Multi-Walled Carbon Nanotubes Modified NiCo2S4 for the Efficient Photocatalytic Reduction of Hexavalent Chromium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Composite Photocatalysts
2.2. Photocatalytic Performance Measurements
3. Results
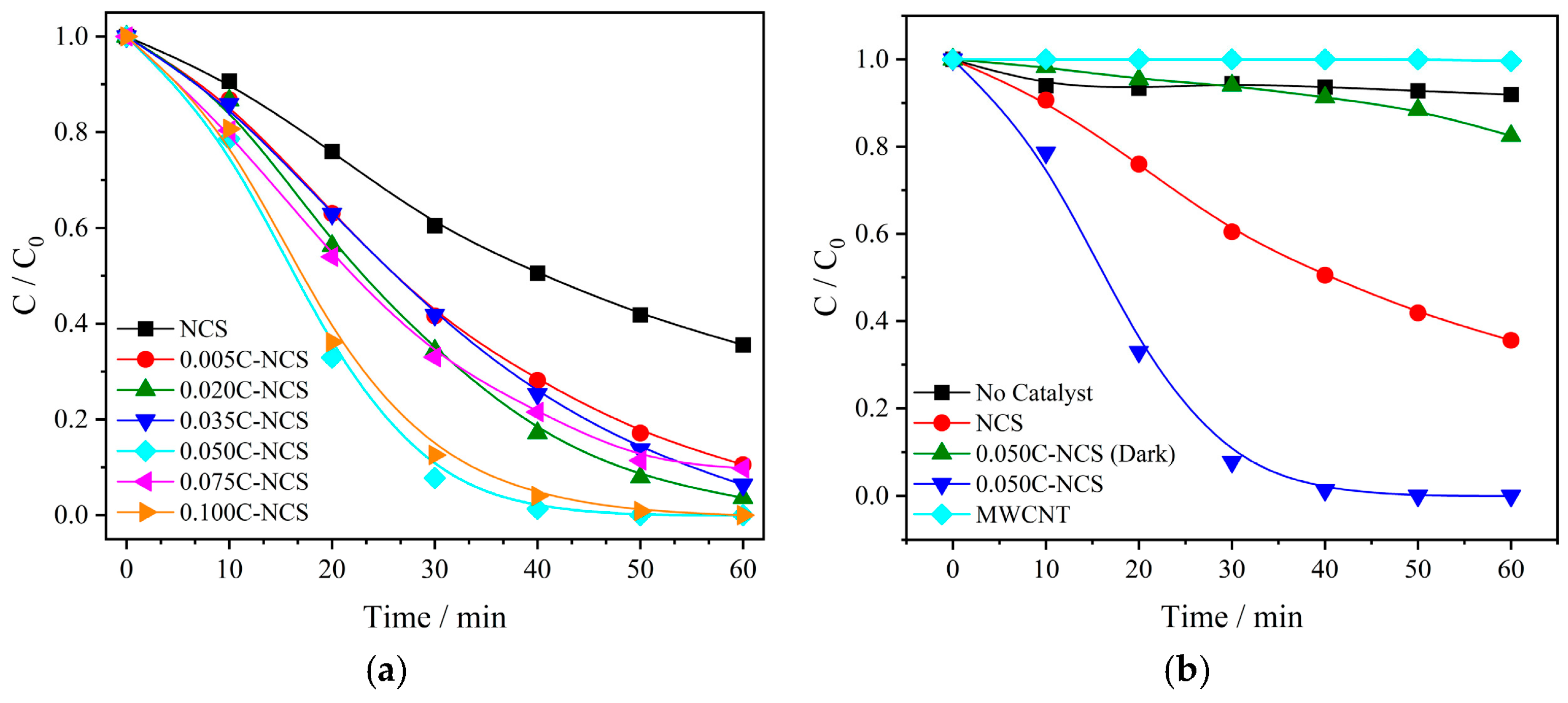
3.1. Photocatalytic Performances
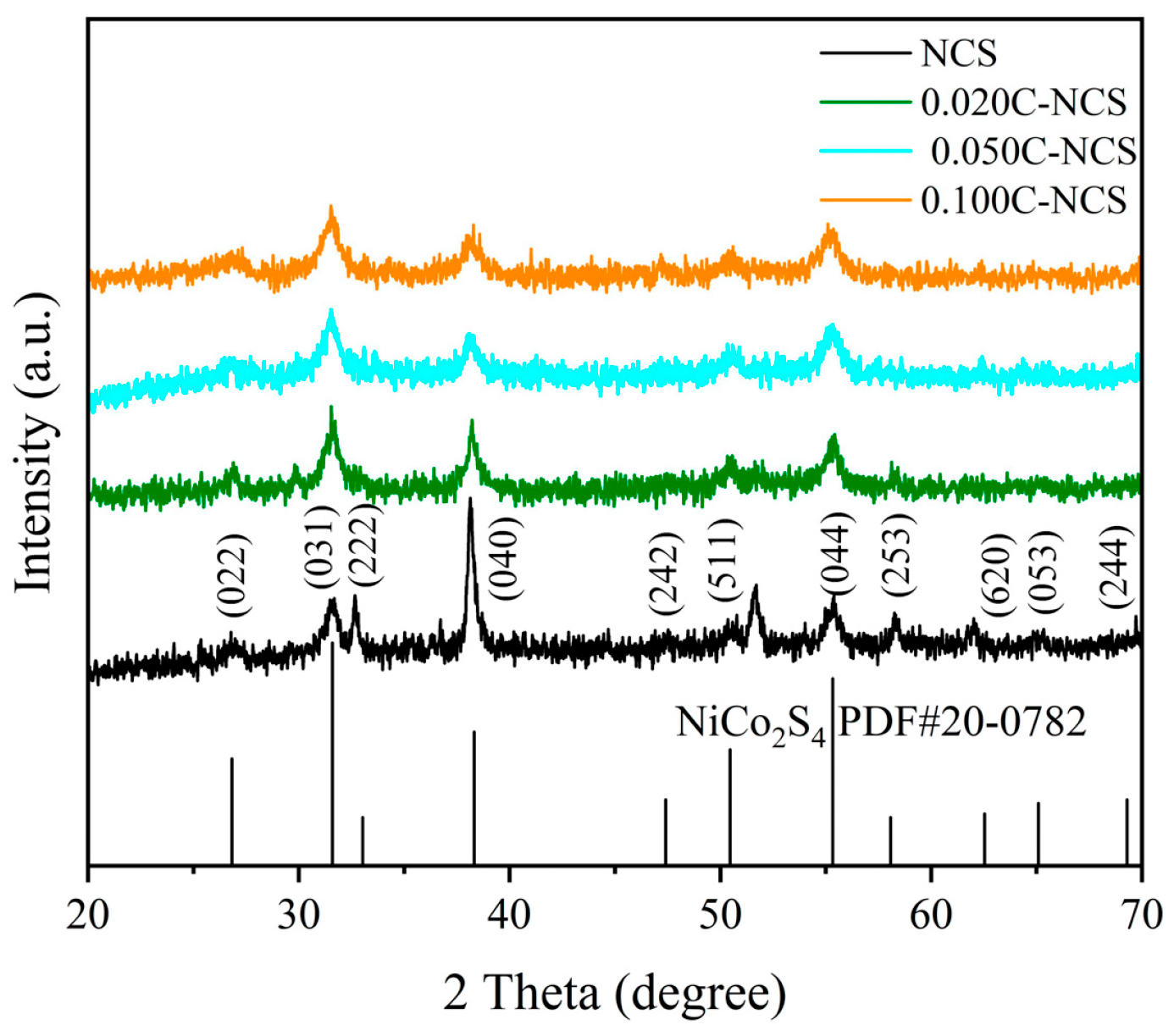
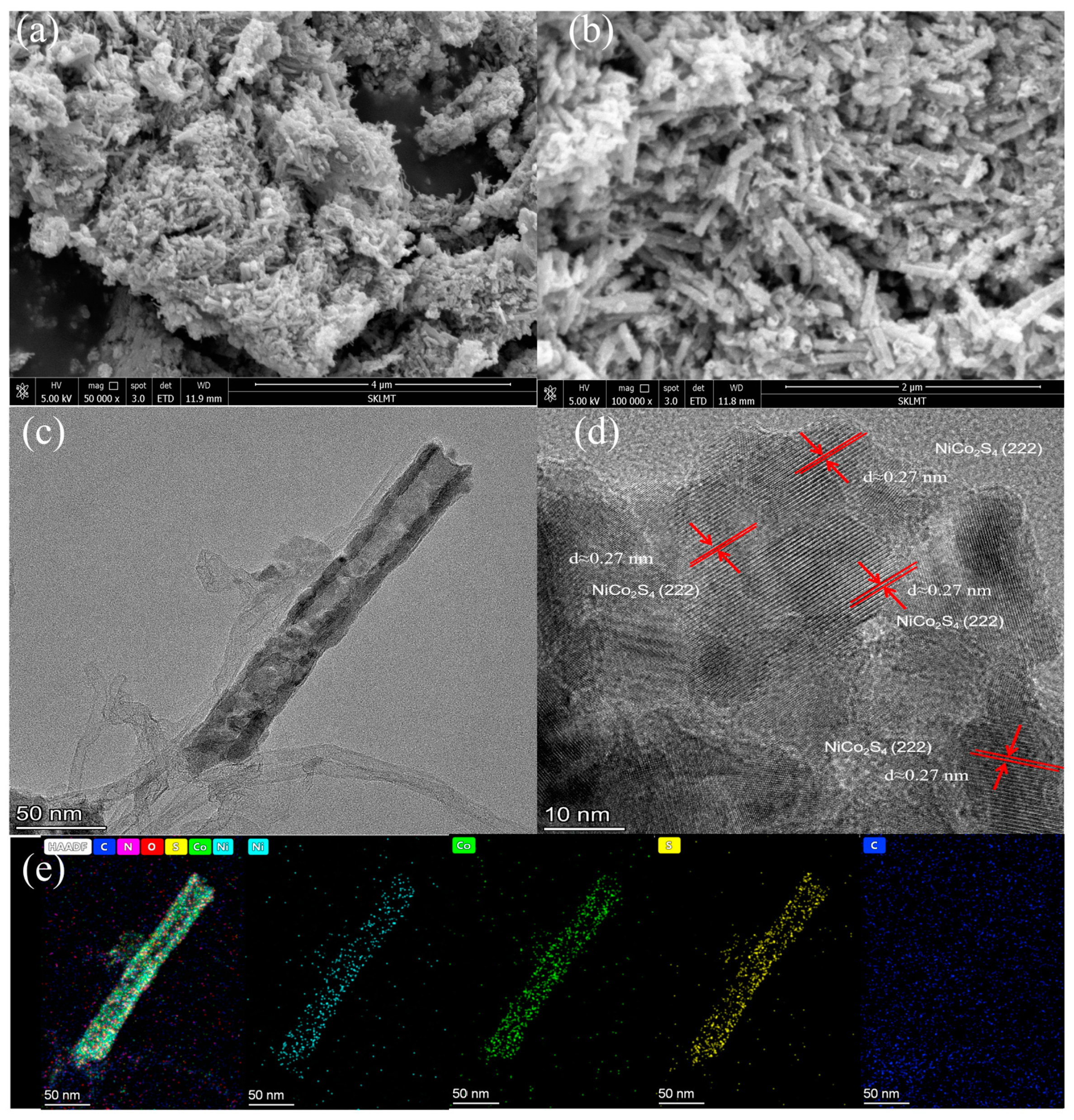
3.2. Structural and Morphological Analysis
3.3. Nitrogen Adsorption–Desorption Experiment
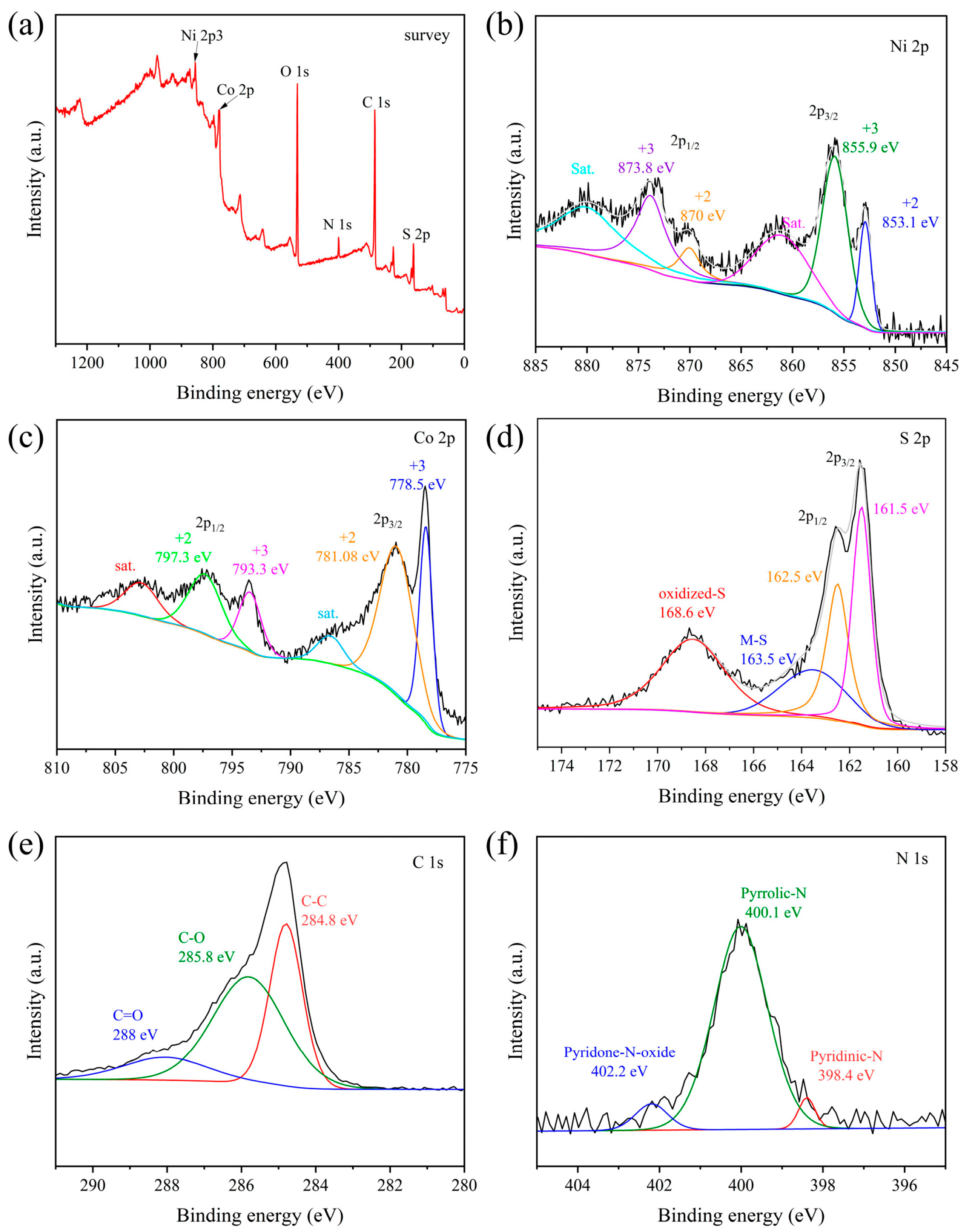
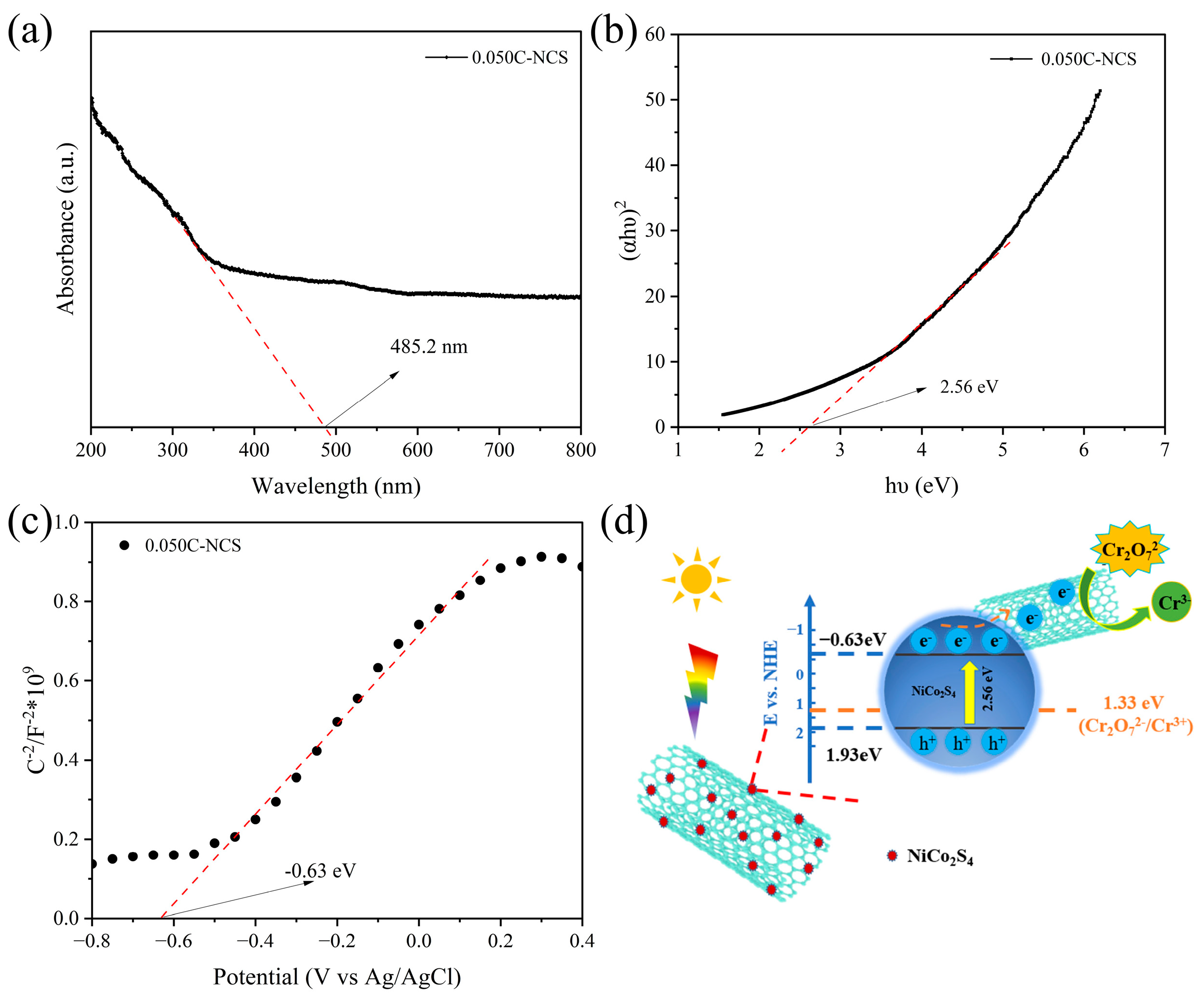
3.4. Composition and Band Structure Analysis
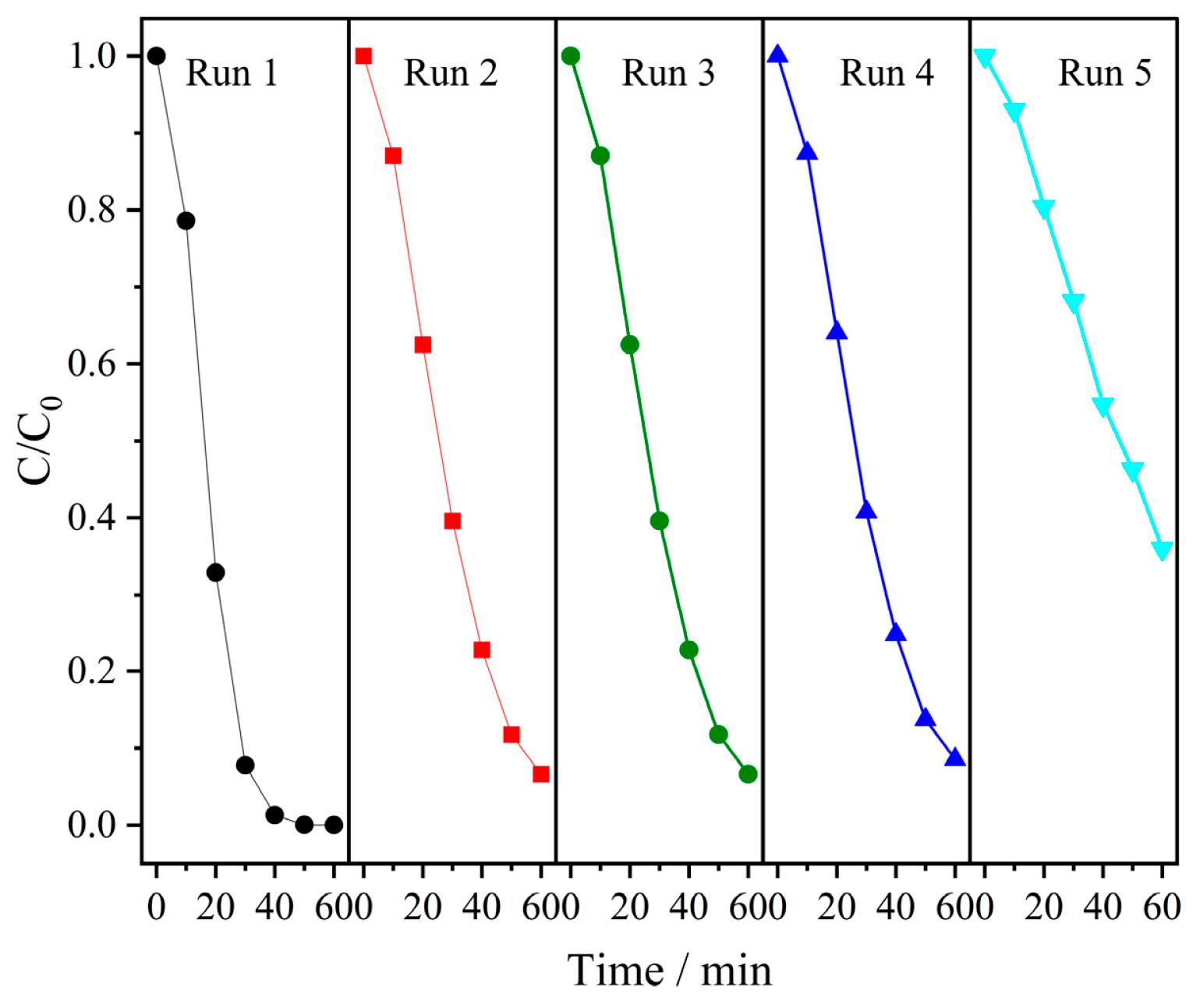
3.5. Stability of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; You, J.; Wang, Z.; Zhao, Y.; Xu, J.; Li, X.; Zhang, H. Application of alpha-Fe2O3-based heterogeneous photo-fenton catalyst in wastewater treatment: A review of recent advances. J. Environ. Chem. Eng. 2022, 10, 108329. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y.; Li, N.; Zhang, P.; Li, X. Rationally designed Ta3N5/BiOCl S-scheme heterojunction with oxygen vacancies for elimination of tetracycline antibiotic and Cr(VI): Performance, toxicity evaluation and mechanism insight. J. Mater. Sci. Technol. 2022, 123, 177–190. [Google Scholar] [CrossRef]
- Kholisa, B.; Matsena, M.; Chirwa, E.M.N. Evaluation of Cr(VI) reduction using indigenous bacterial consortium isolated from a municipal wastewater sludge: Batch and kinetic studies. Catalysts 2021, 11, 1100. [Google Scholar] [CrossRef]
- Cheng, L.; He, R.L.; Min, D.; Li, W.W.; Liu, D.F.; Yu, H.Q. Engineering a rhamnose-inducible system to enhance the extracellular electron transfer ability of Shewanella genus for improved Cr(VI) reduction. ACS EST Eng. 2021, 1, 842–850. [Google Scholar] [CrossRef]
- Jin, Q.; Dai, M.; Zhan, X.; Wang, S.; He, Z. Carbon nanotubes and graphene composites used in Cr(VI) detection techniques: A review. J. Alloys Compd. 2022, 922, 166268. [Google Scholar] [CrossRef]
- Djouider, F. Radiolytic formation of non-toxic Cr(III) from toxic Cr(VI) in formate containing aqueous solutions: A system for water treatment. J. Hazard. Mater. 2012, 223, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Meinhold, V.; Hoehlich, D.; Mehner, T.; Lampke, T. Electrodeposition of thick and crack-free Fe-Cr-Ni coatings from a Cr (III) electrolyte. Coatings 2022, 12, 56. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, M.; Cui, Y.-L.; Zhao, L.; Liu, S. Novel reduction of Cr(VI) from wastewater using a naturally derived microcapsule loaded with rutin-Cr(III) complex. J. Hazard. Mater. 2015, 285, 336–345. [Google Scholar] [CrossRef]
