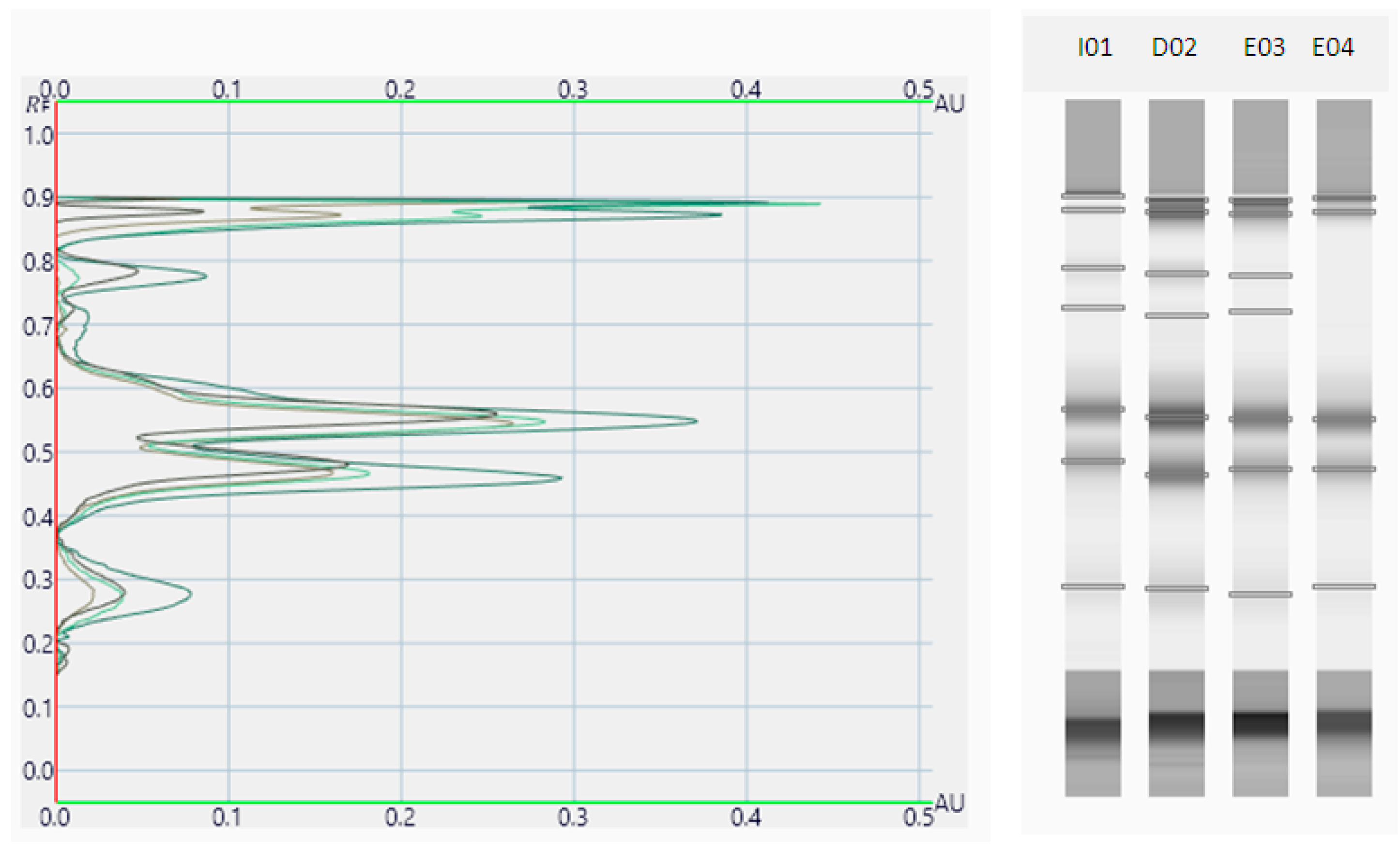
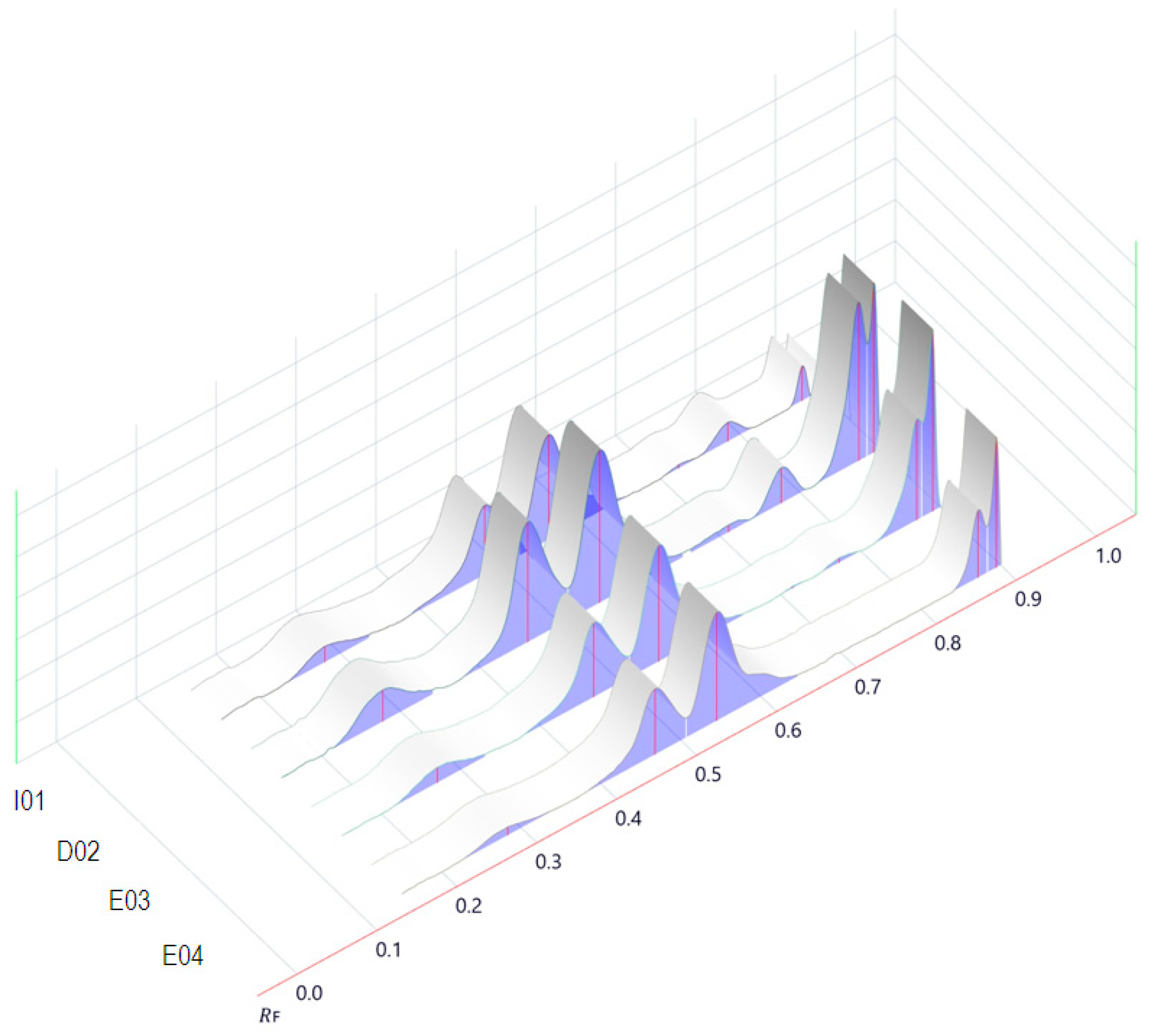
2.2. Oregano Extract Analysis
2.3. Allium Cepa Assay
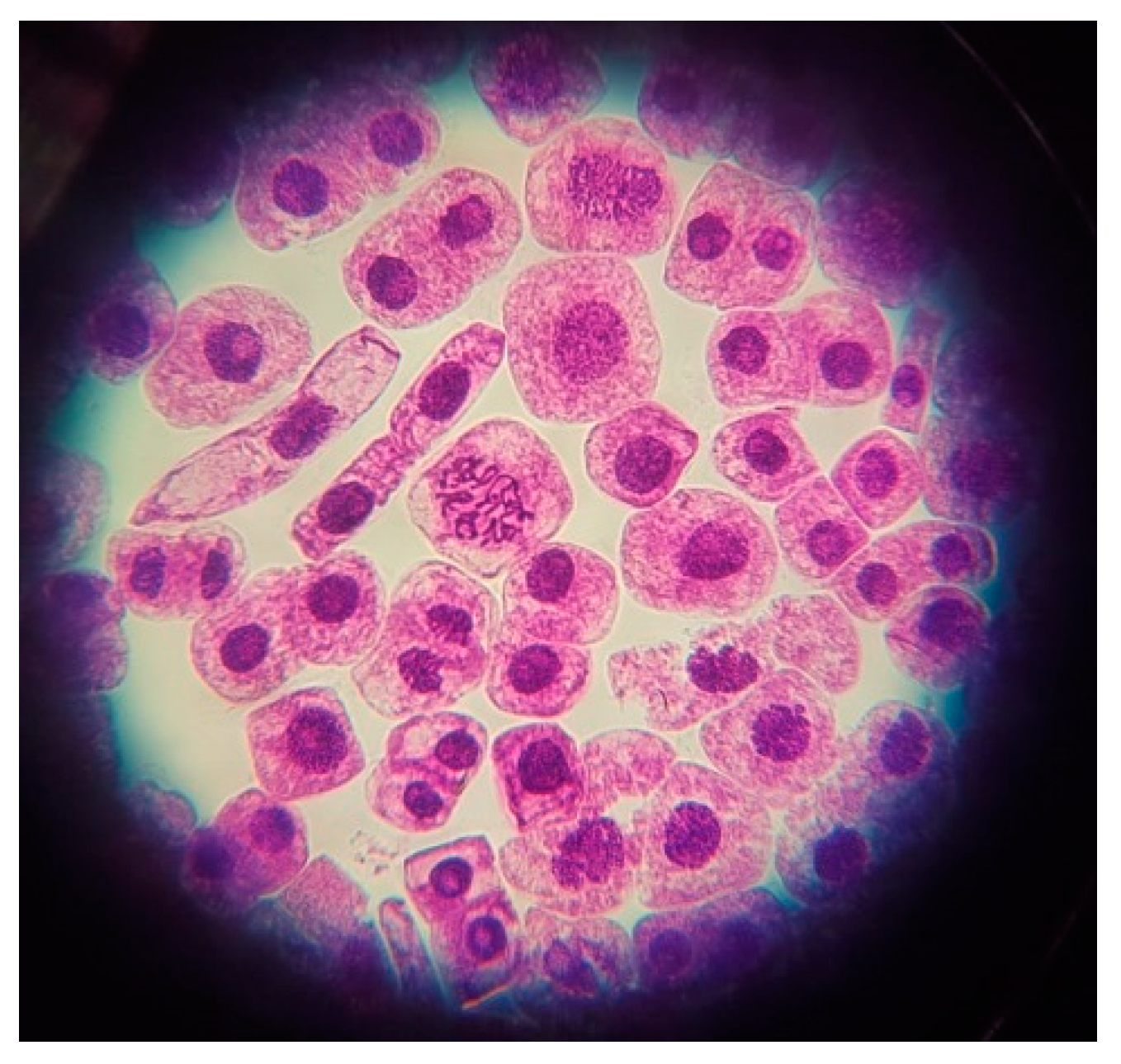
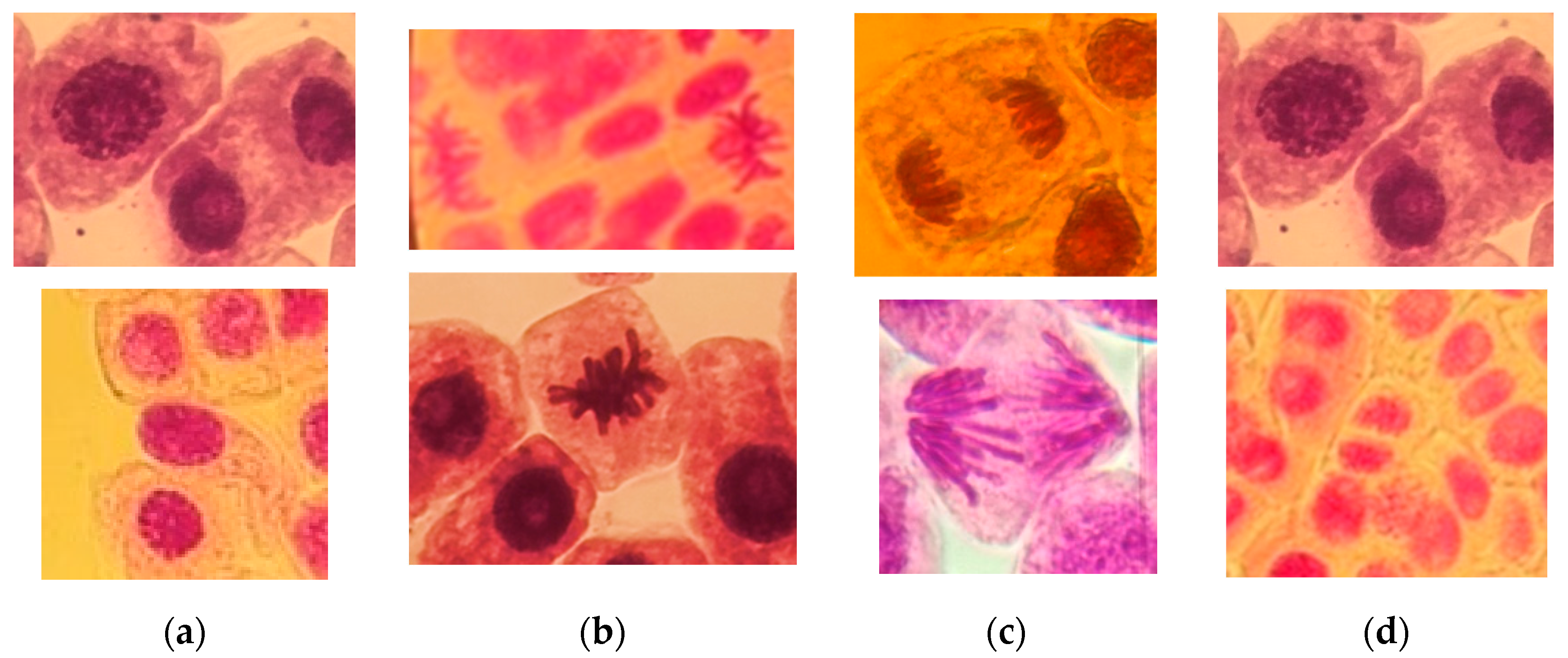
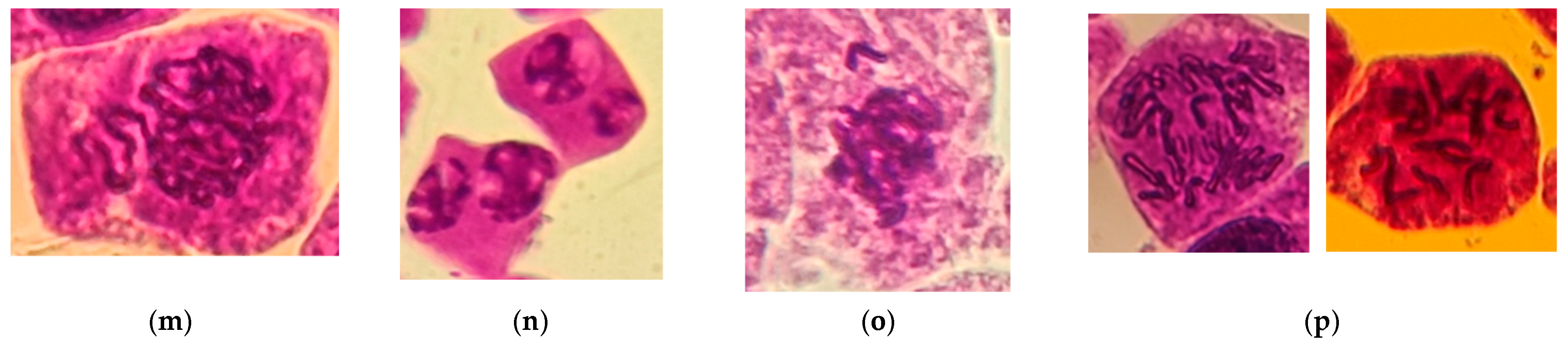
2.4. Cytogenetic Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Oregano Extract Analysis
3.1.1. Physicochemical Parameters, Total Phenolic Content, and Color Intensity of Origanum vulgare L. Extracts
3.1.2. Phytochemical Screening of Origanum vulgare L. Extracts
3.2. Cytotoxicity of the Oregano Extracts (Mitotic Index MI)
3.3. Genototoxicity of the Oregano Extracts (Chromosomal Aberration CA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Cepan, C.; Grozescu, I.; Cziple, F.; Olariu, S.; Ratiu, S.; Lazar, V.; Murariu, S.M.; Velciov, S.M.; Marti, T.D. Therapeutic use of some Romanian medicinal plants. In Pharmacognosy—Medicinal Plants; Perveen, A., Al-Taweel, A., Eds.; InTech: London, UK, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Avramescu, S.M.; Dinu-Pirvu, C.E.; Ionescu, D. Romanian aromatic and medicinal plants: From tradition to science. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H., Ed.; InTech: London, UK, 2017; pp. 151–173. [Google Scholar] [CrossRef]
