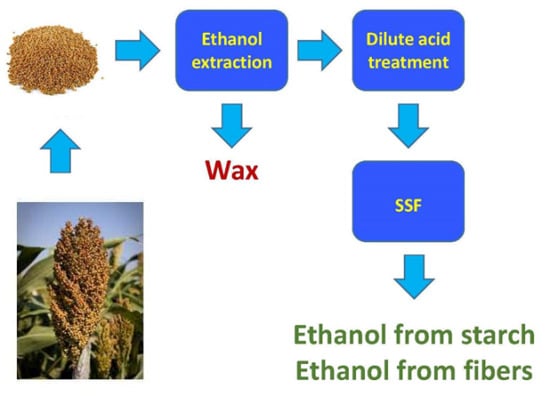
Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sorghum Grains
2.1.2. Enzymes and Chemicals
2.2. Methods
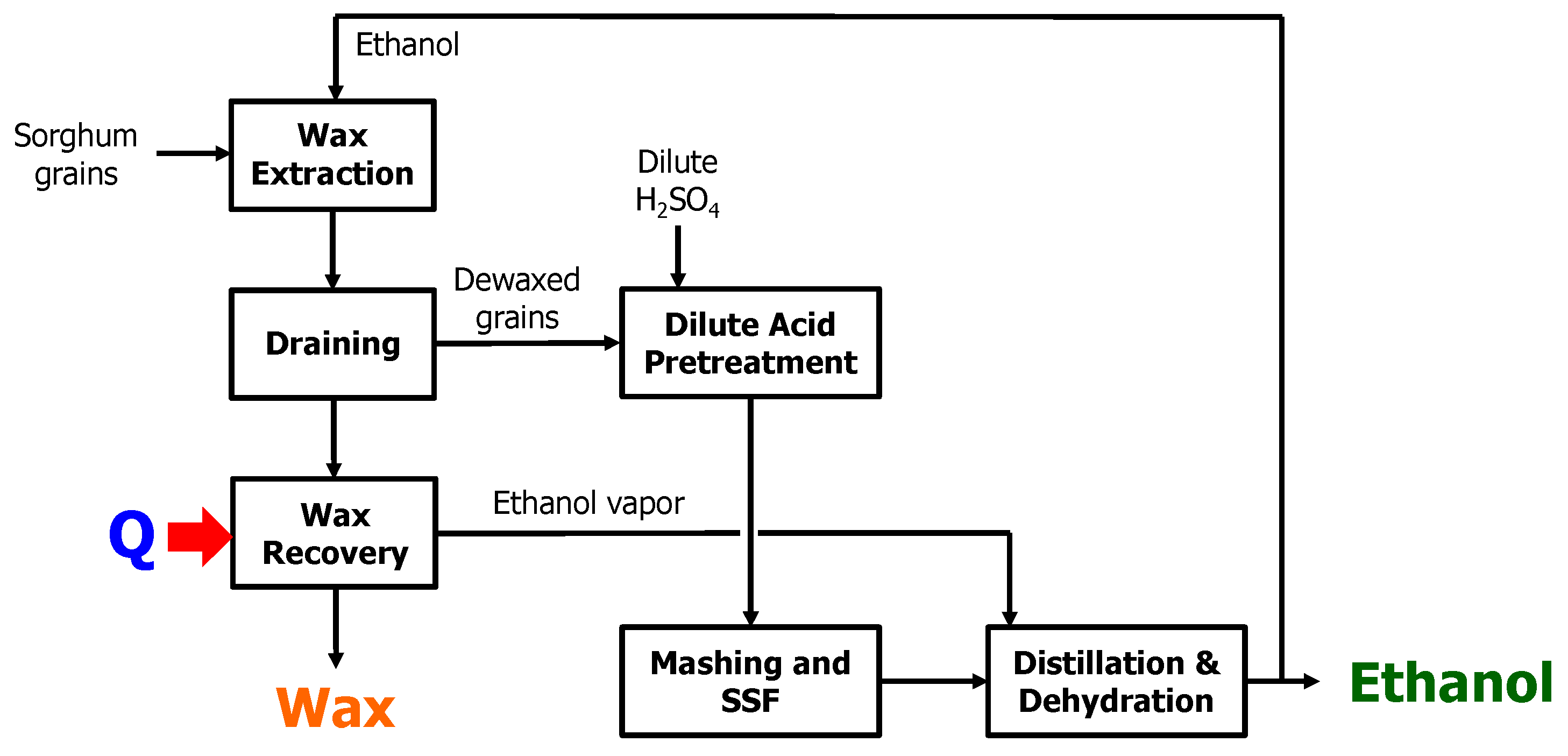
2.2.1. Wax Extraction
2.2.2. Dilute H2SO4 Treatment
2.2.3. Ethanol Fermentation of Raw and Dewaxed Sorghums
Mashing
Simultaneous Saccharification and Fermentation
Viscosity Reduction
2.2.4. Ethanol Fermentation of Dewaxed and H2SO4-Treated Sorghums
2.3. Analytical Methods.
2.3.1. Moisture Determination
2.3.2. Wax Characterization
2.3.3. Starch Determination
2.3.4. Compositional Analysis of BRM Sorghum Fiber
2.3.5. Analysis of Fermentation Samples
3. Results and Discussion
3.1. Wax Extraction and Characterization
3.2. Starch Contents
3.3. SSF of Raw and Dewaxed Sorghum Grains
3.4. SSF of Dewaxed and H2SO4-Treated BRM Sorghum
3.5. The Proposed Integrated Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Calculations of Ethanol Yields
- Basis: 1000 g of 25 wt % raw BRM sorghum mash.
- Total starch available: 250 g × 0.701 = 175.25 g.
- Water consumed by hydrolysis of starch: 175.25 g × 0.11 = 19.28 g or 19.28 mL since the density of water is 1.
- Let VF be the final liquid volume in mL. Since the final ethanol concentration was 86.1 g/L, the additional volume (in mL) contributed by the ethanol produced was
- 0.0861 g/mL × VF (mL) ÷ 0.789 g/mL = 0.109 VF
- Mass balance will give
- VF = 750 mL − 19.28 mL + 0.109 VF
