A Bacterial Laccase for Enhancing Saccharification and Ethanol Fermentation of Steam-Pretreated Biomass
Abstract
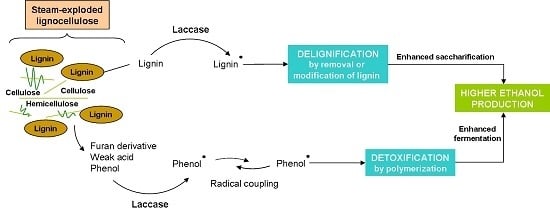
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Steam Explosion Pretreatment
2.2. Enzymes
2.3. Microorganism and Growth Conditions
2.4. Laccase Treatment and Saccharification of the WIS Fraction
2.5. Laccase Treatment and Fermentation of the Whole Slurry
2.6. Analytical Methods
3. Results and Discussion
3.1. Pretreated Biomass Composition
3.2. Laccase Treatment and Saccharification of the WIS Fraction
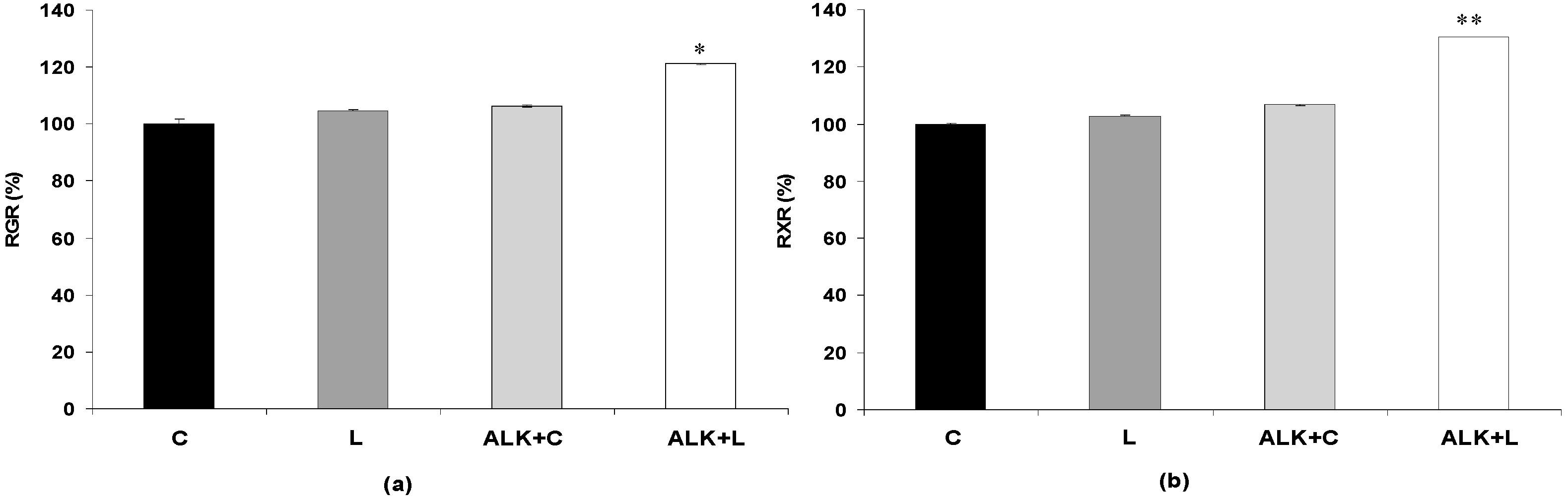
3.2.1. Effect of Bacterial Laccase Treatment on the Chemical Composition of WIS
3.2.2. Effect of Bacterial Laccase Treatment on Saccharification Yields
3.3. Laccase Treatment and Fermentation of the Whole Slurry
3.3.1. Effect of Bacterial Laccase Treatment on Inhibitory Compounds
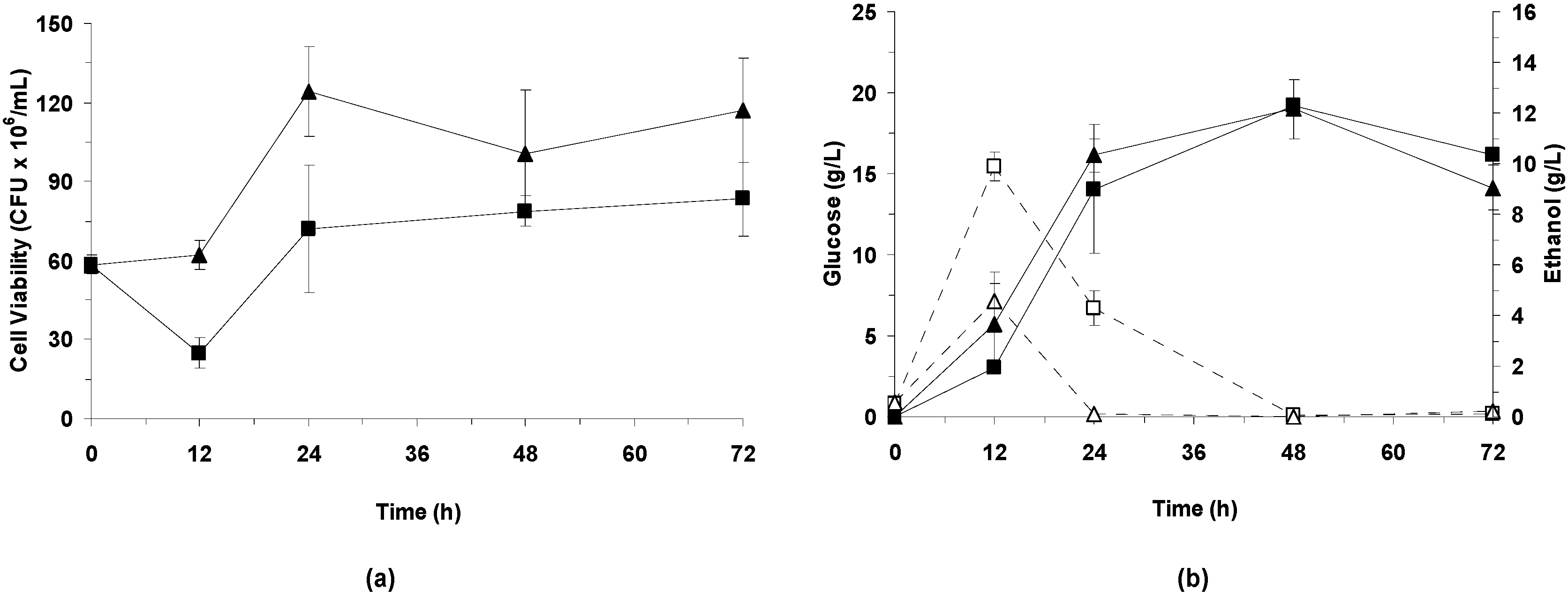
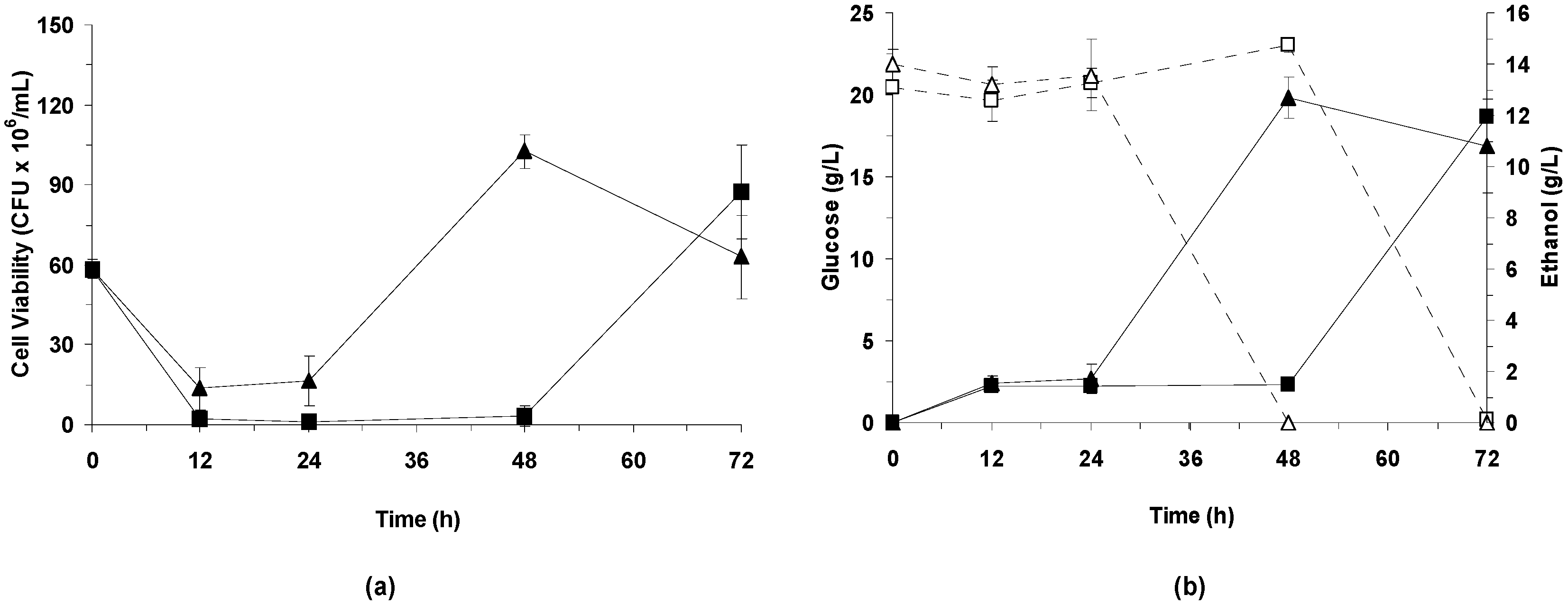
3.3.2. Effect of Bacterial Laccase Treatment on Cell Viability and Ethanol Production
LSSF
LPSSF
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| WIS | Water Insoluble Solids fraction |
| NREL-LAP | National Renewable Energies Laboratory-Laboratory Analytical Procedures |
| SSF | Simultaneous Saccharification and Fermentation |
| CFU | Colony Forming Units |
| DW | Dry Weight |
| LAP | Laboratory Analytical Procedures |
| IU | International Units |
| FPU | Filter Paper Units |
| TS | Total Solids |
| RGR | Relative Glucose Recovery |
| RXR | Relative Xylose Recovery |
