Impact of Mechanical Stirring and Percolate Recirculation on the Performances of Dry Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
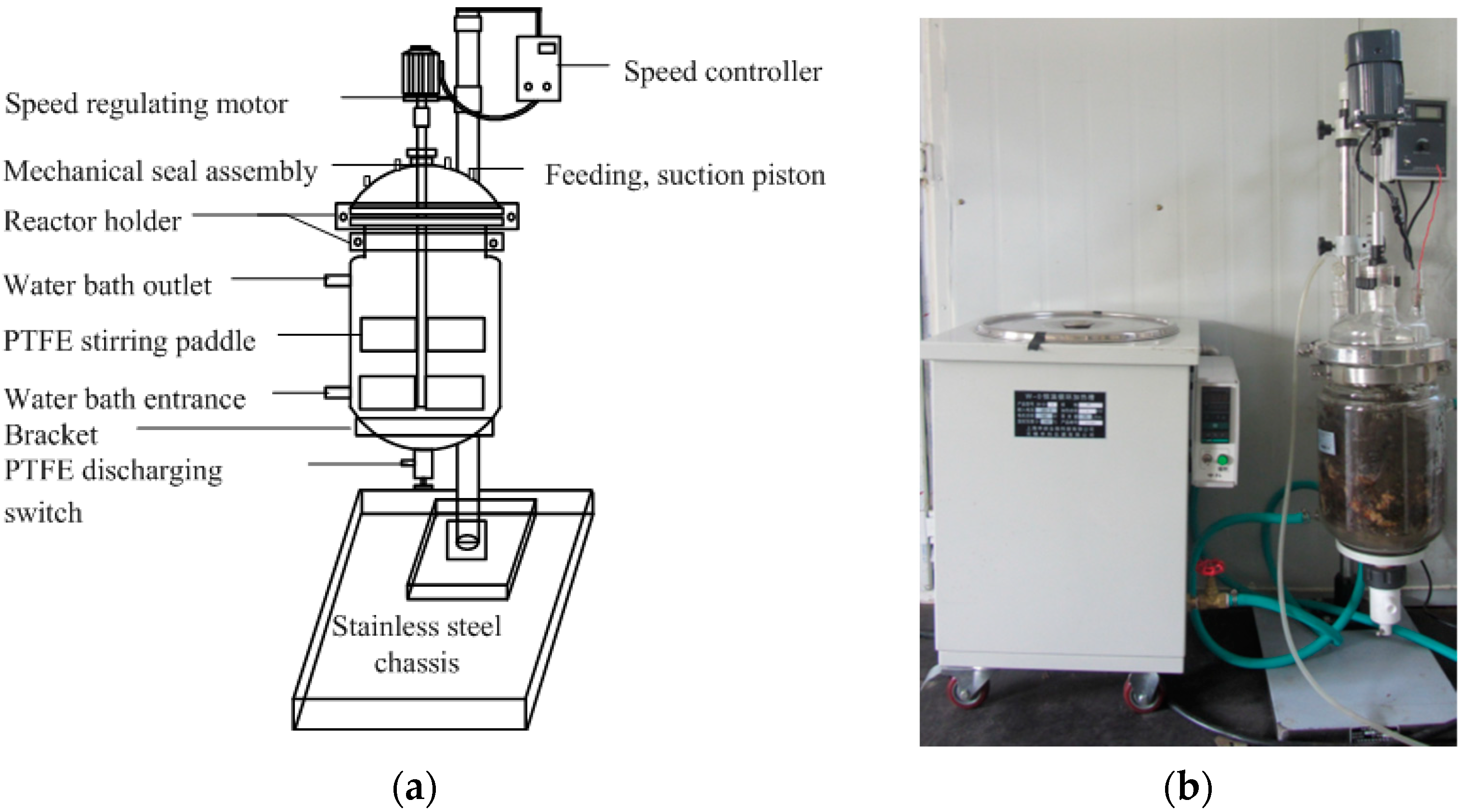
2.2. Experimental Setup and Procedures
- (1)
- The influence of mixing intensity on the DAD process
- (2)
- The effect of height-to-diameter ratio (H/D) on the DAD process.