- Horablaga, N.M.; Cozma, A.; Alexa, E.; Obistioiu, D.; Cocan, I.; Poiana, M.-A.; Lalescu, D.; Pop, G.; Imbrea, I.M.; Buzna, C. Influence of sample preparation/extraction method on the phytochemical profile and antimicrobial activities of 12 commonly consumed medicinal plants in Romania. Appl. Sci. 2023, 13, 2530. [Google Scholar] [CrossRef]
- Sarkar, A.; Sarkar, D.; Poddar, K. Plant metabolites as new leads to drug discovery: Approaches and challenges. In Medicinal Plants—Chemistry, Pharmacology, and Therapeutic Applications, 1st ed.; Swamy, M.K., Patra, J.K., Rudramurthy, G.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–70. [Google Scholar] [CrossRef]
- Singletary, K. Oregano—Overview of the literature on health benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Bharti, V.; Vasudeva, N. Oreganum vulgare linn. leaf: An extensive pharmacognostical and phytochemical quality assessment. Adv. Pharm. Bull. 2013, 3, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, antibacterial, and cytotoxic activities of the ethanolic Origanum vulgare extract and its major constituents. Oxid. Med. Cell. Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Cör, D.; Simonovska, J.; Knez, Ž.; Kavrakovski, Z.; Rafajlovska, V. Extraction techniques and analytical methods for characterization of active compounds in Origanum species. Molecules 2020, 25, 4735. [Google Scholar] [CrossRef] [PubMed]