- Xu, J.-J.; Gu, H.-Y.; Chen, M.-D.; Li, X.-P.; Zhao, H.-W.; Yang, H.-B. Dual Z-scheme Bi3TaO7/Bi2S3/SnS2 photocatalyst with high performance for Cr(VI) reduction and TC degradation under visible light irradiation. Rare Metals 2022, 41, 2417–2428. [Google Scholar] [CrossRef]
- Qin, C.; Pan, G.; Zhang, Y.; Ding, F.; Qu, J.; Xu, X.; Su, X. Efficient reduction of Cr (VI) to Cr (III) over a TiO2-supported palladium catalyst using formic acid as a reductant. Catalysts 2022, 12, 179. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, Z.L.; Song, D.G.; Huang, H.; Yu, J.; Wen, Y.C.; Lu, J.L.; Bian, Z.F.; Qian, X.F. Interstitial compound Fe3C-soped Fe(0) nanoparticles embedded in mesoporous carbon boosting Cr(VI) removal. ACS EST Eng. 2022, 3, 131–137. [Google Scholar] [CrossRef]
- Yang, D.Z.; Chu, Z.T.; Feng, X.Z.; Ge, Q.Y.; Wang, R.H.; Zhang, J.; Li, S.Y.; Zheng, R.J.; Wei, W.F.; Yi, S.P.; et al. Dual ions neutralized and stabilized red mud for chromium(VI) polluted soil remediation. ACS EST Eng. 2022, 2, 913–923. [Google Scholar] [CrossRef]
- Zarras, P.; Miller, C.E.; Webber, C.; Anderson, N.; Stenger-Smith, J.D. Laboratory and field studies of Poly(2,5-bis(N-methyl-N-hexylamino)phenylene vinylene) (BAM-PPV): A potential wash primer replacement for army military vehicles. Coatings 2014, 4, 687–700. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, X.; Lu, X.; Li, M.; Wang, L.; Wang, Y. Kinetics and mechanisms of Cr(VI) removal by nZVI: Influencing parameters and modification. Catalysts 2022, 12, 999. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, W.; Yang, W.; Li, Q. Palladium nanoparticles supported on amine-functionalized glass fiber mat for fixed-bed reactors on the effective removal of hexavalent chromium by catalytic reduction. J. Mater. Sci. Technol. 2018, 34, 961–968. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, S.; Zhu, X. Biomass cellulose component and Fe mineral catalysis help Cr(VI) to realize almost 100% pyrolysis reduction efficiency. ACS EST Eng. 2021, 1, 1441–1448. [Google Scholar] [CrossRef]
- Vaddi, D.R.; Gurugubelli, T.R.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. Bio-stimulated adsorption of Cr(VI) from aqueous solution by groundnut shell activated carbon@Al embedded material. Catalysts 2022, 12, 290. [Google Scholar] [CrossRef]
- Rahmat, S.T.; Alias, N.; Kumar, R.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Electrophoretic deposition of graphene oxide and reduced graphene oxide on the rutile phase of TiO2 nanowires for rapid reduction of Cr (VI) under simulated sunlight irradiation. Catalysts 2022, 12, 1282. [Google Scholar] [CrossRef]