| LSSF | consecutive Laccase treatment and Simultaneous Saccharification and Fermentation |
| LPSSF | consecutive Laccase treatment with Presaccharification and Simultaneous Saccharification and Fermentation |
| 5-HMF | 5-hydroxymethylfurfural |
| C | Control treatment |
| CP | Control treatment with Presaccharification |
| L | Laccase treatment |
| LP | Laccase treatment with Presaccharification |
| Alk | Alkaline extraction |
| ATP/ADP | Adenosine-5′-triphosphate/Adenosine-5′-diphosphate |
References
- Ballesteros, M. Enzymatic hydrolysis of lignocellulosic biomass. In Bioalcohol Production. Biochemical Conversion of Lignocellulosic Biomass; Waldron, K., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 159–177. [Google Scholar]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Negro, M.; Oliva, J.M.; Manzanares, P.; Ballesteros, M. Ethanol production from steam-explosion pretreated wheat straw. Appl. Biochem. Biotechnol. 2006, 130, 496–508. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V.; Kubo, S. Inhibition of cellulase, xylanase and β-glucosidase activities by sofwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanism of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Enguita, F.J.; Matias, P.M.; Martins, L.O.; Placido, D.; Henriques, A.O.; Carrondo, M.A. Spore-coat laccase CotA from Bacillus subtilis: Crystallization and preliminary X-ray characterization by the MAD method. Acta Crystallogr. D Biol. Cristallogr. 2002, 58, 1490–1493. [Google Scholar] [CrossRef]
