Responses of Fermentation Characteristics and Microbial Communities to Vitamin B12 Supplementation in In Vitro Ruminal Cultures
Abstract
:1. Introduction
2. Material and Methods
2.1. In Vitro Incubation
2.2. Sample Collection and Analysis
2.3. Microbial DNA Extraction and 16S rRNA Genes Sequencing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effects of Vitamin B12 on Fermentation Characteristics
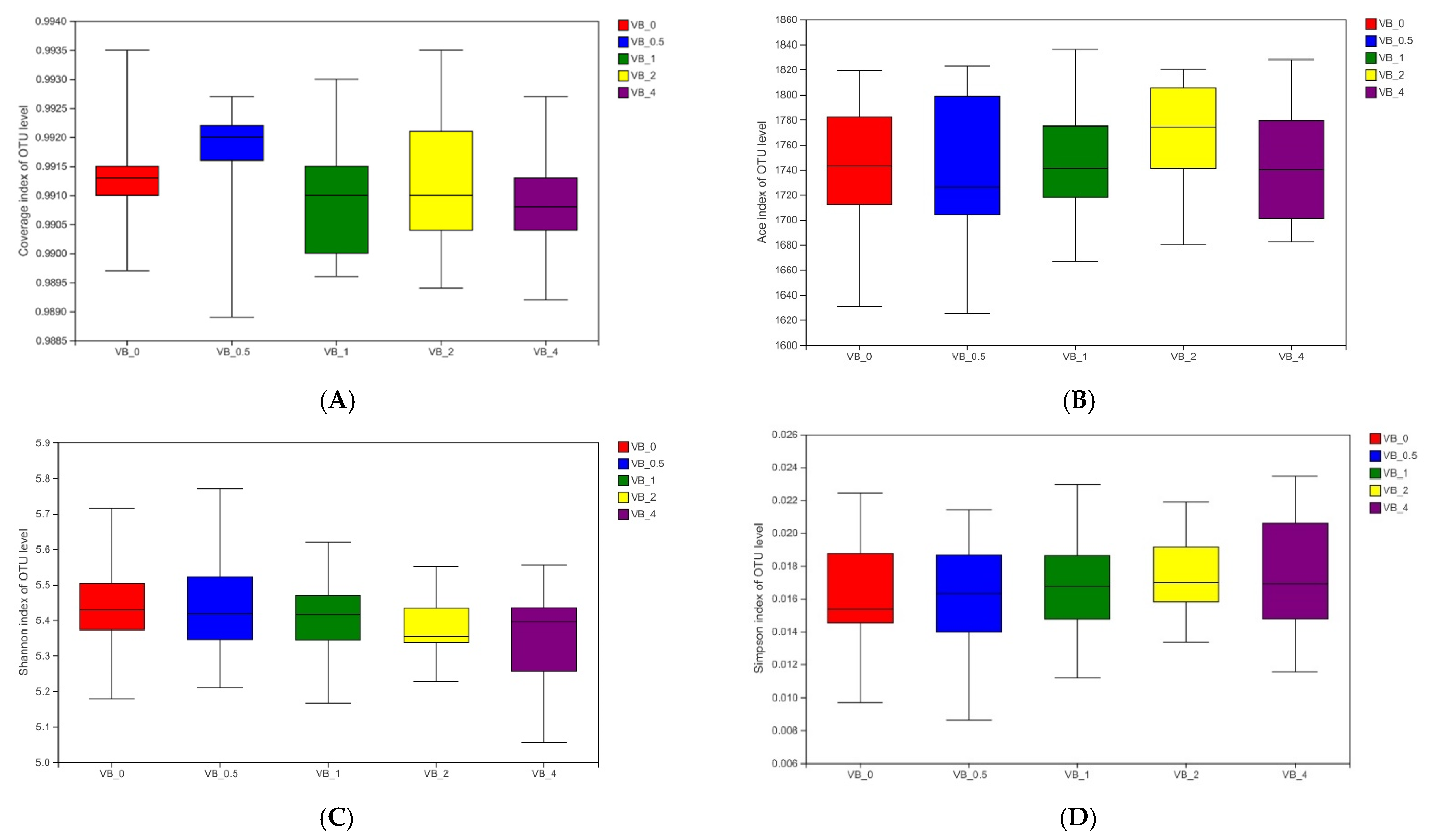
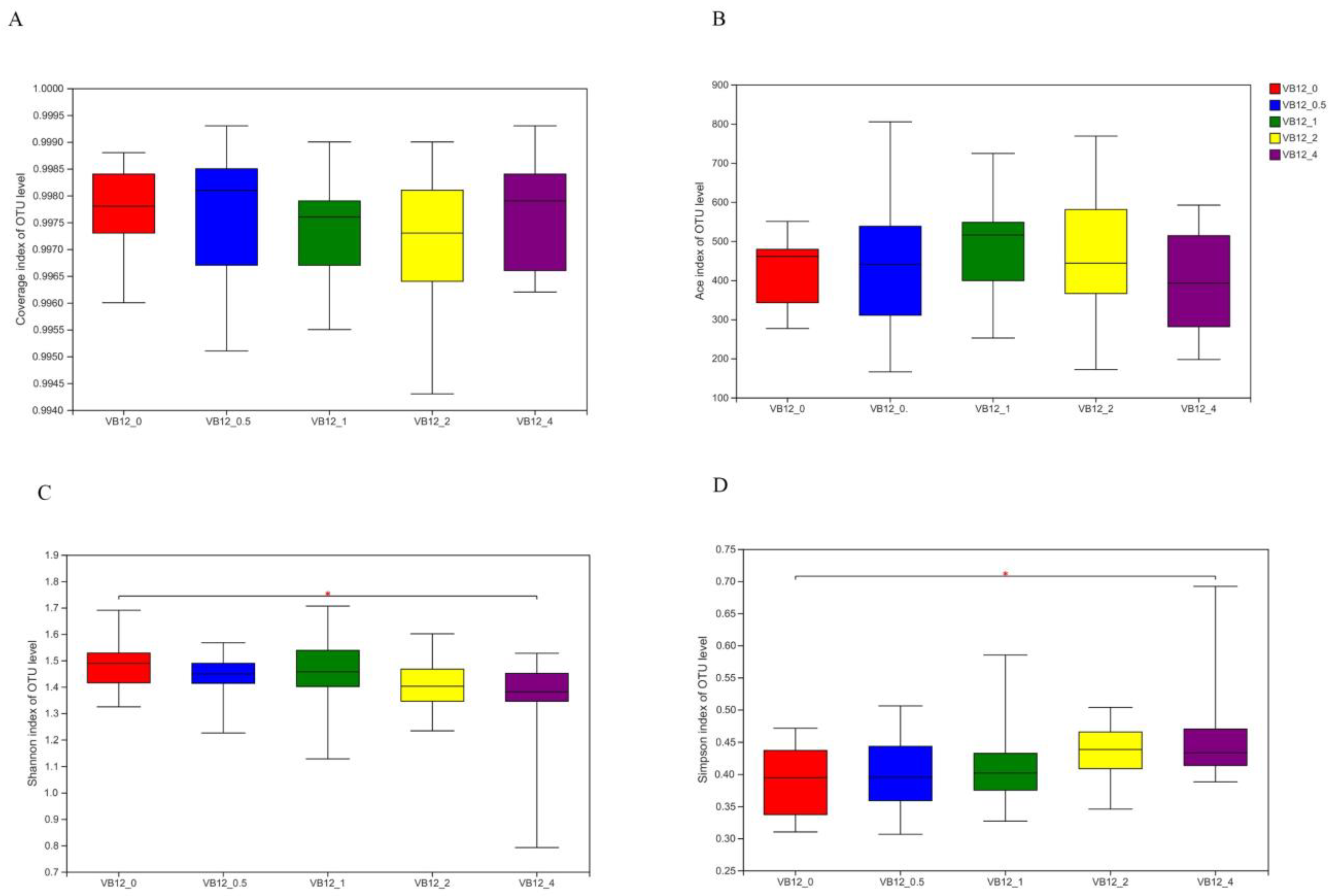
3.2. Microbial Community
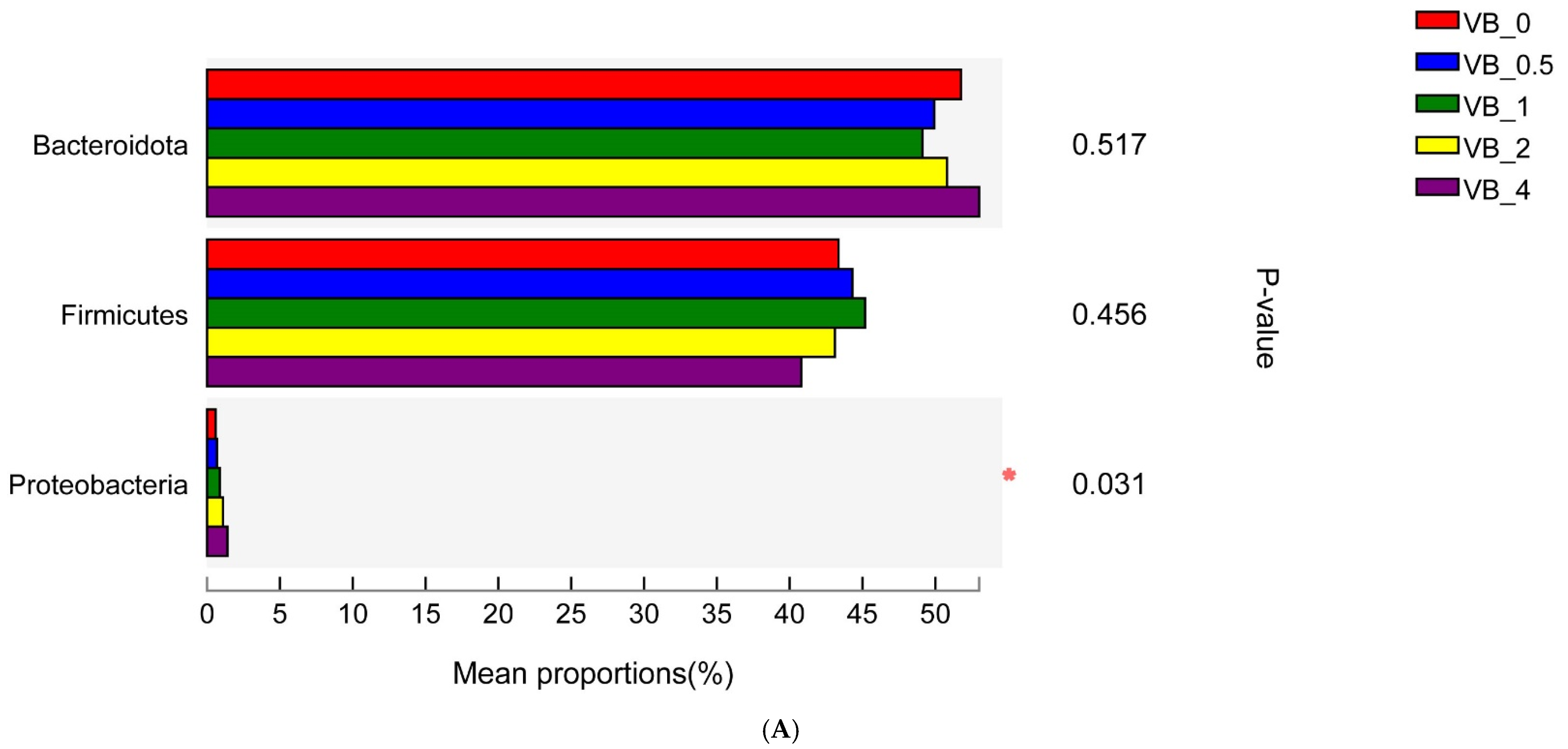
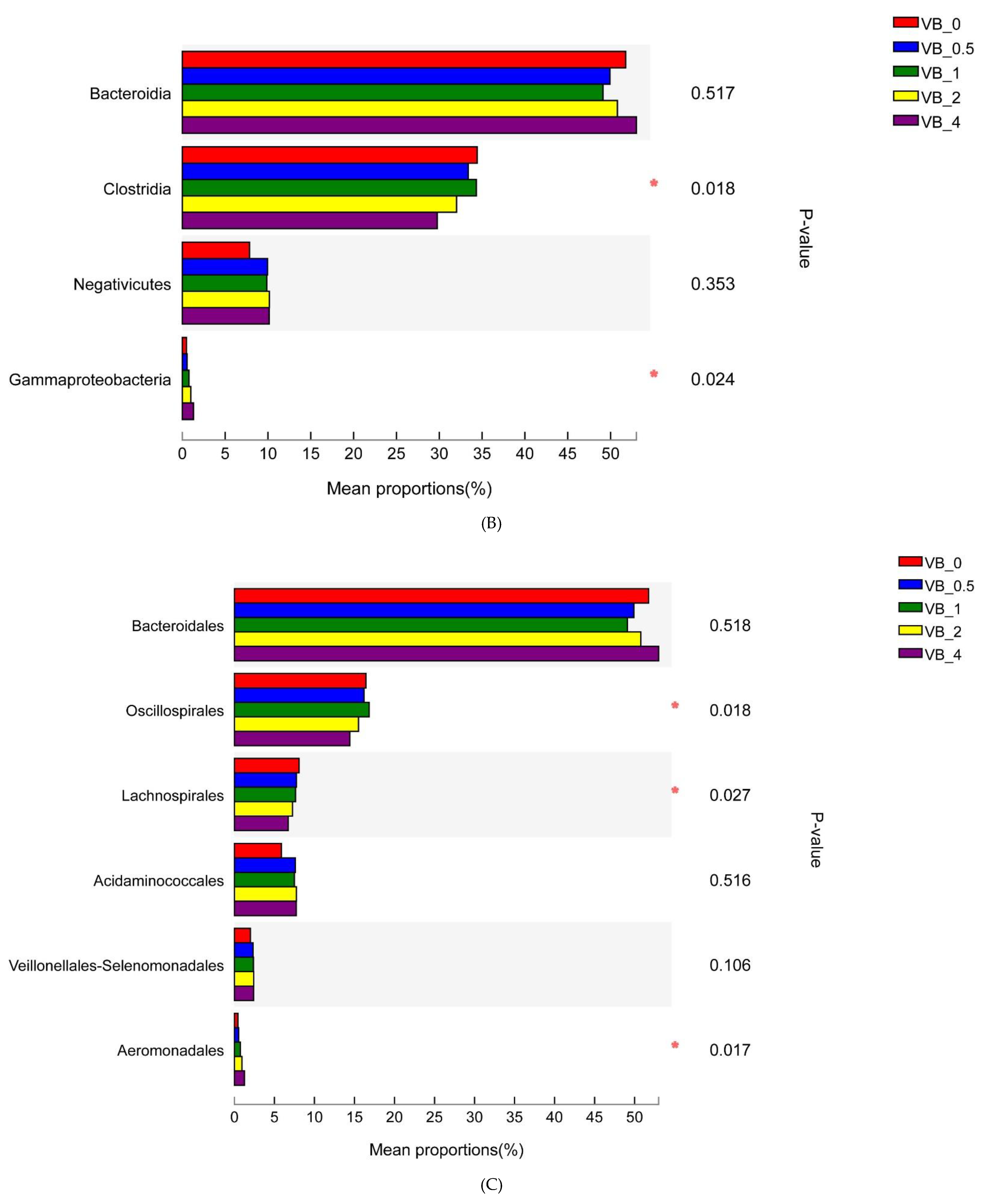
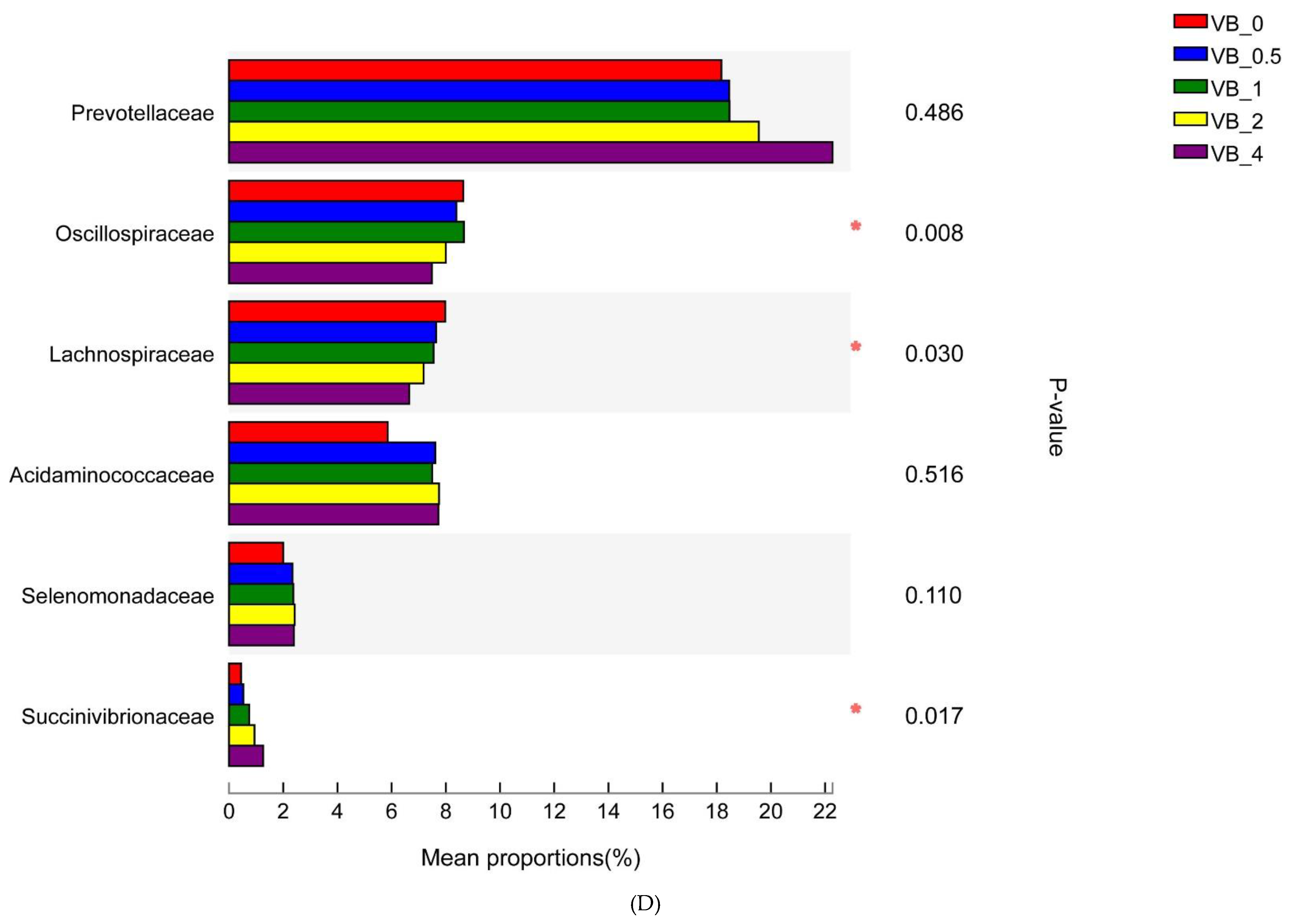
3.2.1. Bacterial Community
Proteobacteria
Firmicutes and Bacteroidetes
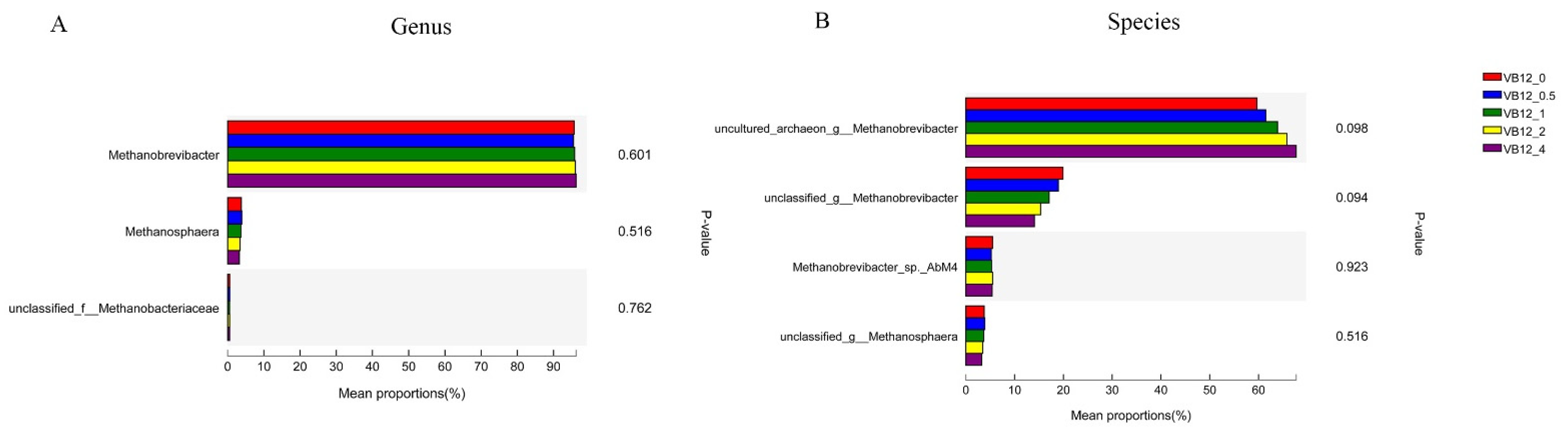
3.2.2. Archaeal Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, J.R.; Lawrence, J.G.; Bobik, T.A. Cobalamin (coenzyme B-12): Synthesis and biological significance. Annu. Rev. Microbiol. 1996, 50, 137–181. [Google Scholar] [CrossRef] [PubMed]
- Sobczynska-Malefora, A.; Delvin, E.; McCaddon, A.; Ahmadi, K.R.; Harrington, D.J. Vitamin B12 status in health and disease: A critical review. Diagnosis of deficiency and insufficiency—Clinical and laboratory pitfalls. Crit. Rev. Clin. Lab. Sci. 2021, 58, 399–429. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Herbert, V. Vitamin B-12: Plant sources, requirements, and assay. Am. J. Clin. Nutr. 1988, 48, 852–858. [Google Scholar] [CrossRef]
- Farquharson, J.; Adams, J. The forms of vitamin B12 in foods. Br. J. Nutr. 1976, 36, 127–136. [Google Scholar] [CrossRef]