- Wan, Z.; Mao, Q.; Xiang, J.; Ma, D.; Tang, H. Greatly increased visible-light photocatalytic activity of SnS2/carbon nanotube composite for Cr(VI) reduction: Insights into effects of solid acid structure. J. Mater. Sci. Technol. 2023, 161, 233–244. [Google Scholar] [CrossRef]
- Yu, M.D.; Mao, X.H.; He, X.S.; Zheng, M.X.; Meng, Y.; He, F.; Xi, B.D. Enhanced sequestration of chromium by mechanochemically silicified microscale zerovalent iron: Role of the silicate-modified surface. ACS EST Eng. 2023, 3, 1604–1613. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Karakassides, A.; Baikousi, M.; Gioti, C.; Moschovas, D.; Avgeropoulos, A.; Bourlinos, A.B.; Douvalis, A.P.; Salmas, C.E.; Karakassides, M.A. Nanoporous carbon magnetic hybrid derived from waterlock polymers and its application for hexavalent chromium removal from aqueous solution. C-J. Carbon Res. 2021, 7, 69. [Google Scholar] [CrossRef]
- Smith, V.A.; Rivera, J.F.A.; Bello, R.; Rodriguez-Aguado, E.; Elshaer, M.R.; Wodzinski, R.L.; Bashkova, S. The role of surface chemistry and polyethylenimine grafting in the removal of Cr (VI) by activated carbons from cashew nut shells. C-J. Carbon Res. 2021, 7, 27. [Google Scholar] [CrossRef]
- Feng, H.-F.; Yu, Y.-X.; Jiang, S.-Q.; Shang, J.; Cheng, Y.; Wang, L.; Hao, W.-C.; Wang, T.-M. Synthesis of magnetic core-shell iron nanochains for potential applications in Cr(VI) ion pollution treatment. Rare Metals 2021, 40, 176–179. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y. Effect of acidic surface functional groups on Cr(VI) removal by activated carbon from aqueous solution. Rare Metals 2010, 29, 333–338. [Google Scholar] [CrossRef]
- Yu, J.X.; Fu, Q.; Yang, H.S.; Ouyang, G.F.; Wang, J.H.; Hao, Z.P. Synergistic catalytic organic pollutants degradation and Cr(VI) reduction by carbon nanotubes through an electron-transfer mechanism without external energy or chemical input. ACS EST Eng. 2022, 2, 1221–1228. [Google Scholar] [CrossRef]
- Mu, F.H.; Dai, B.L.; Zhao, W.; Zhou, S.J.; Huang, H.B.; Yang, G.; Xia, D.H.; Kong, Y.; Leung, D.Y.C. Construction of a novel Ag/Ag3PO4 /MIL-68(In)-NH2 plasmonic heterojunction photocatalyst for high-efficiency photocatalysis. J. Mater. Sci. Technol. 2022, 101, 37–48. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.Y.; Xu, S.; Peng, J.F.; Liu, F.Y.; Wang, L.H. Enhancing the intrinsic properties of flower-like BiOI by S-doping toward excellent photocatalytic performances. J. Mater. Sci. Technol. 2022, 118, 181–189. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Dong, Y.; Hu, C.; Xiang, X.; Zeng, X.; Jia, J.; Jin, C.; Ding, L.; Chen, X. In-situ synthesis of 0D/1D CeO2/Zn0.4Cd0.6S S-scheme heterostructures for boosting photocatalytic remove of antibiotic and chromium. Ceram. Int. 2023, 49, 5842–5853. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, Y.; Peng, M.; Chen, X.; Zheng, Q.; Liao, J.; Chen, W. Band gap-controllable N, P co-doped carbon@ZnxCd1−xIn2S4 for photocatalytic reduction of chromium(VI) and degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2023, 322, 124294. [Google Scholar] [CrossRef]
- Shearer, C.J.; Alvino, J.F.; Batmunkh, M.; Metha, G.E. Pt nanocluster co-catalysts for photocatalytic water splitting. C-J. Carbon Res. 2018, 4, 64. [Google Scholar] [CrossRef]
- Ratova, M.; Sawtell, D.; Kelly, P.J. Micro-patterning of magnetron sputtered titanium dioxide coatings and their efficiency for photocatalytic applications. Coatings 2020, 10, 68. [Google Scholar] [CrossRef]
- Roveri, M.; Goidanich, S.; Toniolo, L. Artificial ageing of photocatalytic nanocomposites for the protection of natural stones. Coatings 2020, 10, 729. [Google Scholar] [CrossRef]
- Shen, R.; Hao, L.; Chen, Q.; Zheng, Q.; Zhang, P.; Li, X. P-Doped g-C3N4 nanosheets with highly dispersed Co0.2Ni1.6Fe0.2P cocatalyst for efficient photocatalytic hydrogen evolution. Acta Phys.-Chim. Sin. 2022, 38, 2110014. [Google Scholar]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and development of novel composites containing nickel ferrites supported on activated carbon derived from agricultural wastes and its application in water remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Raditoiu, V.; Raditoiu, A.; Raduly, M.F.; Amariutei, V.; Gifu, I.C.; Anastasescu, M. Photocatalytic behavior of water-based styrene-acrylic coatings containing TiO2 sensitized with metal-phthalocyanine tetracarboxylic acids. Coatings 2017, 7, 229. [Google Scholar] [CrossRef]
- Huang, G.Q.; Ye, W.N.; Lv, C.X.; Butenko, D.S.; Yang, C.; Zhang, G.L.; Lu, P.; Xu, Y.; Zhang, S.C.; Wang, H.W.; et al. Hierarchical red phosphorus incorporated TiO2 hollow sphere heterojunctions toward superior photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 108, 18–25. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, J.; Qiu, J.; Wang, D.; Zhao, Z.; Lu, Z.; Huang, G.; Liu, X.; Mei, Y. Gold nanoparticles decorated titanium oxide nanotubes with enhanced antibacterial activity driven by photocatalytic memory effect. Coatings 2022, 12, 1351. [Google Scholar] [CrossRef]