- Bajpai, P. Biological bleaching of chemical pulps. Crit. Rev. Biotechnol. 2004, 24, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Paice, M.G.; Bourbonnais, R.; Reid, I.D.; Archibald, F.S.; Jurasek, L. Oxidative bleaching enzymes: A review. J. Pulp Pap. Sci. 1995, 21, 280–284. [Google Scholar]
- Ibarra, D.; Camarero, S.; Romero, J.; Martínez, M.J.; Martínez, A.T. Integrating laccase-mediator treatment into an industrial-type sequence for totally chlorine free bleaching of eucalipt kraft pulp. J. Chem. Technol. Biotechnol. 2006, 81, 1159–1165. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Hernández, M.; Moya, R.; Martín-Sampedro, R.; Villar, J.C.; Arias, M.E. Evaluation of a new laccase produced by Streptomyces ipomoea on biobleaching and ageing of kraft pulps. BioResources 2011, 6, 3231–3241. [Google Scholar]
- Chandra, R.; Chowdhary, P. Properties of bacterial laccases and their application in biorremediation of industrial wastes. Environ. Sci. Process. Impacts 2015, 17, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Milstein, O.; Haars, A.; Majcherczyk, A.; Tautz, D.; Zanker, H.; Hüttermann, A. Removal of chlorophenols and chlorolignins from bleaching effluents by combined chemical and biological treatment. Water Sci. Technol. 1988, 20, 161–170. [Google Scholar]
- Jönsson, L.J.; Palmqvist, E.; Nilvebrant, N.-O.; Hahn-Hägerdal, B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1998, 49, 691–697. [Google Scholar] [CrossRef]
- Jurado, M.; Prieto, A.; Martínez-Alcalá, A.; Martínez, A.T.; Martínez, M.J. Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour. Technol. 2012, 100, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, D.; Dhiman, S.S.; Kim, H.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochem. 2012, 47, 671–678. [Google Scholar] [CrossRef]