- Scott, K.J.; Bishop, D.R.; Zechalko, A.; Edwards-Webb, J.D. Nutrient content of liquid milk: II. Content of vitamin C, riboflavin, folic acid, thiamin, vitamins B12 and B6 in pasteurized milk as delivered to the home and after storage in the domestic refrigerator. J. Dairy Res. 1984, 51, 51–57. [Google Scholar] [CrossRef]
- Sun, L.L.; Liu, G.T.; Xu, D.M.; Wu, Z.H.; Ma, L.; Victoria, S.F.M.; Baumgard, L.H.; Bu, D.P. Milk selenium content and speciation in response to supranutritional selenium yeast supplementation in cows. Anim. Nutr. 2021, 7, 1087–1094. [Google Scholar] [CrossRef]
- Bu, D.P.; Wang, J.Q.; Dhiman, T.R.; Liu, S.J. Effectiveness of oils rich in linoleic and linolenic acids to enhance conjugated linoleic acid in milk from dairy cows. J. Dairy Sci. 2007, 90, 998–1007. [Google Scholar] [CrossRef]
- Duplessis, M.; Gervais, R.; Lapierre, H.; Girard, C. Combined biotin, folic acid, and vitamin B12 supplementation given during the transition period to dairy cows: Part II. Effects on energy balance and fatty acid composition of colostrum and milk. J. Dairy Sci. 2022, 105, 7097–7110. [Google Scholar] [CrossRef] [PubMed]
- Molano, R.A.; Girard, C.L.; Van Amburgh, M.E. Effect of dietary supplementation of 2 forms of a B vitamin and choline blend on the performance of Holstein calves during the transition and postweaning phase. J. Dairy Sci. 2021, 104, 10812–10827. [Google Scholar] [CrossRef]
- Preynat, A.; Lapierre, H.; Thivierge, M.C.; Palin, M.F.; Matte, J.J.; Desrochers, A.; Girard, C.L. Influence of methionine supply on the response of lactational performance of dairy cows to supplementary folic acid and vitamin B12. J. Dairy Sci. 2009, 92, 1685–1695. [Google Scholar] [CrossRef]