- Therefore, VF = 820.2 mL.
- Total ethanol production: 86.1 g/L × 0.820 L = 70.62 g.
- Ethanol yield: 70.62 g ÷ 250 g = 0.283 g ethanol/g sorghum.
- Assume 1 bu of sorghum weighs 56 lb and has moisture content of 15 wt%. The mass of 1 bu of BRM sorghum in g is 56 lb × 0.85 × 454 g/lb = 21610 g.
- Ethanol yield per bu of sorghum is 0.283 g/g × 21610 g/bu = 6104 g ethanol/bu or 2.04 gal/bu.
- Similar calculations are performed for dewaxed and dewaxed/H2SO4-treated BRM sorghum. The ethanol yields are 2.59 gal/bu and 2.92 gal/bu, respectively.
Appendix A.2. Calculations of Potential Economic Benefits
- Design basis: A plant to produce 50 million gallons ethanol per year.
- Annual feedstock requirement for raw sorghum: 50 × 106 gal ÷ 2.04 gal/bu = 24.5 × 106 bu.
- Similarly, for dewaxed sorghum and dewaxed/H2SO4-treated sorghum, the annual feedstock requirements are 19.3 × 106 bu and 17.1 × 106 bu.
- For the dewaxed sorghum, the amount of wax that can be extracted is 0.003 × 21.6 kg/bu = 0.065 kg/bu.
- The total quantity of wax that can be extracted is 0.065 kg/bu × 19.3 × 106 bu = 1.251 × 106 kg or 1251 MT.
- The value of the extracted wax is 1.251 × 106 kg × $6/kg = $7.5 × 106.
- The saving on feedstock is (24.5 × 106 bu − 19.3 × 106 bu) × $3.10/bu = $16.1 × 106.
- Similar calculations are performed for the dewaxed/H2SO4-treated sorghum and the calculated results are shown in Table 7.