- Li, J.; Sun, F.; Li, X.; Yan, Z.; Yuan, Y.; Liu, X.F. Enhanced saccharification of corn straw pretreated by alkali combining crude ligninolytic enzymes. J. Chem. Technol. Biotecnhol. 2012, 87, 1687–1693. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; García, J.C.; López, F.; Villar, J.C.; Díaz, M.J. Steam explosion and enzymatic pre-treatments as an approach to improve the enzymatic hydrolysis of Eucalytpus globulus. Biomass Bioenergy 2012, 42, 97–106. [Google Scholar] [CrossRef]
- Moilanen, U.; Kellock, M.; Galkin, S.; Viikari, L. The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzym. Microb. Technol. 2011, 49, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Fernández, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J. Chem. Technol. Biotehcnol. 2015. [Google Scholar] [CrossRef]
- Palonen, H.; Viikari, L. Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol. Bioeng. 2004, 86, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Jiang, S.; Zheng, Z.; Shuizhong, L.; Luo, S.; Pan, L. Effect of alkali and laccase pretreatment of Brassica campestris straw: Architecture, crystallisation, and saccharification. Polym. Renew. Resour. 2011, 2, 21–34. [Google Scholar]
- National Renewable Energy Laboratory. Chemical Analysis and Testing Laboratory Analytical Procedures; National Renewable Energy Laboratory: Golden, CO, USA, 2007. Available online: www.1.ere.energy.gov/biomass/analytical_procedures.html (accessed on 15 March 2015).