- Abdelaali, B.; El Menyiy, N.; El Omari, N.; Benali, T.; Guaouguaou, F.E.; Salhi, N.; Naceiri Mrabti, H.; Bouyahya, A. Phytochemistry, toxicology, and pharmacological properties of Origanum elongatum. Evid.-Based Complement. Altern. Med. 2021, 2021, 6658593. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Gîrd, C.E.; Duţu, L.E.; Costea, T.; Nencu, I.; Popescu, M.L.; Tudorel, O.O. Preliminary research concerning the obtaining of herbal extracts with potential neuroprotective activity note I. Obtaining and characterization of a selective Origanum vulgare L. dry extract. Farmacia 2016, 64, 680–687. [Google Scholar]
- Bran, P.E.; Nicuță, D.; Grosu, L.; Patriciu, O.-I.; Alexa, I.-C. Investigation regarding the potential application of grape pomace extracts on in vitro plant growth and development. Ovidius Univ. Ann. Chem. 2022, 33, 135–142. [Google Scholar] [CrossRef]
- de Almeida, P.; Blanco-Pascual, N.; Rosolen, D.; Cisilotto, J.; Creczynski-Pasa, T.; Laurindo, J. Antioxidant and antifungal properties of essential oils of oregano (Origanum vulgare) and mint (Mentha arvensis) against Aspergillus flavus and Penicillium commune for use in food preservation. Food Sci. Technol. 