- Alasri, T.M.; Ali, S.L.; Salama, R.S.; Alshorifi, F.T. Band-structure engineering of TiO2 photocatalyst by AuSe quantum dots for efficient degradation of malachite green and phenol. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1729–1740. [Google Scholar] [CrossRef]
- Zhang, S.J.; He, Z.L.; Xu, S.S.; Li, X.; Zhang, J.; Zhan, X.P.; Dai, M.; Wang, S.G. In situ liquid-phase growth strategies of g-C3N4 solar-driven heterogeneous catalysts for environmental applications. Sol. RRL 2021, 5, 2100233. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Hao, X. Ni, Co-based selenide anchored g-C3N4 for boosting photocatalytic hydrogen evolution. Acta Phys.-Chim. Sin. 2021, 37, 1912033. [Google Scholar]
- Fernandez-Catala, J.; Greco, R.; Navlani-Garcia, M.; Cao, W.; Berenguer-Murcia, A.; Cazorla-Amoros, D. g-C3N4-based direct Z-scheme photocatalysts for environmental applications. Catalysts 2022, 12, 1137. [Google Scholar] [CrossRef]
- Dai, M.; Yu, H.; Chen, W.; Qu, K.-A.; Zhai, D.; Liu, C.; Zhao, S.; Wang, S.; He, Z. Boosting photocatalytic activity of CdLa2S4/ZnIn2S4 S-scheme heterojunctions with spatial separation of photoexcited carries. Chem. Eng. J. 2023, 470, 144240. [Google Scholar] [CrossRef]
- Xiong, Z.; Hou, Y.; Yuan, R.; Ding, Z.; Ong, W.-J.; Wang, S. Hollow NiCo2S4 Nanospheres as a cocatalyst to support ZnIn2S4 nanosheets for visible-light-driven hydrogen production. Acta Phys.-Chim. Sin. 2022, 38, 2111021. [Google Scholar]
- Zeng, J.-Y.; Wang, X.-S.; Xie, B.-R.; Li, Q.-R.; Zhang, X.-Z. Large pi-conjugated metal-organic frameworks for infrared-light-driven CO2 reduction. J. Am. Chem. Soc. 2022, 144, 1218–1231. [Google Scholar] [CrossRef]
- Lan, C.; Meng, L.; Xu, N. One-pot synthesis of the direct Z-scheme AgInS2/AgIn5S8 QDs heterojunction for efficient photocatalytic reduction of Cr6+ in neutral condition. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127762. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4-ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- He, Z.; Byun, J.-H.; Zhou, G.; Park, B.-J.; Kim, T.-H.; Lee, S.-B.; Yi, J.-W.; Um, M.-K.; Chou, T.-W. Effect of MWCNT content on the mechanical and strain-sensing performance of thermoplastic polyurethane composite fibers. Carbon 2019, 146, 701–708. [Google Scholar] [CrossRef]
- He, Z.; Zhou, G.; Byun, J.H.; Lee, S.K.; Um, M.K.; Park, B.; Kim, T.; Lee, S.B.; Chou, T.W. Highly stretchable multi-walled carbon nanotube/thermoplastic polyurethane composite fibers for ultrasensitive, wearable strain sensors. Nanoscale 2019, 11, 5884–5890. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Zhang, J. Controlled synthesis of carbon nanotubes: Past, present and future. Acta Phys.-Chim. Sin. 2020, 36, 1907021. [Google Scholar] [CrossRef]