- Ghose, T.K. Measurement of cellulase activity. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Ballesteros, I.; Ballesteros, M.; Cabañas, A.; Carrasco, J.; Martín, C.; Negro, M.J.; Saez, F.; Saez, R. Selection of thermotolerant yeasts for simultaneous saccharification and fermentation (SSF) of cellulose to ethanol. Appl. Biochem. Biotechnol. 1991, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolic with phosphomolibdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Oliva, J.M.; Saez, F.; Ballesteros, I.; González, A.; Negro, M.J.; Manzanares, P.; Ballesteros, M. Effect of lignocellulosic degradation compounds from steam explosion pretreatment on ethanol fermentation by thermotolerant yeast Kluyveromyces marxianus. Appl. Biochem. Biotechnol. 2003, 105, 141–154. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Ballesteros, I.; Fernández, J.L.; Ballesteros, M. Ethanol from laccase-detoxified lignocellulose by the thermotolerant yeast Kluyveromyces marxianus—Effects of steam pretreatment conditions, process configurations and substrate loadings. Biochem. Eng. J. 2013, 79, 94–103. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Capanema, E.A.; Hoeger, I.; Villar, J.C.; Rojas, O.J. Lignin changes after steam explosion and laccase-mediator treatment of eucalytpus wood chips. J. Agric. Food Chem. 2011, 59, 8761–8769. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Taravilla, A.; Moreno, A.D.; Demuez, M.; Ibarra, D.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour. Technol. 2015, 175, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.C.; Mendes, J.A.S.; Gravitis, J.; Evtuguin, D.V.; Xavier, A.M.R.B. Enzymatic saccharification and bioethanol production from Cynara cardunculus pretreated by steam explosion. Bioresour. Technol. 2015, 186, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Boussaid, A.; Mansfield, S.D.; Gregg, D.J.; Sadler, J.N. Fast and efficient alkaline peroxide treatment to enhance the enzymatic digestibility of steam-exploded softwood substrates. Biotechnol. Bioeng. 2002, 77, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pretreatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzym. Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Sieber, V.; Amann, M.; Faulstich, M.; Schieder, M. Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour. Technol. 2012, 104, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, M.; Kruus, K.; Boer, H.; Kemell, M.; Andberg, M.; Viikari, L.; Sipilä, J. The effect of lignin model compound structure on the rate of oxidation catalyzed by two different fungal laccases. J. Mol. Catal. B Enzym. 2009, 57, 204–210. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, J. A modified method for calculating practical ethanol yield at high lignocellulosic solids content and high ethanol titer. Bioresour. Technol. 2012, 116, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Oliva, J.M.; González, A.; Ballesteros, I.; Ballesteros, M. Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 2009, 88, 2142–2147. [Google Scholar] [CrossRef]