2022, 42, e64921. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunesa, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.d.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Basilio Heredia, B.J. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.H.; Saleem, U.; Ahmad, B.; Rashid, M. Phytochemical and toxicological evaluation of Zephyranthes citrine. Front. Pharmacol. 2022, 13, 1007310. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Armentano, M.F.; Monica, C.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic activity of Origanum Vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Grbović, F.; Stanković, M.S.; Ćurčić, M.; Đorđević, N.; Šeklić, D.; Topuzović, M.; Marković, S. In Vitro cytotoxic activity of Origanum vulgare L. on HCT-116 and MDA-MB-231 Cell Lines. Plants 2013, 2, 371–378. [Google Scholar] [CrossRef]
- Pour, B.M.; Latha, L.Y.; Sasidharan, S. Cytotoxicity and oral acute toxicity studies of Lantana camara leaf extract. Molecules 2011, 16, 3663–3674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Naz, S.; Ma, Y.; Ullah, Q.; Khan, M.Z.; Wang, J.; Lu, X.; Luosang, D.-Z.; Tabassum, S.; Chatha, A.M.M.; et al. An overview of comet assay application for detecting DNA damage in aquatic animals. Agriculture 2023, 13, 623. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Habibi, E.; Shadboorestan, A.; Chabra, A.; Ahmadi, A. The protective effects of Origanum vulgare L. extract on genetic damage of cyclophosphamide in mice blood lymphocytes using micronucleus test. Pharm. Biomed. Res. 2020, 6, 297–302. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow cytometry: An Overview. Curr. Protoc. Immunol. 2019, 120, 511–5111. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Chauvey, L.; Kallerhoff, J.; Pinelli, E.; Morard, M.; Silvestre, J. A tool derived from the Vicia faba micronucleus assay, to assess genotoxicity, cytotoxicity or biostimulation of novel compound used in agriculture. Agronomy 2021, 11, 321. [Google Scholar] [CrossRef]
- Santos, C.L.V.; Pourrut, B.; Ferreira de Oliveira, J.M.P. The use of comet assay in plant toxicology: Recent advances. Front. Genet. 2015, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, D.G. A review about phytotoxicity with a focus on the Allium test. Biostudent 2019, 2, 65–74. Available online: https://cbg.uvt.ro/wp-content/uploads/2021/10/A-review-about-phytotoxicity-with-a-focus-on-the-Allium-testBIOSTUDENT_December2019_Ciobanu_65-74.pdf (accessed on 15 November 2023).