References
- Renewable Fuels Association. Ethanol Industry Outlook. 2017. Available online: http://www.ethanolrfa.org/wp-content/uploads/2017/02/Ethanol-Industry-Outlook-2017.pdf (accessed on 30 November 2017).
- USDA. Feed Outlook Reports. Available online: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1273 (accessed on 30 November 2017).
- Nhuan, P.N.; Montanti, J.; Johnston, D.B. Sorghum as a renewable feedstock for production of fuels and industrial chemicals. Bioengineering 2016, 3, 75–91. [Google Scholar]
- Hwang, K.T.; Cuppett, S.L.; Weller, C.L.; Hanna, H.A. Properties, composition and analysis of grain sorghum wax. J. Am. Oil Chem. Soc. 2002, 79, 521–527. [Google Scholar] [CrossRef]
- Hwang, K.T.; Cuppert, S.L.; Weller, C.L.; Hanna, M.A. HPLC of grain sorghum wax classes highlighting separation of aldehydes from wax esters and steryl esters. J. Sep. Sci. 2002, 25, 619–623. [Google Scholar] [CrossRef]
- Harron, A.F.; Powell, M.J.; Nunez, A.; Moreau, R.A. Analysis of sorghum wax and carnauba wax by reversed phase liquid chromatography mass spectrometry. Ind. Crops Prod. 2017, 98, 116–129. [Google Scholar] [CrossRef]
- Steinle, J.V. CARNAUBA WAX an expedition to its source. Ind. Eng. Chem. 1936, 28, 1004–1008. [Google Scholar] [CrossRef]
- Carnauba Wax. Available online: https://www.alibaba.com/product-detail/Carnauba-Wax_60433586534.html (accessed on 12 February 2018).
- Carnauba Wax Market Analysis by Product. Available online: http://www.grandviewresearch.com/industry-analysis/carnauba-wax-market (accessed on 12 February 2018).
- Carnauba Wax Market Size Projected to Reach $334.9 Million by 2024. Available online: https://www.grandviewresearch.com/press-release/global-carnauba-wax-market (accessed on 12 February 2018).
- Cai, D.; Chang, Z.; Wang, C.; Ren, W.; Wang, Z.; Qin, P.; Tan, T. Impact of sweet sorghum cuticular waxes (SSCW) on acetone-butanol-ethanol fermentation using Clostridium acetobutylicum ABE 1201. Bioresour. Technol. 2013, 149, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.S.; Blessin, C.W. Composition and structure of sorghum grains. Cereal Sci. Today 1969, 14, 264–271. [Google Scholar]