- Wang, K.; Nan, X.; Tong, J.; Zhao, G.; Jiang, L.; Xiong, B. Steam Explosion Pretreatment Changes Ruminal Fermentation in vitro of Corn Stover by Shifting Archaeal and Bacterial Community Structure. Front. Microbiol. 2020, 11, 2027. [Google Scholar] [CrossRef]
- Wang, K.; Nan, X.; Chu, K.; Tong, J.; Yang, L.; Zheng, S.; Zhao, G.; Jiang, L.; Xiong, B. Shifts of Hydrogen Metabolism From Methanogenesis to Propionate Production in Response to Replacement of Forage Fiber With Non-forage Fiber Sources in Diets in vitro. Front. Microbiol. 2018, 9, 2764. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhao, S.; Zheng, N.; Bu, D.; Beckers, Y.; Denman, S.E.; McSweeney, C.S.; Wang, J. Differences in Ureolytic Bacterial Composition between the Rumen Digesta and Rumen Wall Based on ureC Gene Classification. Front. Microbiol. 2017, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Wolin, M.; Miller, T.; Stewart, C. Microbe-microbe interactions. In The Rumen Microbial Ecosystem; Springer: Berlin, Germany, 1997; pp. 467–491. [Google Scholar]
- Shelton, A.N.; Seth, E.C.; Mok, K.C.; Han, A.W.; Jackson, S.N.; Haft, D.R.; Taga, M.E. Uneven distribution of cobamide biosynthesis and dependence in bacteria predicted by comparative genomics. ISME J. 2019, 13, 789–804. [Google Scholar] [CrossRef]
- Santschi, D.; Chiquette, J.; Berthiaume, R.; Martineau, R.; Matte, J.; Mustafa, A.; Girard, C. Effects of the forage to concentrate ratio on B-vitamin concentrations in different ruminal fractions of dairy cows. Can. J. Anim. Sci. 2005, 85, 389–399. [Google Scholar] [CrossRef]