2.3. Analytical Methods
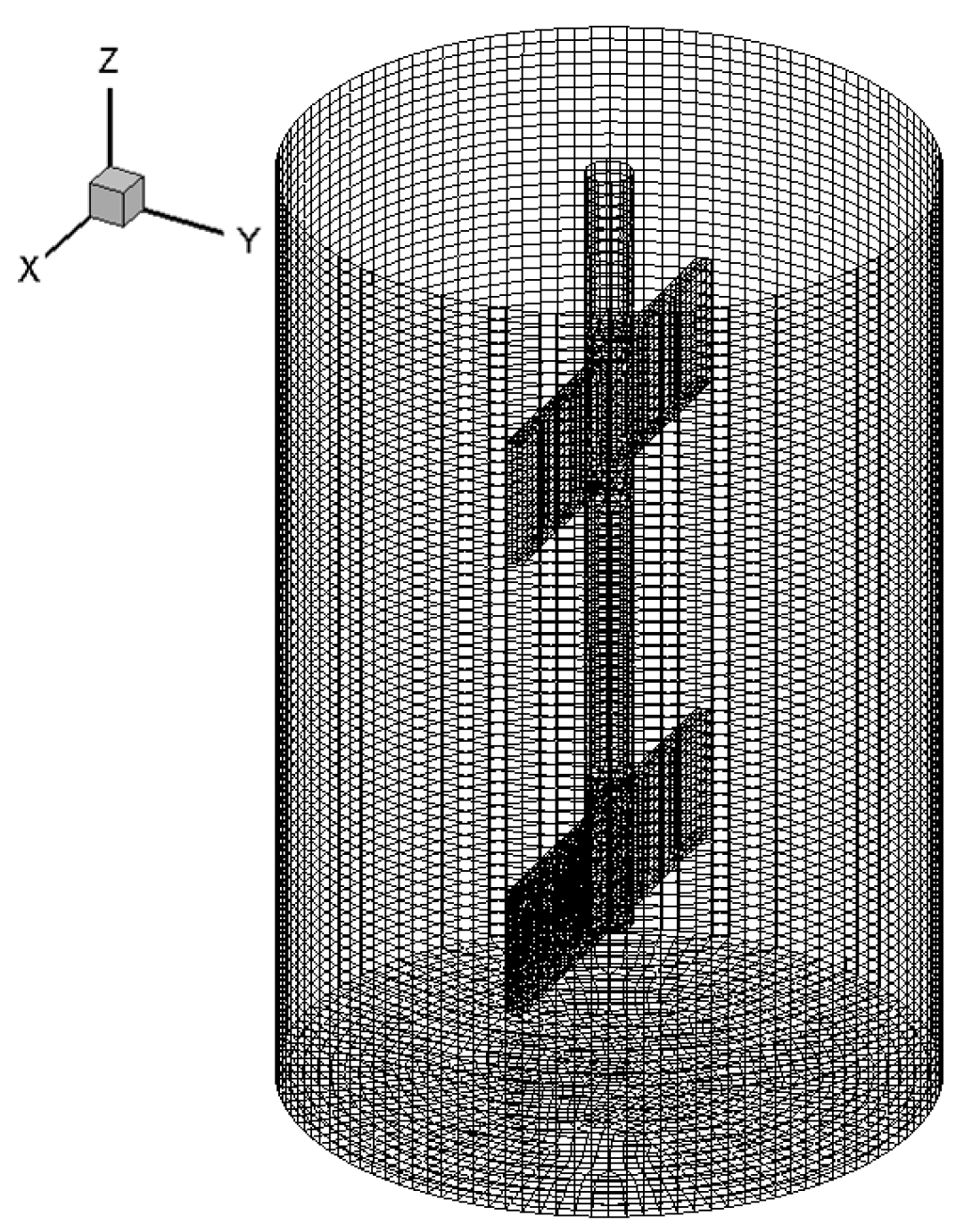
2.4. CFD Simulation
3. Results
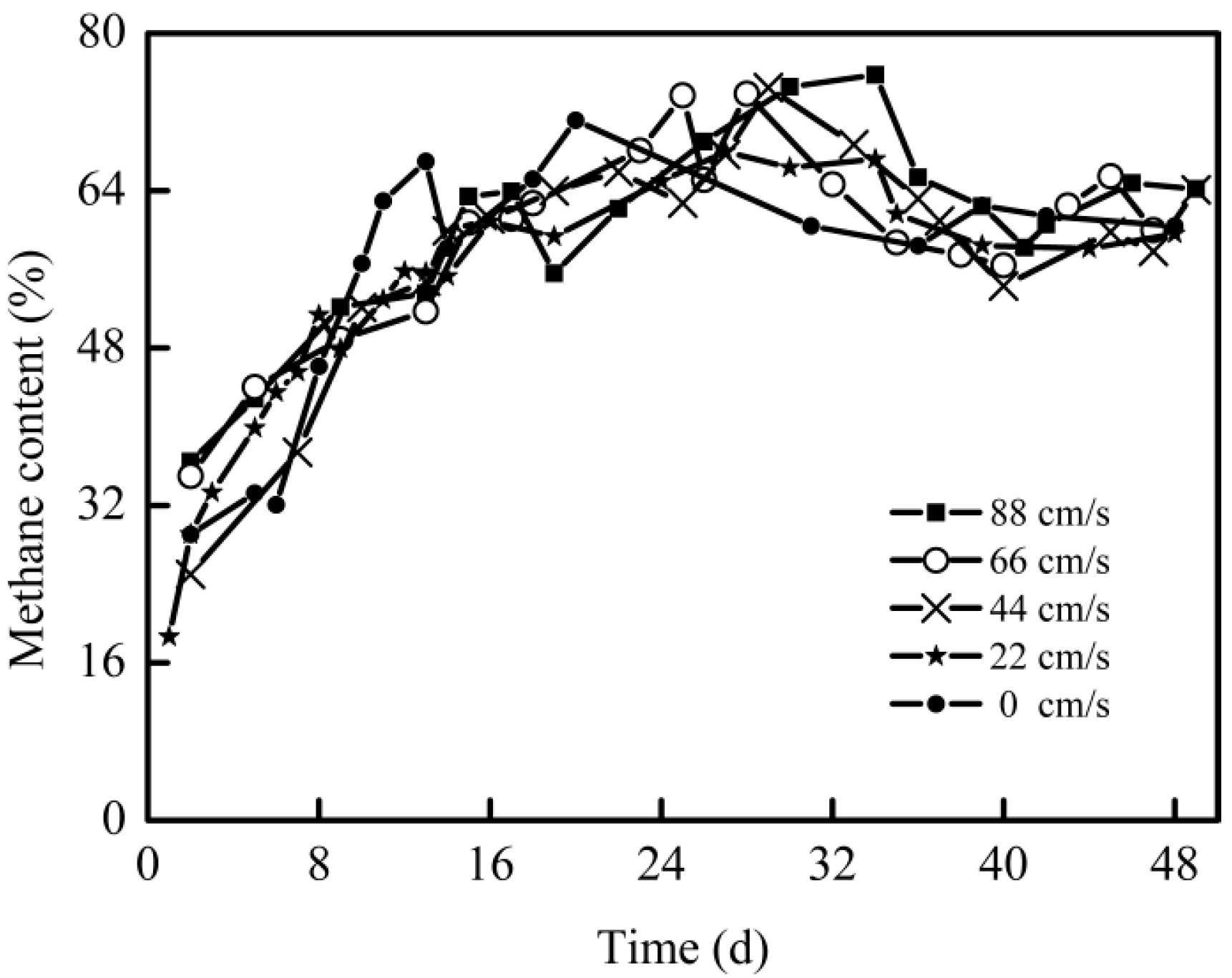
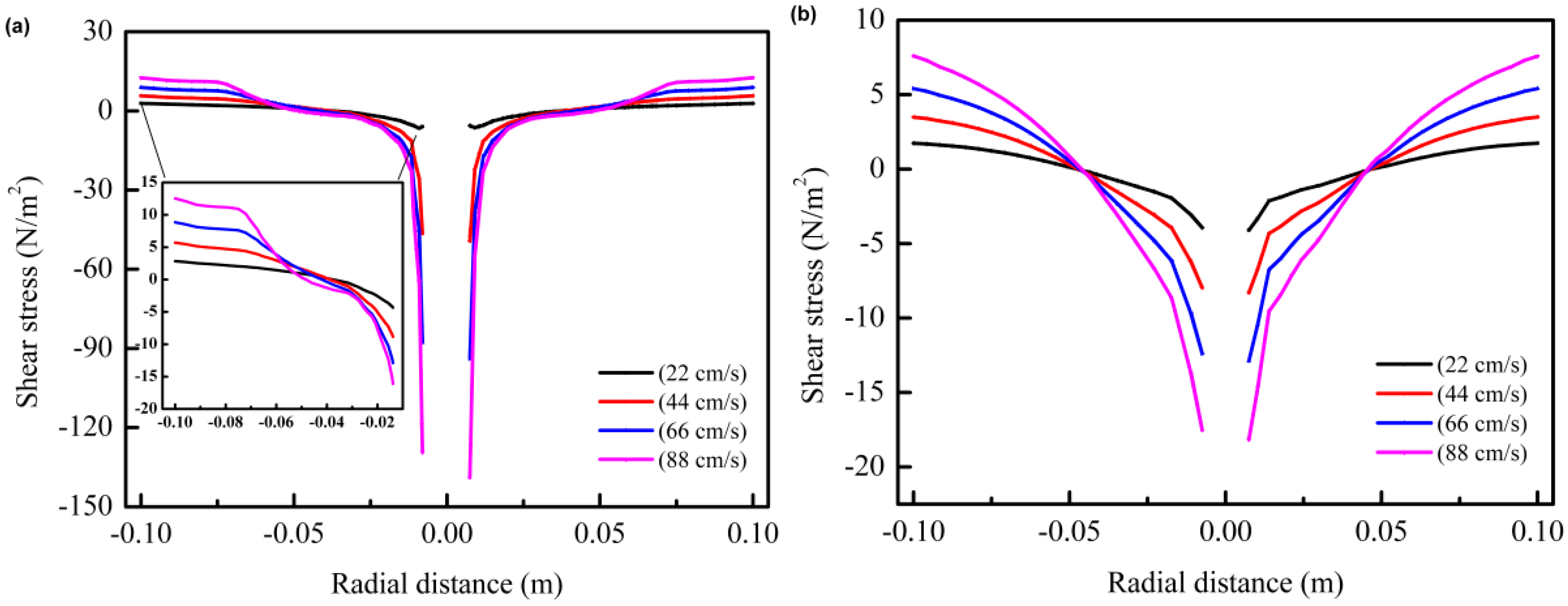
3.1. The Effect of Mechanical Stirring Velocity on DAD
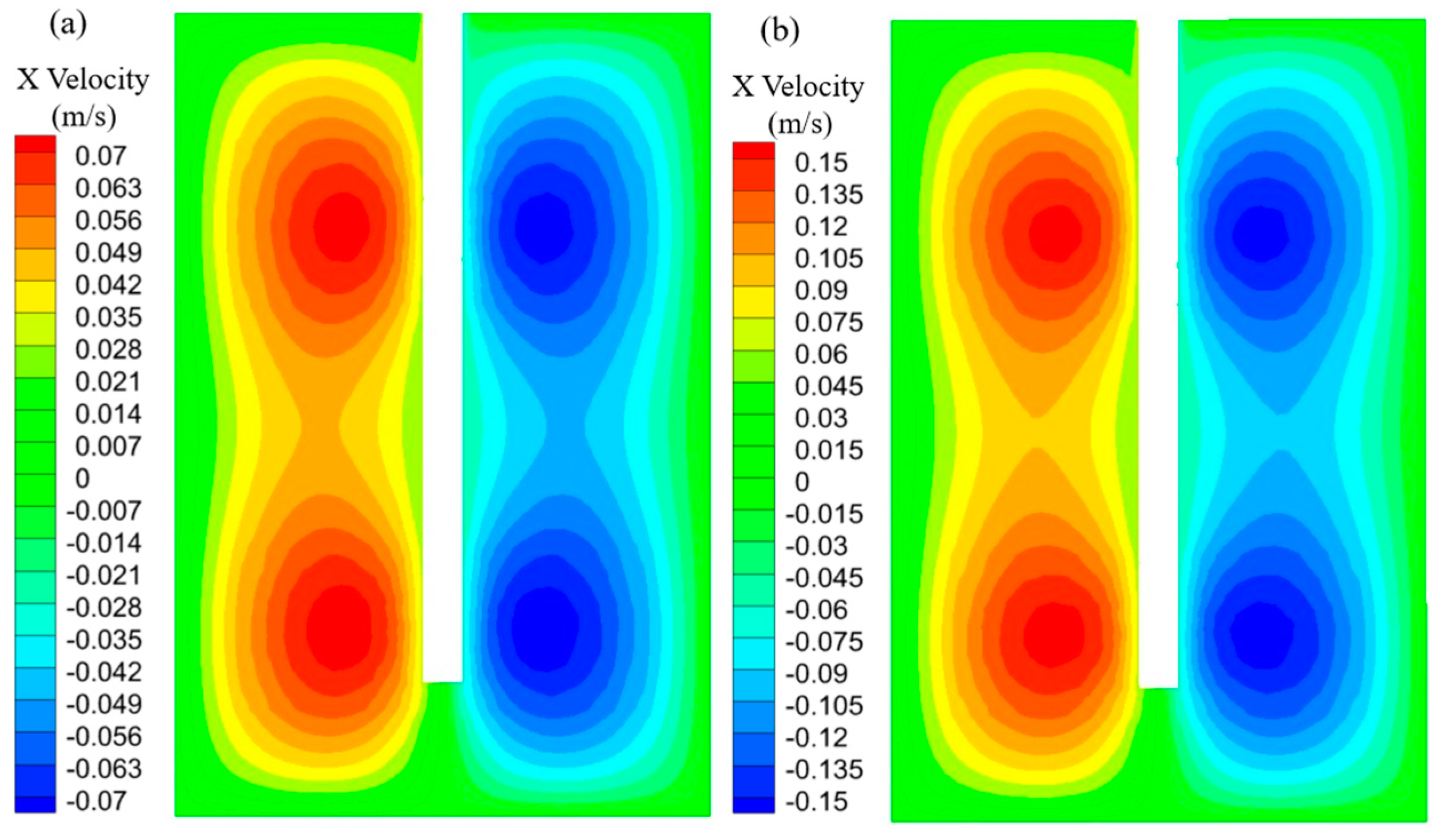
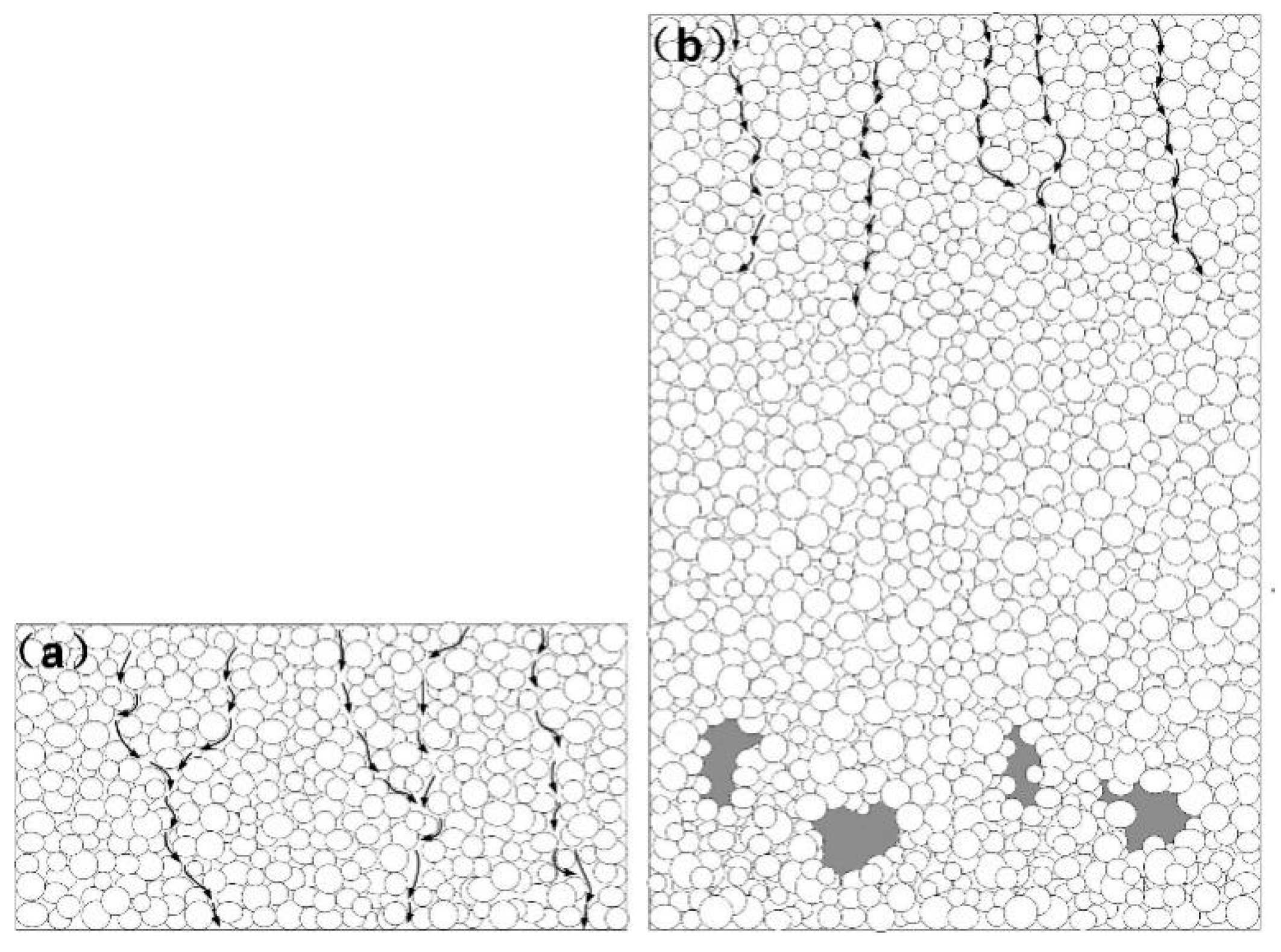
3.2. CFD Simulation
3.3. The Influence of Different Height to Diameter Ratios on Dry Digestion
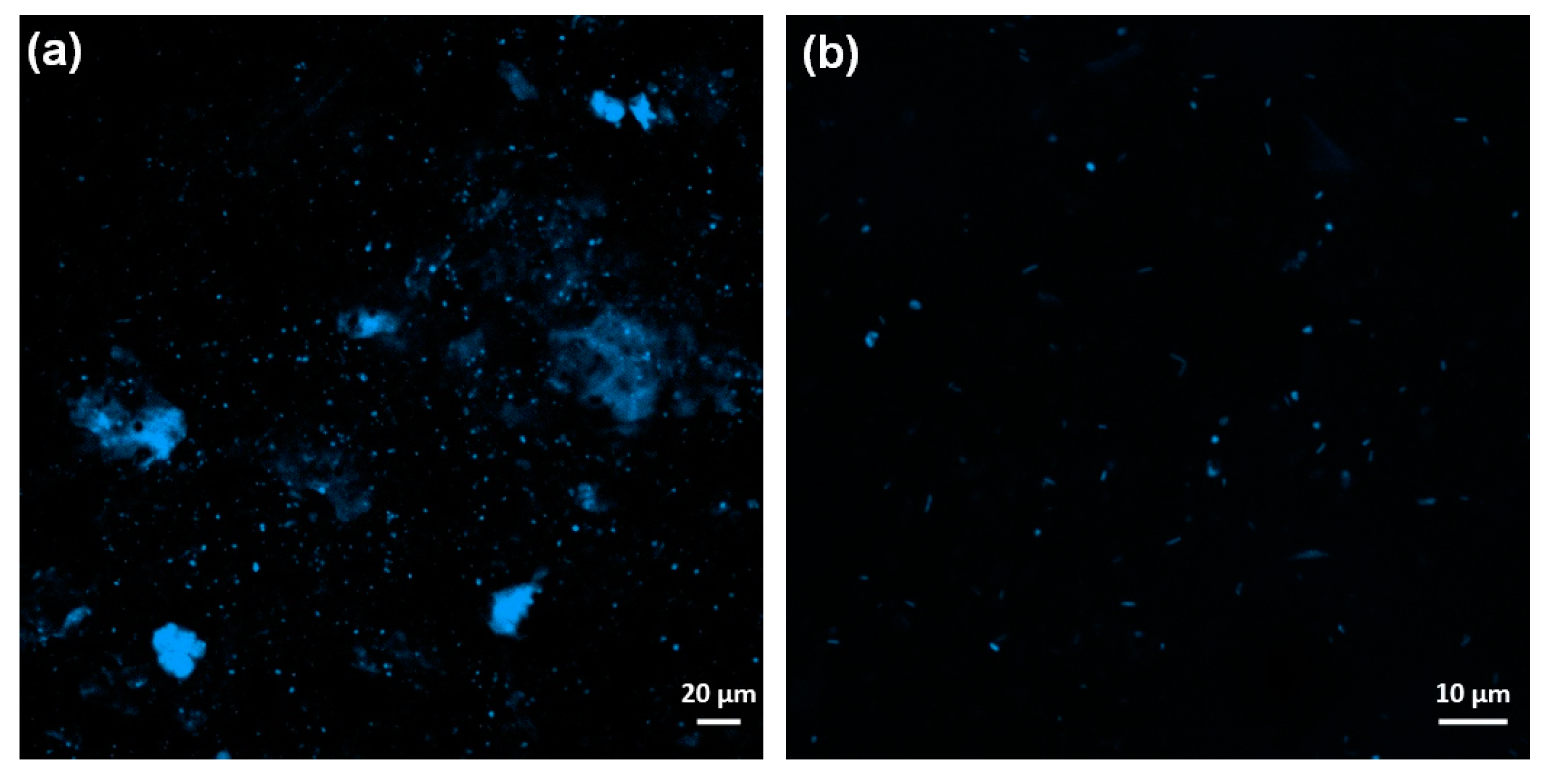
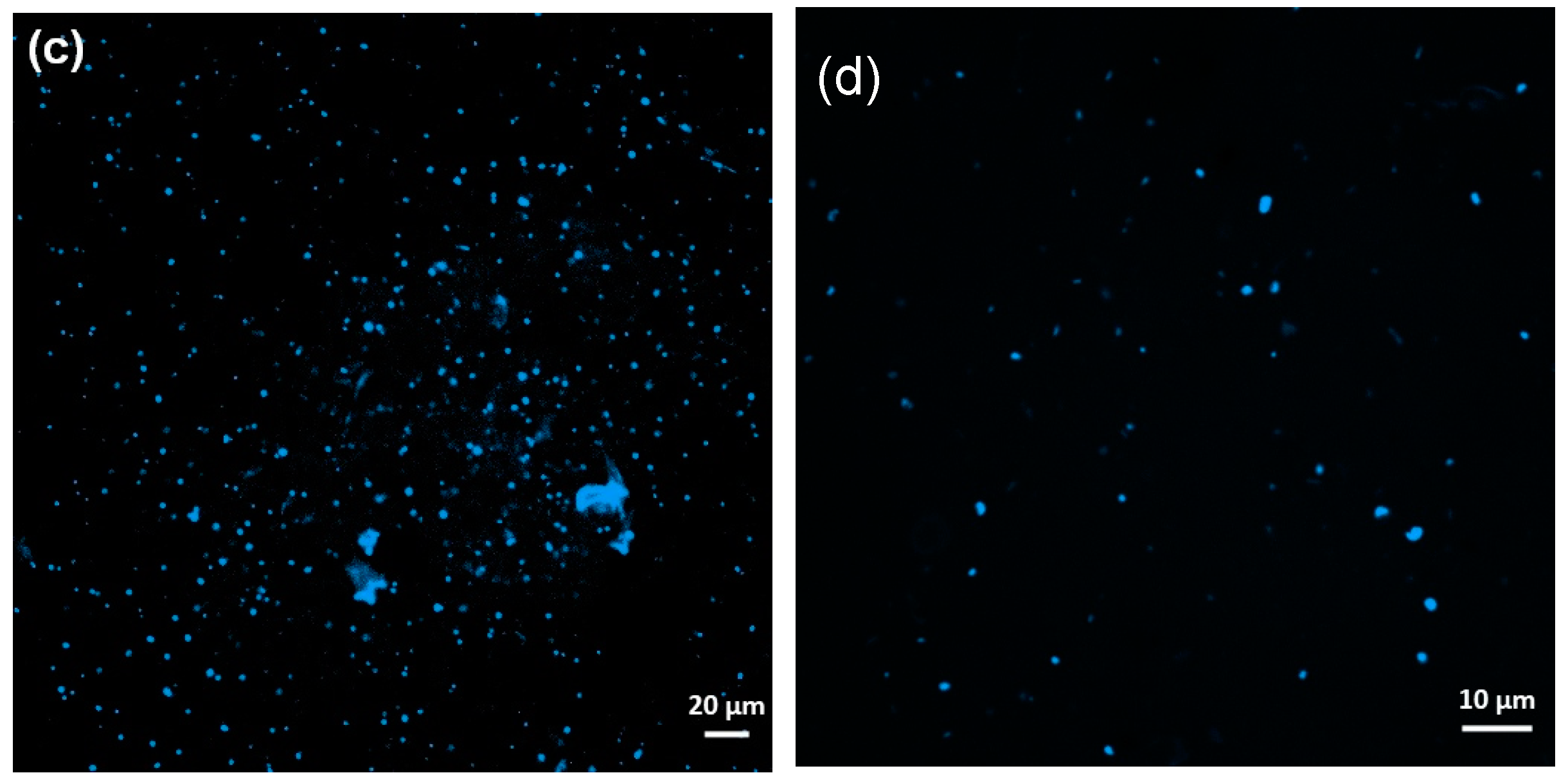
3.4. Comparison and Combination of Mechanical Mixing and Percolate Recirculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Kuroki, A.; Loh, K.-C.; Seok, J.K.; Dai, Y.; Tong, Y.W. Highly efficient anaerobic co-digestion of food waste and horticultural waste using a three-stage thermophilic bioreactor: Performance evaluation, microbial community analysis, and energy balance assessment. Energy Convers. Manag. 2020, 223, 113290. [Google Scholar] [CrossRef]
- El Ibrahimi, M.; Khay, I.; El Maakoul, A.; Bakhouya, M. Techno-economic and environmental assessment of anaerobic co-digestion plants under different energy scenarios: A case study in Morocco. Energy Convers. Manag. 2021, 245, 114553. [Google Scholar] [CrossRef]
- Sun, J.; Kosaki, Y.; Kawamura, K.; Watanabe, N. Operational load enhancement for an anaerobic membrane bioreactor through ethanol fermentation pretreatment of food waste. Energy Convers. Manag. 2021, 249, 114840. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Li, W.; Li, C.; Xu, G. Enhanced biogas production from sorghum stem by co-digestion with cow manure. Int. J. Hydrog. Energy 2016, 41, 9153–9158. [Google Scholar] [CrossRef]