- Adusei, P.K.; Johnson, K.; Kanakaraj, S.N.; Zhang, G.; Fang, Y.; Hsieh, Y.-Y.; Khosravifar, M.; Gbordzoe, S.; Nichols, M.; Shanov, V. Asymmetric fiber supercapacitors based on a FeC2O4/FeOOH-CNT hybrid material. C-J. Carbon Res. 2021, 7, 62. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, M.; Zhang, S.; Dai, M.; Zhang, P.; Wang, S.; He, Z. Recent progress on carbon-nanotube-based materials for photocatalytic applications: A review. Sol. RRL 2022, 6, 2200243. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Wang, S.; Dai, G.-P.; Lu, J.; Liu, K. Enhanced visible-light photocatalytic activity and stability of nano-sized Ag2CO3 combined with carbon nanotubes. Acta Phys.-Chim. Sin. 2014, 30, 2121–2126. [Google Scholar]
- Toloman, D.; Stefan, M.; Macavei, S.; Barbu-Tudoran, L.; Popa, A. Photocatalytic self-cleaning PVDF membrane blended with MWCNT-ZnO nanocomposites for RhB removal. Coatings 2023, 13, 594. [Google Scholar] [CrossRef]
- Miklec, K.; Grcic, I.; Radetic, L.; Cingesar, I.K.; Vrsaljko, D. Photocatalytic oxidation of amoxicillin in CPC reactor over 3D printed TiO2-CNT@PETG static mixers. Coatings 2023, 13, 386. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Iniesta, J.; Fermin, D.J.; Ania, C.O. Photoelectrochemical response of WO3/nanoporous carbon anodes for photocatalytic water oxidation. C-J. Carbon Res. 2018, 4, 45. [Google Scholar] [CrossRef]
- Falara, P.P.; Ibrahim, I.; Zourou, A.; Sygellou, L.; Sanchez, D.E.; Romanos, G.E.; Givalou, L.; Antoniadou, M.; Arfanis, M.K.; Han, C.; et al. Bi-functional photocatalytic heterostructures combining titania thin films with carbon quantum dots (C-QDs/TiO2) for effective elimination of water pollutants. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Kong, Q.; Long, R.; Wang, C.; Jiang, J.; Xiong, Y. Toward enhanced photocatalytic oxygen evolution: Synergetic utilization of plasmonic effect and schottky junction via interfacing facet selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef]
- Yuan, K.; Gao, T.-J.; Yang, Y.; Luo, W.; Li, S.; Zhang, C.-Y.; Xu, J.-X.; Li, N.; Zhu, Y.-R. Template sacrificial controlled synthesis of hierarchical nanoporous carbon@NiCo2S4 microspheres for highperformance hybrid supercapacitors. Rare Metals 2023, 42, 2643–2657. [Google Scholar] [CrossRef]
- Rashid, M.; Hassan, W.; Aadil, M.; Somaily, H.H.; Mahdi, N.M.; Lataef, R.; Taki, A.G.; Srithilat, K.; Baamer, D.F.; Albukhari, S.M.; et al. Solar-light-driven and magnetically recoverable doped nano-ferrite: An ideal photocatalyst for water purification applications. Opt. Mater. 2023, 135, 113192. [Google Scholar] [CrossRef]