- Franco-Lopez, J.; Duplessis, M.; Bui, A.; Reymond, C.; Poisson, W.; Blais, L.; Chong, J.; Gervais, R.; Rico, D.E.; Cue, R.I.; et al. Correlations between the Composition of the Bovine Microbiota and Vitamin B12 Abundance. mSystems 2020, 5, e00107-20. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: A meta-analysis. Front. Microbiol. 2015, 6, 37. [Google Scholar] [CrossRef]
- Belanche, A.; Pinloche, E.; Preskett, D.; Newbold, C.J. Effects and mode of action of chitosan and ivy fruit saponins on the microbiome, fermentation and methanogenesis in the rumen simulation technique. FEMS Microbiol. Ecol. 2016, 92, fiv160. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Wu, J.; Seyedsalehi, M.; Hu, Y.Y.; Tian, G. Novel insights of impacts of solid content on high solid anaerobic digestion of cow manure: Kinetics and microbial community dynamics. Bioresour. Technol. 2021, 333, 125205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Azman, S.; Khadem, A.F.; Van Lier, J.B.; Zeeman, G.; Plugge, C.M. Presence and role of anaerobic hydrolytic microbes in conversion of lignocellulosic biomass for biogas production. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2523–2564. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, J.; Li, H.; Poncin, S.; Wang, K.; Zuo, J. Novel insight into high solid anaerobic digestion of swine manure after thermal treatment: Kinetics and microbial community properties. J. Environ. Manag. 2019, 235, 169–177. [Google Scholar] [CrossRef]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A Structural and Functional Elucidation of the Rumen Microbiome Influenced by Various Diets and Microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef]