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Owolarafe, T.A.; Salawu, K.; Ihegboro, G.O.; Ononamadu, C.J.; Alhassan, A.J.; Wudil, A.M. Investigation of cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam) leaf Allium cepa model. Toxicol. Rep. 2020, 7, 816–821. [Google Scholar] [CrossRef]
- Ihegboroa, G.O.; Alhassanb, A.J.; Ononamadua, C.J.; Owolarafea, T.A.; Sule, M.S. Evaluation of the biosafety potentials of methanol extracts/fractions of Tapinanthus bangwensis and Moringa oleifera leaves using Allium cepa model. Toxicol. Rep. 2020, 7, 671–679. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Van Staden, J. Cytotoxic and genotoxic effects of water extract of Distephanus angulifolius on Allium cepa Linn. S. Afr. J. Bot. 2014, 92, 147–150. [Google Scholar] [CrossRef]
- Akinboro, A.; Bakare, A.A. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J. Ethnopharmacol. 2007, 112, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Dragoeva, A.P.; Koleva, V.P.; Nanova, Z.D.; Kaschieva, M.Z. Allelopathy of cold water extracts from Origanum vulgare ssp. vulgare L. JACEN 2014, 3, 144–150. [Google Scholar] [CrossRef]
- Dragoeva, A.P.; Koleva, V.P.; Nanova, Z.D.; Kaschieva, M.Z.; Yotova, I.R. Allelopathic and cytotoxic activity of Origanum Vulgare ssp. vulgare growing wild in Bulgaria. Chem. Bulg. J. Sci. Educ. 2014, 23, 914–924. Available online: https://www.researchgate.net/publication/287350745 (accessed on 15 November 2023).
- Economou, G.; Travlos, I.S.; Folinas, A.; Karamanos, A.J. Greek oregano (Origanum vulgare ssp. hirtum) as allelopathic plant. JFAE 2007, 5, 348–351. Available online: https://www.wflpublisher.com/Abstract/790 (accessed on 16 November 2023).
- Grondona, E.; Gatti, G.; López, A.G.; Sánchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-efficacy of the essential oil of oregano (Origanum vulgare Lamiaceae. ssp. Hirtum). Plant. Foods Hum. Nutr. 2014, 69, 351–357. [Google Scholar] [CrossRef]
- AdNatura. Available online: https://adnatura.ro/magazin/ceaiuri-vrac/ceaiuri-simple-vrac/ceai-de-sovarf/ (accessed on 13 March 2024).
- Prodalcom. Available online: https://prodalcom.ro/alcohol-production/ (accessed on 12 March 2024).
- Apa Bucovina. Available online: https://apabucovina.ro/ (accessed on 12 March 2024).
- Official Methods of Analysis of AOAC International (22). AOAC Official Method 922.01 Sampling of Plants. AOAC 930.04 Loss on Drying (Moisture) in Plants; Latimer, G.W., Jr., Beine, R.L., Eds.; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.W. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA) Press: Washington, DC, USA, 2005. [Google Scholar]
- Saad, R.; Asmani, F.; Saad, M.; Hussain, M.; Khan, J.; Kaleemullah, M.; Bin Othman, N.; Tofigh, A.; Yusuf, E. A new approach for predicting antioxidant property of herbal extracts. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 166–174. Available online: http://impactfactor.org/PDF/IJPPR/7/IJPPR,Vol7,Issue1,Article25.pdf (accessed on 20 November 2023).