- Li, W.; Xu, G. Enhancement of anaerobic digestion of grass by pretreatment with imidazolium-based ionic liquids. Environ. Technol. 2016, 38, 1843–1851. [Google Scholar] [CrossRef]
- Li, W.; Loh, K.-C.; Zhang, J.; Tong, Y.W.; Dai, Y. Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl. Energy 2018, 209, 400–408. [Google Scholar] [CrossRef]
- Mavridis, S.; Voudrias, E.A. Using biogas from municipal solid waste for energy production: Comparison between anaerobic digestion and sanitary landfilling. Energy Convers. Manag. 2021, 247, 114613. [Google Scholar] [CrossRef]
- Guendouz, J.; Buffière, P.; Cacho, J.; Carrère, M.; Delgenes, J.-P. Dry anaerobic digestion in batch mode: Design and operation of a laboratory-scale, completely mixed reactor. Waste Manag. 2010, 30, 1768–1771. [Google Scholar] [CrossRef]
- Li, W.; Lu, C.; An, G.; Chang, S. Comparison of Alkali-Buffering Effects and Co-digestion on High-Solid Anaerobic Digestion of Horticultural Waste. Energy Fuels 2017, 31, 10990–10997. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. State of the art on mixing in an anaerobic digester: A review. Renew. Energy 2019, 141, 922–936. [Google Scholar] [CrossRef]
- Zhai, X.; Kariyama, I.D.; Wu, B. Investigation of the effect of intermittent minimal mixing intensity on methane production during anaerobic digestion of dairy manure. Comput. Electron. Agric. 2018, 155, 121–129. [Google Scholar] [CrossRef]
- Wu, B. CFD simulation of gas and non-Newtonian fluid two-phase flow in anaerobic digesters. Water Res. 2010, 44, 3861–3874. [Google Scholar] [CrossRef]