- Snelling, T.J.; Auffret, M.D.; Duthie, C.A.; Stewart, R.D.; Watson, M.; Dewhurst, R.J.; Roehe, R.; Walker, A.W. Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Anim. Microb. 2019, 1, 16. [Google Scholar] [CrossRef]
- Dieho, K.; van den Bogert, B.; Henderson, G.; Bannink, A.; Ramiro-Garcia, J.; Smidt, H.; Dijkstra, J. Changes in rumen microbiota composition and in situ degradation kinetics during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 2017, 100, 2695–2710. [Google Scholar] [CrossRef]
- Wallace, R.J.; Rooke, J.A.; McKain, N.; Duthie, C.A.; Hyslop, J.J.; Ross, D.W.; Waterhouse, A.; Watson, M.; Roehe, R. The rumen microbial metagenome associated with high methane production in cattle. BMC Genom. 2015, 16, 839. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.S.; Cormican, P.; Keogh, K.; O’Connor, A.; O’Hara, E.; Palladino, R.A.; Kenny, D.A.; Waters, S.M. Illumina MiSeq phylogenetic amplicon sequencing shows a large reduction of an uncharacterised Succinivibrionaceae and an increase of the Methanobrevibacter gottschalkii clade in feed restricted cattle. PLoS ONE 2015, 10, e0133234. [Google Scholar] [CrossRef] [PubMed]
- Hespell, R.B. The Genera Succinivibrio and Succinimonas. In The Prokaryotes; Springer: Berlin, Germany, 1992; pp. 3979–3982. [Google Scholar]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Emerson, E.L.; Weimer, P.J. Fermentation of model hemicelluloses by Prevotella strains and Butyrivibrio fibrisolvens in pure culture and in ruminal enrichment cultures. Appl. Microbiol. Biotechnol. 2017, 101, 4269–4278. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Kittelmann, S.; Pinares-Patino, C.S.; Seedorf, H.; Kirk, M.R.; Ganesh, S.; McEwan, J.C.; Janssen, P.H. Two different bacterial community types are linked with the low-methane emission trait in sheep. PLoS ONE 2014, 9, e103171. [Google Scholar] [CrossRef]
- Danielsson, R.; Dicksved, J.; Sun, L.; Gonda, H.; Muller, B.; Schnurer, A.; Bertilsson, J. Methane Production in Dairy Cows Correlates with Rumen Methanogenic and Bacterial Community Structure. Front. Microbiol. 2017, 8, 226. [Google Scholar] [CrossRef]
- Van Gylswyk, N. Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Evol. Microbiol. 1995, 45, 297–300. [Google Scholar] [CrossRef]
- Paynter, M.; Elsden, S. Mechanism of propionate formation by Selenomonas ruminantium, a rumen micro-organism. Microbiology 1970, 61, 1–7. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Koch, A.-M.; Lambert, W.; Michiels, J.; Chalvon-Demersay, T. The Effect of Amino Acids on Production of SCFA and bCFA by Members of the Porcine Colonic Microbiota. Microorganisms 2022, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, gen. nov., sp. nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Veneman, J.B.; Muetzel, S.; Hart, K.J.; Faulkner, C.L.; Moorby, J.M.; Perdok, H.B.; Newbold, C.J. Does Dietary Mitigation of Enteric Methane Production Affect Rumen Function and Animal Productivity in Dairy Cows? PLoS ONE 2015, 10, e0140282. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; Rooke, J.A.; Duthie, C.A.; Hyslop, J.J.; Ross, D.W.; McKain, N.; de Souza, S.M.; Snelling, T.J.; Waterhouse, A.; Roehe, R. Archaeal abundance in post-mortem ruminal digesta may help predict methane emissions from beef cattle. Sci. Rep. 2014, 4, 5892. [Google Scholar] [CrossRef]