- Drapcho, C.M.; Nghiem, N.P.; Walker, T.H. Biofuels Engineering Process Technology; McGraw-Hill: New York, NY, USA, 2008; pp. 134–143. [Google Scholar]
- Total Starch Assay Procedure, Megazyme. Available online: https://secure.megazyme.com/files/Booklet/K-TSTA_DATA.pdf (accessed on 12 February 2018).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Hagerman, A.E.; Butler, L.G. Condensed tannin purification and characterization of tannin-associated proteins. J. Agric. Food Chem. 1980, 28, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.G.; Riedl, D.J.; Lebryk, D.G.; Blytt, H.J. Interaction of proteins with sorghum tannin: Mechanism, specificity and significance. J. Am. Oil Chem. Soc. 1980, 61, 916–920. [Google Scholar] [CrossRef]
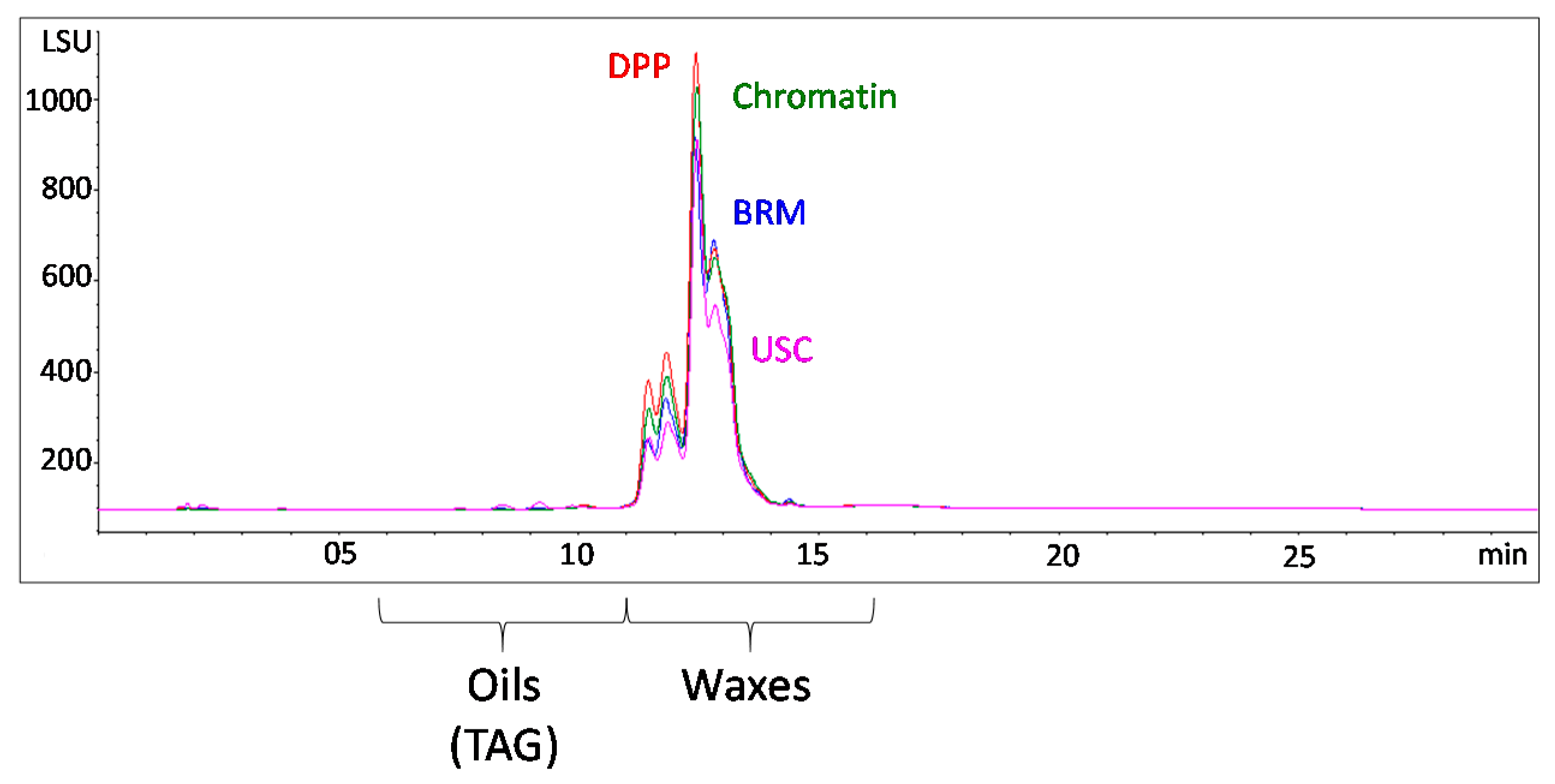
| Source | Description |
|---|---|
| Bob’s Red Natural Foods (BRM) (Milwaukee, OR, USA) | Commercial product; unknown variety |
| United Sorghum Checkoff (USC) (Lubbock, TX, USA) | Blend of two varieties, Sorghum Partner SP6929 and Terral RV9782; the majority is SP6929 but the exact proportion is unknown |
| DuPont Pioneer (DPP) (Johnston, IA, USA) | Pioneer 83P56 |
| Chromatin (Chicago, IL, USA) | SP7715 |
| Sorghum Type | Initial Dry Weight (g) | Wax Extracted (% of Initial Weight) |
|---|---|---|
| BRM | 176.64 | 0.29 |
| USC | 171.86 | 0.19 |
| DPP | 174.26 | 0.17 |
| Chromatin | 174.51 | 0.29 |
| Treatment | Starch (wt % Dry Basis) |
|---|---|
| BRM Raw | 59.7 ± 3.0 |
| BRM Dewaxed | 67.4 ± 2.1 |
| USC Raw | 58.4 ± 7.8 |
| USC Dewaxed | 70.2 ± 3.5 |
| DPP Raw | 59.1 ± 1.8 |
| DPP Dewaxed | 65.3 ± 0.6 |
| Chromatin Raw | 58.7 ± 3.8 |
| Chromatin Dewaxed | 64.7 ± 1.6 |
| Feedstock | Final Ethanol (g/L) | Yield (% Theoretical) |
|---|---|---|
| BRM Raw | 86.1 ± 2.0 | 71.1 |
| BRM Dewaxed | 106.2 ± 0.6 | 90.2 |
| BRM Dewaxed with Accellerase | 107.8 ± 1.9 | 91.8 |
| USC Raw | 102.2 ± 0.9 | 83.9 |
| USC Dewaxed | 107.9 ± 0.5 | 89.3 |