- Nazik, G.; Aadil, M.; Zulfiqar, S.; Hassan, W.; Rahman, A.; Ibrahim, S.M.; Naseem, K.; Sheikh, T.A.; Akhtar, M.N. Synthesis of doped metal sulfide nanoparticles and their graphene reinforced nanohybrid for Pb(II) detection. Z. Für Phys. Chem. 2023, 237, 1257–1285. [Google Scholar] [CrossRef]
- Aadil, M.; Zulfiqar, S.; Agboola, P.O.; Aboud, M.F.A.; Shakir, I.; Warsi, M.F. Fabrication of graphene supported binary nanohybrid with multiple approaches for electrochemical energy storage applications. Synth. Met. 2021, 272, 116645. [Google Scholar] [CrossRef]
- Tariq, R.; Zulfiqar, S.; Somaily, H.H.; Warsi, M.F.; Ayman, I.; Hanif, F.; Akhtar, M.; Aadil, M. Synthesis of carbon supported iron oxide nanochips and their composite with glutathione: A novel electrochemical sensitive material. Surf. Interfaces 2022, 34, 102350. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Cao, W.; Zhang, J.; Chen, W.; Jin, Q.; Que, W.; Wang, S. Rational construction of S-scheme BN/MXene/ZnIn2S4 heterojunction with interface engineering for efficient photocatalytic hydrogen production and chlorophenols degradation. Sep. Purif. Technol. 2023, 309, 123004. [Google Scholar] [CrossRef]
- Guo, M.-L.; Wu, Z.-Y.; Zhang, M.-M.; Huang, Z.-J.; Zhang, K.-X.; Wang, B.-R.; Tu, J.-C. Coupling interface constructions of FeOOH/NiCo2S4 by microwave-assisted method for efficient oxygen evolution reaction. Rare Metals 2023, 42, 1847–1857. [Google Scholar] [CrossRef]
- Aadil, M.; Hassan, W.; Somaily, H.H.; Ejaz, S.R.; Abass, R.R.; Jasem, H.; Hachim, S.K.; Adhab, A.H.; Abood, E.S.; Alsafari, I.A. Synergistic effect of doping and nanotechnology to fabricate highly efficient photocatalyst for environmental remediation. J. Alloys Compd. 2022, 920, 165876. [Google Scholar] [CrossRef]
- Tamam, N.; Aadil, M.; Hassan, W.; Ejaz, S.R.; Najm, Z.M.; Alsafari, I.A.; Aman, S.; Trukhanov, A.V.; Al-Buriahi, M.S.; Boukhris, I. Surfactant assisted synthesis of nanostructured Mn-doped CuO: An efficient photocatalyst for environmental remediation. Ceram. Int. 2022, 48, 29589–29600. [Google Scholar] [CrossRef]
- Ishfaq, M.; Aadil, M.; Ejaz, S.R.; Hassan, W.; Panduro-Tenazoa, N.M.; El Sayed, M.E.; Murshed, M.N.; El-Bahy, Z.M. Synthesis of binary metal doped CeO2 via the subcritical hydrothermal method for photo-mineralizing methyl orange dye. J. Alloys Compd. 2023, 960, 170661. [Google Scholar] [CrossRef]