- Shi, W.; Moon, C.D.; Leahy, S.C.; Kang, D.; Froula, J.; Kittelmann, S.; Fan, C.; Deutsch, S.; Gagic, D.; Seedorf, H.; et al. Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome. Genome Res. 2014, 24, 1517–1525. [Google Scholar] [CrossRef]
- Freitag, T.E.; Prosser, J.I. Correlation of methane production and functional gene transcriptional activity in a peat soil. Appl. Environ. Microbiol. 2009, 75, 6679–6687. [Google Scholar] [CrossRef]
- Wang, K.; Nan, X.M.; Zhao, Y.G.; Tong, J.J.; Jiang, L.S.; Xiong, B.H. Effects of propylene glycol on in vitro ruminal fermentation, methanogenesis, and microbial community structure. J. Dairy Sci. 2021, 104, 2924–2934. [Google Scholar] [CrossRef]

| Items | VB12 (mg/g DM) 1 | SEM 4 | p Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 4 | Trt | L | Q | ||
| pH | 6.57 b | 6.58 ab | 6.59 a | 6.60 a | 6.60 a | 0.004 | 0.048 | 0.008 | 0.336 |
| DMD 3 | 0.726 | 0.712 | 0.711 | 0.714 | 0.713 | 0.003 | 0.441 | 0.233 | 0.224 |
| Gas production (mL) | 147.1 | 144.8 | 142.0 | 141.7 | 141.3 | 0.998 | 0.292 | 0.041 | 0.447 |
| CH4 (mL) | 9.08 a | 8.77 ab | 8.20 b | 8.22 b | 8.13 b | 0.131 | 0.045 | 0.008 | 0.329 |
| Lactate, μg/mL | 2.38 a | 1.55 b | 1.52 b | 1.52 b | 1.51 b | 0.096 | 0.009 | 0.006 | 0.268 |
| Oxaloacetate, μg/mL | 139.9 | 136.23 | 136.32 | 134.74 | 133.97 | 1.479 | 0.761 | 0.216 | 0.736 |
| Fumarate, μg/mL | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.002 | 0.607 | 0.457 | 0.467 |
| Succinate, μg/mL | 0.16 a | 0.12 ab | 0.12 ab | 0.11 ab | 0.10 b | 0.007 | 0.046 | 0.011 | 0.422 |