| USC Dewaxed with Accellerase | 108.2 ± 0.9 | 89.6 |
| DPP Raw | 104.1 ± 1.0 | 85.1 |
| DPP Dewaxed | 107.0 ± 3.8 | 87.8 |
| DPP Dewaxed with Accellerase | 109.6 ± 1.0 | 90.3 |
| Chromatin Raw | 98.7 ± 1.5 | 80.8 |
| Chromatin Dewaxed | 98.0 ± 5.4 | 80.2 |
| Chromatin Dewaxed with Accellerase | 99.7 ± 1.0 | 81.7 |
| Component (wt % of Total Mass, Dry Basis) | |||||
|---|---|---|---|---|---|
| Glucan | Xylan | Arabinan | AI Lignin | AS Lignin | Ash |
| 56.9 ± 1.6 | 4.9 ± 0.1 | 3.6 ± 0.0 | 10.7 ± 0.8 | 2.1 ± 0.0 | 0.1 ± 0.0 |
| Experiment | Final Ethanol (g/L) | Yield (% Theoretical) * |
|---|---|---|
| Bob’s Red Mill raw | 86.1 ± 2.0 | 62.7 |
| Bob’s Red Mill dewaxed | 106.2 ± 0.6 | 77.4 |
| Bob’s Red Mill dewaxed and treated with 1 wt % sulfuric acid with CTec2 addition in SSF | 117.8 ± 0.1 | 85.7 |
| Bob’s Red Mill dewaxed and treated with 2 wt % sulfuric acid with CTec2 addition in SSF | 112.5 ± 4.5 | 82.0 |
| Base Case | Dewaxed Only | Dewaxed/H2SO4 Treated | |
|---|---|---|---|
| Ethanol yield (gal/bu) | 2.04 | 2.59 | 2.92 |
| Sorghum feedstock needed (million bu) | 24.5 | 19.3 | 17.1 |
| Total wax co-product (MT) | 0 | 1252 | 1110 |
| Wax co-product value (million $) | 0 | 7.5 | 6.6 |
| Sorghum feedstock savings (million $) | 0 | 16.1 | 22.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nghiem, N.P.; O’Connor, J.P.; Hums, M.E. Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains. Fermentation 2018, 4, 12. https://doi.org/10.3390/fermentation4010012
Nghiem NP, O’Connor JP, Hums ME. Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains. Fermentation. 2018; 4(1):12. https://doi.org/10.3390/fermentation4010012
Chicago/Turabian StyleNghiem, Nhuan P., James P. O’Connor, and Megan E. Hums. 2018. "Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains" Fermentation 4, no. 1: 12. https://doi.org/10.3390/fermentation4010012
APA StyleNghiem, N. P., O’Connor, J. P., & Hums, M. E. (2018). Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains. Fermentation, 4(1), 12. https://doi.org/10.3390/fermentation4010012