- Cioroi, M. Study on total polyphenols and reducing power of aqueous extracts from selected Lamiaceae species. J. Agroaliment. Process Technol. 2009, 15, 521–524. Available online: https://journal-of-agroalimentary.ro/admin/articole/91369L8_Maria_Cioroi_Vol.4_521-524.pdf (accessed on 23 November 2023).
- Glories, Y. La couleur des vins rouges. 2e partie: Mesure, origine et interpretation. J. Int. Sci. Vigne du Vin 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Fredes, S.N.; Ruiz, L.Á.; Recio, J.A. Modeling phenols, anthocyanins and color intensity of wine using pre-harvest Sentinel-2 images. Remote Sens. 2021, 13, 4951. [Google Scholar] [CrossRef]
- Remini, H.; Mertz, C.; Belbahi, A.; Achir, N.; Dornier, M.; Madani, K. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurized blood orange juice during storage. Food Chem. 2015, 173, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Teneva, D.; Pencheva, D.; Petrova, A.; Ognyanov, M.; Georgiev, Y.; Denev, P. Addition of medicinal plants increases antioxidant activity, color, and anthocyanin stability of black chokeberry (Aronia melanocarpa) functional beverages. Plants 2022, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Reich, E.; Schibli, A. HPTLC for the Analysis of Medicinal Plants. In High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; Thieme: New York, NY, USA, 2007; pp. 160–174. [Google Scholar] [CrossRef]
- Nowak, R.; Wojciak-Kosior, M.; Sowa, I.; Sokolowska-Krzaczek, A.; Pietrzak, W.; Szczodra, A.; Kocjan, R. HPTLC-densitometry determination of triterpenic acids in Origanum vulgare, Rosmarinus officinalis and Syzygium aromaticum. Acta Pol. Pharm.-Drug Res. 2013, 70, 413–418. Available online: https://www.ptfarm.pl/pub/File/Acta_Poloniae/2013/3/413.pdf (accessed on 15 November 2023).
- Juga, U.; Glavnika, V.; Kranjca, E.; Vovk, I. HPTLC–densitometric and HPTLC–MS methods for analysis of flavonoids. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 329–341. [Google Scholar] [CrossRef]
- Zekic, J.; Vovk, I.; Glavnik, V. Extraction and analyses of flavonoids and phenolic acids from Canadian Goldenrod and Giant Goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Kachbi, A.; Abdelfettah-Kara, D.; Benamor, M.; Senhadji-Kebiche, O. Simultaneous spectrometric determination of caffeic acid, gallic acid, and quercetin in some aromatic herbs, using chemometric tools. J. Korean Chem. Soc. 2021, 65, 254–259. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption coefficients of phenolic structures in different solvents routinely used for experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef]
- Cervato, G.; Carabelli, M.; Gervasio, S.; Cittera, A.; Cazzola, R.; Cestaro, B. Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J. Food Biochem. 2000, 24, 453–465. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kogiannou, D.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef]





| Characteristics | I01 | D02 | E03 | E04 |
|---|---|---|---|---|
| DM [%] | 0.48 ± 0.02 ** | 0.36 ± 0.04 ** | 0.42 ± 0.07 ** | 0.34 ± 0.05 ** |
| pH at 25 °C | 5.69 ± 0.002 ** | 5.98 ± 0.006 ** | 6.93 ± 0.001 ** | 7.25 ± 0.003 * |
| EC [μS/cm] | 638.26 ± 0.50 ** | 768.50 ± 0.26 ** | 329.73 ± 0.41 ** | 164.06 ± 0.49 ** |
| TDS [ppm] | 312.06 ± 0.35 * | 376.16 ± 0.25 * | 157.96 ± 3.53 * | 79.65 ± 0.49 * |
| SAL [psu] | 0.31 ± 0.0005 * | 0.37 ± 0.0005 * | 0.16 ± 0.0006 * | 0.08 ± 0.0006 * |