- Wu, B. CFD Prediction of Mixing Time in Anaerobic Digesters. Trans. ASABE 2010, 53, 553–563. [Google Scholar] [CrossRef]
- Mohammadrezaei, R.; Zareei, S.; Khazaei, N.B. Optimum mixing rate in biogas reactors: Energy balance calculations and computational fluid dynamics simulation. Energy 2018, 159, 54–60. [Google Scholar] [CrossRef]
- Ghanimeh, S.; El Fadel, M.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012, 117, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lebranchu, A.; Delaunay, S.; Marchal, P.; Blanchard, F.; Pacaud, S.; Fick, M.; Olmos, E. Impact of shear stress and impeller design on the production of biogas in anaerobic digesters. Bioresour. Technol. 2017, 245, 1139–1147. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Q.; Hu, K. Influence of mixing time on the performance of anaerobic digestion of sewage sludge. J. Nat. Sci. Heilongjiang Univ. 2011, 28, 383–387. [Google Scholar]
- Lindmark, J.; Eriksson, P.; Thorin, E. The effects of different mixing intensities during anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2014, 34, 1391–1397. [Google Scholar] [CrossRef]
- Lindmark, J.; Thorin, E.; Bel Fdhila, R.; Dahlquist, E. Effects of mixing on the result of anaerobic digestion: Review. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Li, L.-L.; Tong, Z.-H.; Fang, C.-Y.; Chu, J.; Yu, H.-Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Azargoshasb, H.; Mousavi, S.; Amani, T.; Jafari, A.; Nosrati, M. Three-phase CFD simulation coupled with population balance equations of anaerobic syntrophic acidogenesis and methanogenesis reactions in a continuous stirred bioreactor. J. Ind. Eng. Chem. 2015, 27, 207–217. [Google Scholar] [CrossRef]