- Badovinac, I.J.; Peter, R.; Omerzu, A.; Salamon, K.; Saric, I.; Samarzija, A.; Percic, M.; Piltaver, I.K.; Ambrozic, G.; Petravic, M. Grain size effect on photocatalytic activity of TiO2 thin films grown by atomic layer deposition. Thin Solid Films 2020, 709, 138215. [Google Scholar] [CrossRef]
- Li, T.; Aadil, M.; Zulfiqar, S.; Anwar, A.; Yakout, S.M.; Panduro-Tenazoa, N.M.; Mubeen, S. Synthesis of doped and porous CuO with boosted light-harvesting features for the photocatalytic mineralization of azo dyes. Ceram. Int. 2023, 49, 27827–27836. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, S.; Chen, J.; Sun, X.; Zhao, S.; Chang, J.; He, Z. Ti3C2@g-C3N4/TiO2 ternary heterogeneous photocatalyst for promoted photocatalytic degradation activities. Coatings 2023, 13, 655. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Lin, L.H.; Jeon, T.H.; Lin, S.; Wang, X.C.; Choi, W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy 2017, 42, 58–68. [Google Scholar] [CrossRef]
- Yu, H.; Xu, S.; Zhang, S.; Wang, S.; He, Z. In-situ construction of core–shell structured TiB2-TiO2@g-C3N4 for efficient photocatalytic degradation. Appl. Surf. Sci. 2022, 579, 152201. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; Chen, J.; Yin, X.; He, Y.; Ren, J. Photocatalytic degradation of methyl orange over nitrogen-fluorine codoped TiO2 nanobelts prepared by solvothermal synthesis. ACS Appl. Mater. Interfaces 2012, 4, 6816–6826. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Karuturi, S.; Zan, L. Bi2S3-In2S3 heterostructures for efficient photoreduction of highly toxic Cr6+ enabled by facet-coupling and Z-scheme structure. Small 2021, 17, 2101833. [Google Scholar] [CrossRef] [PubMed]


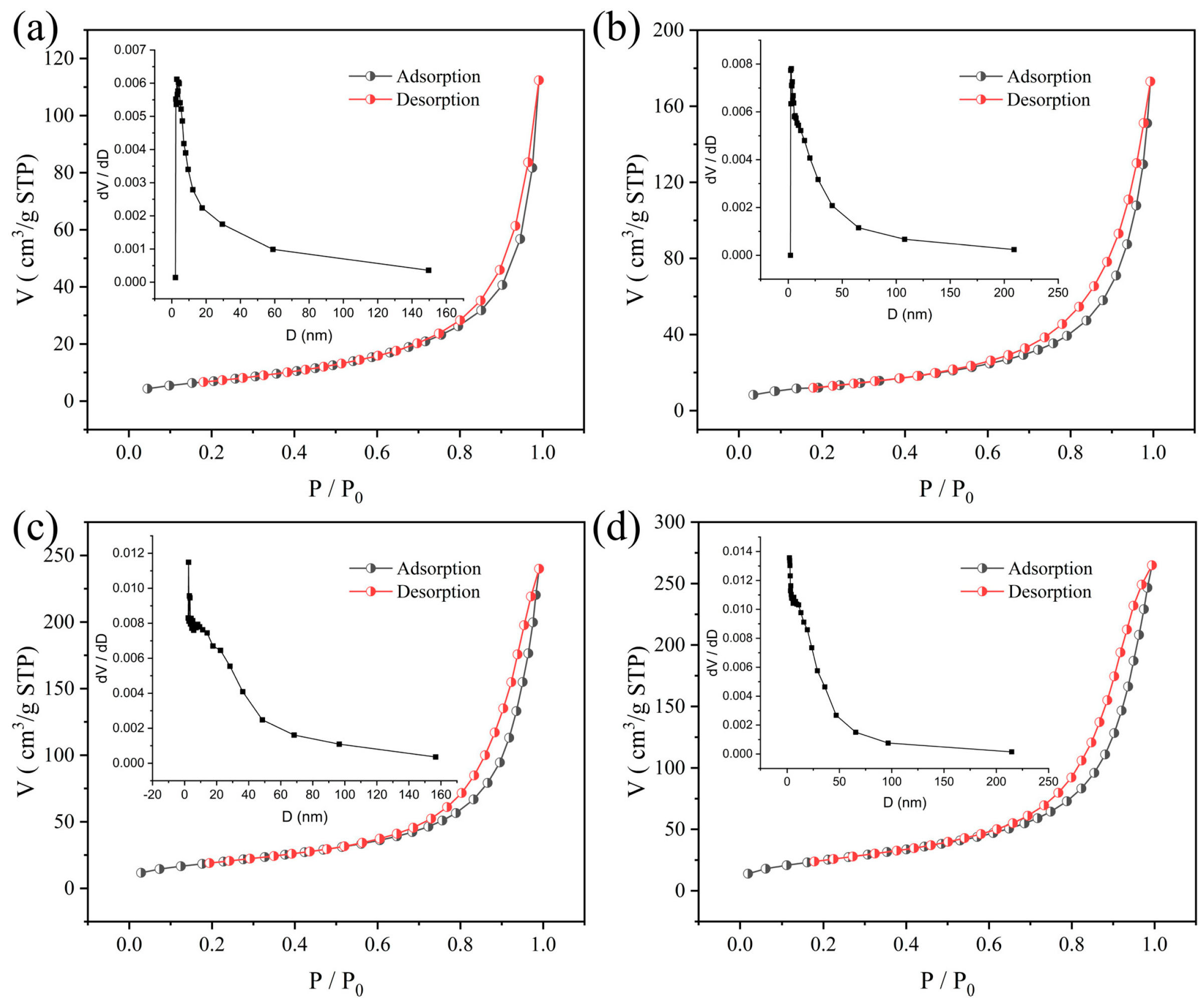
| Sample | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| NCS | 16 | 34 | 0.133 |
| 0.005C-NCS | 22 | 27 | 0.145 |
| 0.020C-NCS | 51 | 22 | 0.290 |
| 0.035C-NCS | 71 | 18 | 0.328 |
| 0.050C-NCS | 91 | 20 | 0.460 |
| 0.075C-NCS | 100 | 18 | 0.439 |
| 0.100C-NCS | 125 | 15 | 0.467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Q.; Zheng, Z.; Feng, Y.; Tian, S.; He, Z. Multi-Walled Carbon Nanotubes Modified NiCo2S4 for the Efficient Photocatalytic Reduction of Hexavalent Chromium. C 2023, 9, 99. https://doi.org/10.3390/c9040099
Jin Q, Zheng Z, Feng Y, Tian S, He Z. Multi-Walled Carbon Nanotubes Modified NiCo2S4 for the Efficient Photocatalytic Reduction of Hexavalent Chromium. C. 2023; 9(4):99. https://doi.org/10.3390/c9040099
Chicago/Turabian StyleJin, Qiu, Ziye Zheng, Yuxiao Feng, Shuang Tian, and Zuoli He. 2023. "Multi-Walled Carbon Nanotubes Modified NiCo2S4 for the Efficient Photocatalytic Reduction of Hexavalent Chromium" C 9, no. 4: 99. https://doi.org/10.3390/c9040099