- Thomsen, M.T.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechnol. 2009, 83, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Juhász, T.; Szengyel, K.; Réczey, K.; Siika-Aho, M.; Viikari, L. Characterization of cellulase and hemicellulases produced by Trichoderma reesei on various carbon sources. Process Biochem. 2005, 40, 3519–3525. [Google Scholar] [CrossRef]
- Dien, B.S.; Ximenes, E.A.; O’Brien, P.J.; Moniruzzaman, M.; Li, X.L.; Balan, V.; Dale, B.; Cotta, M.A. Enzyme characterization for hydrolysis of AFEX and liquid hot water pretreated distillers’grains and their conversion to ethanol. Bioresour. Technol. 2008, 99, 5216–5225. [Google Scholar] [CrossRef] [PubMed]
| WIS | Liquid Fraction | |||||
|---|---|---|---|---|---|---|
| Component | % DW | Sugar Component | Monomeric Form (g/L) | Oligomeric Form (g/L) | Inhibitors | g/L |
| Cellulose | 53.5 ± 1.1 | Glucan | 2.3 ± 0.2 | 12.4 ± 0.3 | Furfural | 0.8 ± 0.0 |
| Hemicellulose | 11.7 ± 0.7 | Xylan | 2.8 ± 0.1 | 29.2 ± 0.7 | 5-HMF | 0.3 ± 0.0 |
| Lignin | 30.4 ± 3.2 | Arabinan | 1.3 ± 0.2 | 1.1 ± 0.0 | Acetic acid | 6.9 ± 0.3 |
| Galactan | 0.4 ± 0.1 | 1.4 ± 0.1 | Formic acid | 6.3 ± 0.2 | ||
| Mannan | n.d. | n.d. | Phenols | 5.9 ± 0.8 | ||
| Assay | Composition (% DW, w/w) a | ||
|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | |
| C | 53.5 ± 1.1 | 11.7 ± 0.7 | 30.4 ± 3.2 |
| L | 54.1 ± 0.6 | 11.2 ± 0.7 | 30.5 ± 3.1 |
| Alk + C | 58.2 ± 2.9 | 12.3 ± 0.5 | 27.6 ± 1.6 |
| Alk + L | 57.9 ± 2.7 | 12.4 ± 0.6 | 27.1 ± 0.6 |
| Assay | Inhibitors (g/L) | ||||
|---|---|---|---|---|---|
| Furfural | 5-HMF | Acetic Acid | Formic Acid | Phenols | |
| C | 0.3 ± 0.0 | 0.1 ± 0.0 | 2.5 ± 0.0 | 2.2 ± 0.1 | 1.6 ± 0.0 |
| L | 0.3 ± 0.0 | 0.1 ± 0.0 | 2.4 ± 0.0 | 2.3 ± 0.4 | 1.3 ± 0.0 ** |
| CP | 0.3 ± 0.0 | 0.1 ± 0.0 | 3.3 ± 0.0 | 2.1 ± 0.0 | 1.8 ± 0.1 |
| LP | 0.3 ± 0.0 | 0.1 ± 0.0 | 3.3 ± 0.0 | 2.1 ± 0.0 | 1.4 ± 0.1 *** |
| Assay | Adaptation Phase (h) | EtOHmax (g/L) | YE/G (g/g) | YE/ET (%) | QEmax (g/L·h) |
|---|---|---|---|---|---|
| C | 12 | 12.3 ± 0.1 a | 0.29 ± 0.00 | 65.1 | 0.58 ± 0.21 c |
| L | <12 | 12.2 ± 1.2 a | 0.29 ± 0.03 | 64.4 | 0.56 ± 0.09 c |
| CP | 48 | 12.0 ± 0.1 b | 0.29 ± 0.1 | 63.4 | 0.43 ± 0.00 d |
| LP | 24 | 12.7 ± 0.8 a | 0.30 ± 0.1 | 67.1 | 0.44 ± 0.02 e |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.D.; Ibarra, D.; Mialon, A.; Ballesteros, M. A Bacterial Laccase for Enhancing Saccharification and Ethanol Fermentation of Steam-Pretreated Biomass. Fermentation 2016, 2, 11. https://doi.org/10.3390/fermentation2020011
Moreno AD, Ibarra D, Mialon A, Ballesteros M. A Bacterial Laccase for Enhancing Saccharification and Ethanol Fermentation of Steam-Pretreated Biomass. Fermentation. 2016; 2(2):11. https://doi.org/10.3390/fermentation2020011
Chicago/Turabian StyleMoreno, Antonio D., David Ibarra, Antoine Mialon, and Mercedes Ballesteros. 2016. "A Bacterial Laccase for Enhancing Saccharification and Ethanol Fermentation of Steam-Pretreated Biomass" Fermentation 2, no. 2: 11. https://doi.org/10.3390/fermentation2020011