- Huang, Y.; Dehkordy, F.M.; Li, Y.; Emadi, S.; Bagtzoglou, A.; Li, B. Enhancing anaerobic fermentation performance through eccentrically stirred mixing: Experimental and modeling methodology. Chem. Eng. J. 2018, 334, 1383–1391. [Google Scholar] [CrossRef]
- Inglès, X.; Pallares, J.; Larre, M.T.; Méndez, L.; Grau, F.X. Experimental and numerical study of turbulent mixing in a model of a polymerization reactor. J. Ind. Eng. Chem. 2012, 19, 1251–1256. [Google Scholar] [CrossRef]
- Wu, B. CFD investigation of turbulence models for mechanical agitation of non-Newtonian fluids in anaerobic digesters. Water Res. 2011, 45, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, C.; Yang, P.; Wang, Y.; Wang, L. Optimal analysis on the mixing condition of sludge in the process of anaerobic digestion. Environ. Sci. Technol. 2015, 38, 100–105. [Google Scholar]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Q.; Li, G.; Xia, C. Comparison of bio-hydrogen and bio-methane production performance in continuous two-phase anaerobic fermentation system between co-digestion and digestate recirculation. Bioresour. Technol. 2020, 318, 124269. [Google Scholar] [CrossRef]
- El Ibrahimi, M.; Khay, I.; El Maakoul, A.; Bakhouya, M. Food waste treatment through anaerobic co-digestion: Effects of mixing intensity on the thermohydraulic performance and methane production of a liquid recirculation digester. Process Saf. Environ. Prot. 2021, 147, 1171–1184. [Google Scholar] [CrossRef]