| Concentrations, mM | |||||||||
| Total volatile fatty acids | 106.48 a | 104.62 ab | 104.22 b | 103.55 b | 103.45 b | 0.421 | 0.049 | 0.017 | 0.348 |
| Acetate | 66.58 | 65.54 | 65.32 | 64.89 | 64.88 | 0.250 | 0.184 | 0.023 | 0.373 |
| Propionate | 21.54 | 21.56 | 21.73 | 21.72 | 21.73 | 0.043 | 0.407 | 0.084 | 0.564 |
| Isobutyrate | 1.06 | 1.03 | 1.03 | 1.03 | 1.03 | 0.006 | 0.598 | 0.177 | 0.420 |
| Butyrate | 13.45 a | 12.72 ab | 12.41 b | 12.21 b | 12.12 b | 0.135 | 0.009 | <0.001 | 0.188 |
| Isovalerate | 1.94 | 1.88 | 1.86 | 1.85 | 1.85 | 0.017 | 0.394 | 0.074 | 0.386 |
| Valerate | 1.92 | 1.88 | 1.87 | 1.85 | 1.85 | 0.011 | 0.220 | 0.027 | 0.430 |
| Acetate/Propionate | 3.09 a | 3.04 ab | 3.01 b | 2.99 b | 2.99 b | 0.009 | 0.008 | <0.001 | 0.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Liu, Z.; Du, C.; Xiong, B.; Yang, L. Responses of Fermentation Characteristics and Microbial Communities to Vitamin B12 Supplementation in In Vitro Ruminal Cultures. Fermentation 2022, 8, 406. https://doi.org/10.3390/fermentation8080406
Wang K, Liu Z, Du C, Xiong B, Yang L. Responses of Fermentation Characteristics and Microbial Communities to Vitamin B12 Supplementation in In Vitro Ruminal Cultures. Fermentation. 2022; 8(8):406. https://doi.org/10.3390/fermentation8080406
Chicago/Turabian StyleWang, Kun, Zihao Liu, Chunmei Du, Benhai Xiong, and Liang Yang. 2022. "Responses of Fermentation Characteristics and Microbial Communities to Vitamin B12 Supplementation in In Vitro Ruminal Cultures" Fermentation 8, no. 8: 406. https://doi.org/10.3390/fermentation8080406