| TPC [mg GAE/100 mL] | 24.66 ± 0.01 * | 31.36 ± 0.009 * | 22.70 ± 0.038 * | 15.93 ± 0.003 * |
| CI a | 1.66 ± 0.001 ** | 3.21 ± 0.004 ** | 2.88 ± 0.004 ** | 1.74 ± 0.002 ** |
| pH | DM | EC | TDS | SAL | TPC | CI | |
|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||
| DM | −0.549 | 1 | |||||
| EC | −0.934 | 0.303 | 1 | ||||
| TDS | −0.935 | 0.299 | 0.999 | 1 | |||
| SAL | −0.932 | 0.294 | 0.999 | 0.999 | 1 | ||
| TPC | −0.774 | 0.162 | 0.940 | 0.938 | 0.941 | 1 | |
| CI | −0.059 | −0.285 | 0.382 | 0.377 | 0.385 | 0.672 | 1 |
| Control Sample | I01 | D02 | E03 | E04 | |
|---|---|---|---|---|---|
| Mitotic index | 28.54 ± 2.43 * | 24.15 ± 2.12 * | 16.25 ± 1.40 * | 22.12 ± 2.64 * | 9.66 ± 0.91 * |
| Mitotic phase | |||||
| % Prophase | 91.83 ± 3.15 | 94.49 ± 4.08 | 93.80 ± 2.77 | 92.27 ± 0.88 | 82.70 ± 1.38 |
| % Metaphase | 2.81 ± 0.77 | 2.35 ± 0.62 | 3.22 ± 0.61 | 2.91 ± 0.30 | 6.62 ± 0.17 |
| % Anaphase | 1.74 ± 0.43 | 0.91 ± 0.25 | 0.97 ± 0.44 | 1.91 ± 0.45 | 4.27 ± 0.79 |
| % Telophase | 3.61 ± 0.89 | 2.25 ± 0.57 | 2.01 ± 0.54 | 2.91 ± 0.51 | 6.41 ± 0.80 |
| Present Work | Dragoeva et al. | ||||||
|---|---|---|---|---|---|---|---|
| Samples | I01 | D02 | E03 | E04 | OCWE * [36] | OWI ** [37] | |
| Extraction conditions | oregano | 2 g | 3.5 g | ||||
| solvent | 200 mL boiling water *** | 200 mL HA mixture a | 200 mL HA mixture b | 1000 mL cold distilled water | 1000 mL boiling distilled water | ||
| time | 5 min at rt | Boiled for 5 min and left for 5 min at rt | stirred at rt at 150 rpm for 30 min | 24 h at rt | 60 min at rt | ||
| MI % | 24.15 ± 2.12 | 16.25 ± 1.40 | 22.12 ± 2.64 | 9.66 ± 0.91 | 5.01 ± 0.22 | 3.71 ± 0.19 | |
| CA % | 1.01 ± 0.03 | 1.99 ± 0.02 | 3.52 ± 0.05 | 4.50 ± 0.04 | 5.02 ± 0.23 | 6.10 ± 0.24 | |
| Chromosomal Aberrations | Control Sample | I01 | D02 | E03 | E04 |
|---|---|---|---|---|---|
| Total aberrant cells [%] | 0.93 ± 0.02 | 1.01 ± 0.03 | 1.99 ± 0.02 | 3.52 ± 0.05 | 4.50 ± 0.04 |
| Spindle disturbance at late prophase | 0.18 | 0.08 | 0.18 | 0.13 | 0.12 |
| Micronucleus at prophase | 0.04 | 0.35 | 0.19 | 0.62 | 1.09 |
| Non-oriented (NO) chromosome at metaphase | 0 | 0 | 0 | 1.06 | 0.55 |
| Ana-telophase with bridges (A-T bridges) | 0.33 | 0.27 | 0.86 | 0.72 | 1.10 |
| Ana-telophase with delayed chromosome (A-T-DC) | 0.10 | 0.04 | 0.34 | 0.41 | 0.44 |
| Ana-telophase with expulsed chromosome (A-T-EC) | 0.28 | 0.22 | 0.37 | 0.55 | 0.88 |
| Ana-telophase with chromosomal fragments (A-T fragments) | 0 | 0.05 | 0.05 | 0.03 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicuță, D.; Grosu, L.; Alexa, I.-C.; Fînaru, A.-L. Sustainable Characterization of Some Extracts of Origanum vulgare L. and Biosafety Evaluation Using Allium cepa Assay. Horticulturae 2024, 10, 504. https://doi.org/10.3390/horticulturae10050504
Nicuță D, Grosu L, Alexa I-C, Fînaru A-L. Sustainable Characterization of Some Extracts of Origanum vulgare L. and Biosafety Evaluation Using Allium cepa Assay. Horticulturae. 2024; 10(5):504. https://doi.org/10.3390/horticulturae10050504
Chicago/Turabian StyleNicuță, Daniela, Luminița Grosu, Irina-Claudia Alexa, and Adriana-Luminița Fînaru. 2024. "Sustainable Characterization of Some Extracts of Origanum vulgare L. and Biosafety Evaluation Using Allium cepa Assay" Horticulturae 10, no. 5: 504. https://doi.org/10.3390/horticulturae10050504