- Pezzolla, D.; Di Maria, F.; Zadra, C.; Massaccesi, L.; Sordi, A.; Gigliotti, G. Optimization of solid-state anaerobic digestion through the percolate recirculation. Biomass Bioenergy 2017, 96, 112–118. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zhang, G.; Xu, G. Impact of pretreatment on solid state anaerobic digestion of yard waste for biogas production. World J. Microbiol. Biotechnol. 2013, 30, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005; pp. 387–388. [Google Scholar]
- Ghanimeh, S.A.; Al-Sanioura, D.N.; Saikaly, P.E.; El-Fadel, M. Correlation between system performance and bacterial composition under varied mixing intensity in thermophilic anaerobic digestion of food waste. J. Environ. Manag. 2018, 206, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Y.; Li, X. Biogasification per formance of anaerobic co- digestion of kitchen residues and cattle manure. Renew. Energy Resour. 2008, 26, 64–68. [Google Scholar]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.; Szumacher-Strabel, M. Review: Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2020, 15, 100060. [Google Scholar] [CrossRef]
- Martin, D.; Potts, L.; Heslop, V. Reaction Mechanisms in Solid-State Anaerobic Digestion. Process Saf. Environ. Prot. 2003, 81, 171–179. [Google Scholar] [CrossRef]
- Martin, D.J. The Site of Reaction in Solid-State Digestion: A New Hypothesis. Process Saf. Environ. Prot. 2001, 79, 29–37. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.; Li, C. Improvement of Solid-State Anaerobic Digestion of Yard Waste by Co-digestion and pH Adjustment. Waste Biomass Valorization 2017, 9, 211–221. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, C.; Huo, S. Influence of fluid dynamics on anaerobic digestion of food waste for biogas production. Environ. Technol. 2016, 38, 1160–1168. [Google Scholar] [CrossRef]
- Kariyama, I.D.; Zhai, X.; Wu, B. Influence of mixing on anaerobic digestion efficiency in stirred tank digesters: A review. Water Res. 2018, 143, 503–517. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, J.; Poncin, S.; Li, H.Z. Effect of hydrodynamic shear on biogas production and granule characteristics in a continuous stirred tank reactor. Process Biochem. 2016, 51, 345–351. [Google Scholar] [CrossRef]
- Nguyen, D.; Wu, Z.; Shrestha, S.; Lee, P.-H.; Raskin, L.; Khanal, S.K. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019, 166, 115080. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Zhang, J. The impact of height/diameter ratio on aerobic granular sludge(AGS) system in domestic sewage. China Environ. Sci. 2019, 39, 141–148. [Google Scholar]
- Zhu, J.; Han, M.; Zhang, G.; Yang, L. Co-digestion of spent mushroom substrate and corn stover for methane production via sol-id-state anaerobic digestion. J. Renew. Sustain. Energy 2015, 7, 023135. [Google Scholar] [CrossRef]







| Parameters | Sorghum Stalks | Inoculum |
|---|---|---|
| Total solids (%, w.b.) | 94.14 ± 0.06 | 11.30 ± 0.05 |
| Volatile solids (%, d.b.) | 92.06 ± 0.18 | 56.02 ± 0.07 |
| Total carbon (%, d.b.) | 43.37 ± 0.25 | |
| Total nitrogen (%, d.b.) | 1.03 ± 0.03 | |
| C/N ratio | 42.27 | |
| Hydrogen (%, d.b.) | 5.90 ± 0.02 | |
| Sulfur (%, d.b.) | 0.22 ± 0.43 | |
| Hemicellulose (%, d.b.) | 40.21 ± 1.10 | |
| Cellulose (%, d.b.) | 35.45 ± 1.53 | |
| Lignin (%, d.b.) | 2.32 ± 2.15 |
| Velocity (cm/s) | Cumulative Biogas Yield (mL/g VS) | COD (mg/L) | Ammonia Nitrogen (mg/L) | pH |
|---|---|---|---|---|
| 88 | 391 ± 14 | 9358 ± 106 | 771 ± 38 | 7.67 ± 0.20 |
| 66 | 380 ± 13 | 8920 ± 61 | 682 ± 26 | 7.61 ± 0.13 |
| 44 | 353 ± 16 | 9299 ± 87 | 759 ± 8 | 7.69 ± 0.08 |
| 22 | 341 ± 5 | 10,907 ± 92 | 654 ± 21 | 7.96 ± 0.16 |
| 0 | 331 ± 11 | 8033 ± 32 | 855 ± 7 | 7.89 ± 0.00 |
| H/D | Cumulative Biogas Yield (mL/g VS) | COD (mg/L) | Ammonia Nitrogen (mg/L) | pH |
|---|---|---|---|---|
| 3:2 | 407 ± 9 | 12,824 ± 107 | 733 ± 28 | 8.09 ± 0.08 |
| 1.5:2 | 371 ± 6 | 7615 ± 82 | 487 ± 5 | 7.92 ± 0.18 |
| 1:2 | 326 ± 18 | 8957 ± 64 | 592 ± 23 | 7.96 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chang, S.; Zhao, S.; Liu, P.; Qian, Y.; Li, W. Impact of Mechanical Stirring and Percolate Recirculation on the Performances of Dry Anaerobic Digestion. Fermentation 2023, 9, 848. https://doi.org/10.3390/fermentation9090848
Zhang Z, Chang S, Zhao S, Liu P, Qian Y, Li W. Impact of Mechanical Stirring and Percolate Recirculation on the Performances of Dry Anaerobic Digestion. Fermentation. 2023; 9(9):848. https://doi.org/10.3390/fermentation9090848
Chicago/Turabian StyleZhang, Zhikai, Shengqiang Chang, Shengyong Zhao, Peng Liu, Yanan Qian, and Wangliang Li. 2023. "Impact of Mechanical Stirring and Percolate Recirculation on the Performances of Dry Anaerobic Digestion" Fermentation 9, no. 9: 848. https://doi.org/10.3390/fermentation9090848





