Multifunctional Drugs-Loaded Carbomol Hydrogel Promotes Diabetic Wound Healing via Antimicrobial and Immunoregulation
Abstract
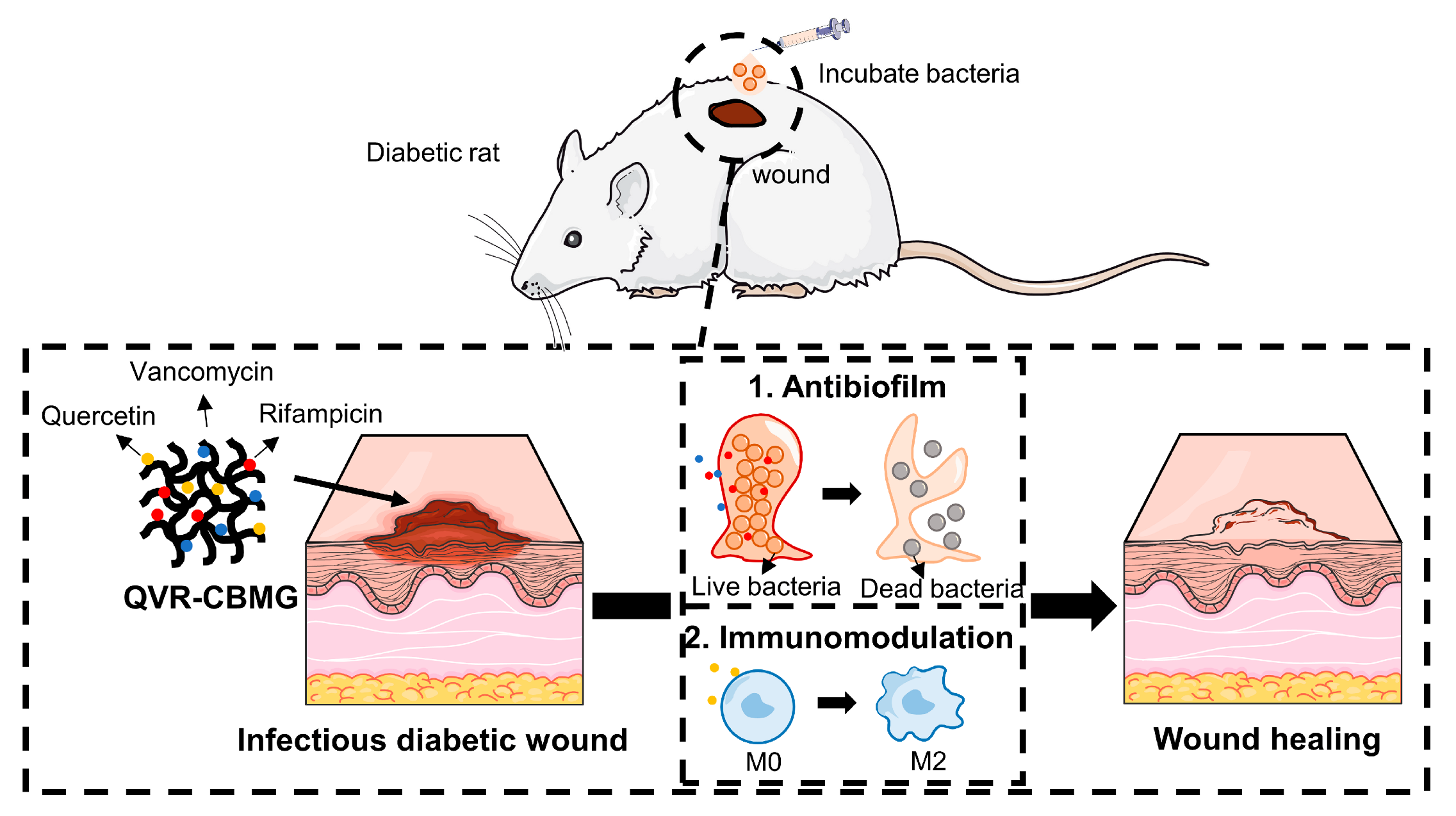
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Hydrogels
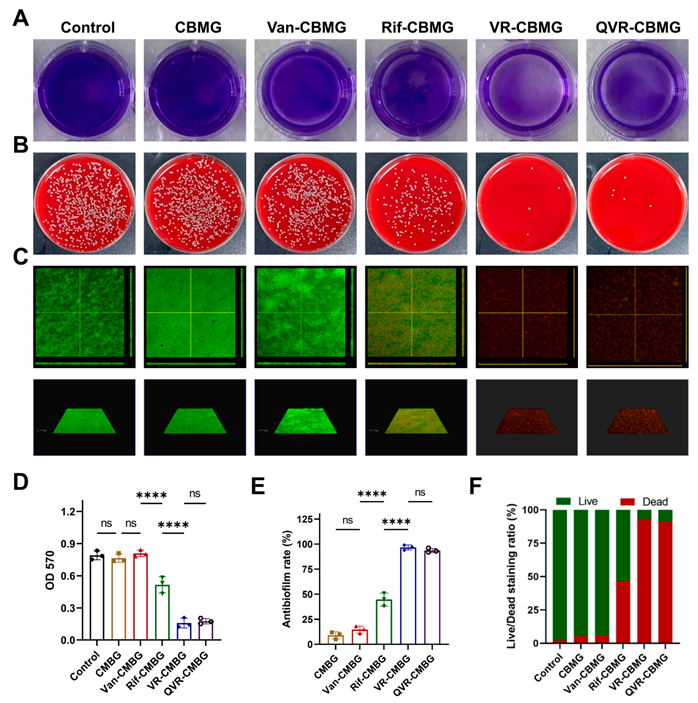
2.2. Cell Biocompatibility and Antibacterial/Antibiofilm Activities of Hydrogels
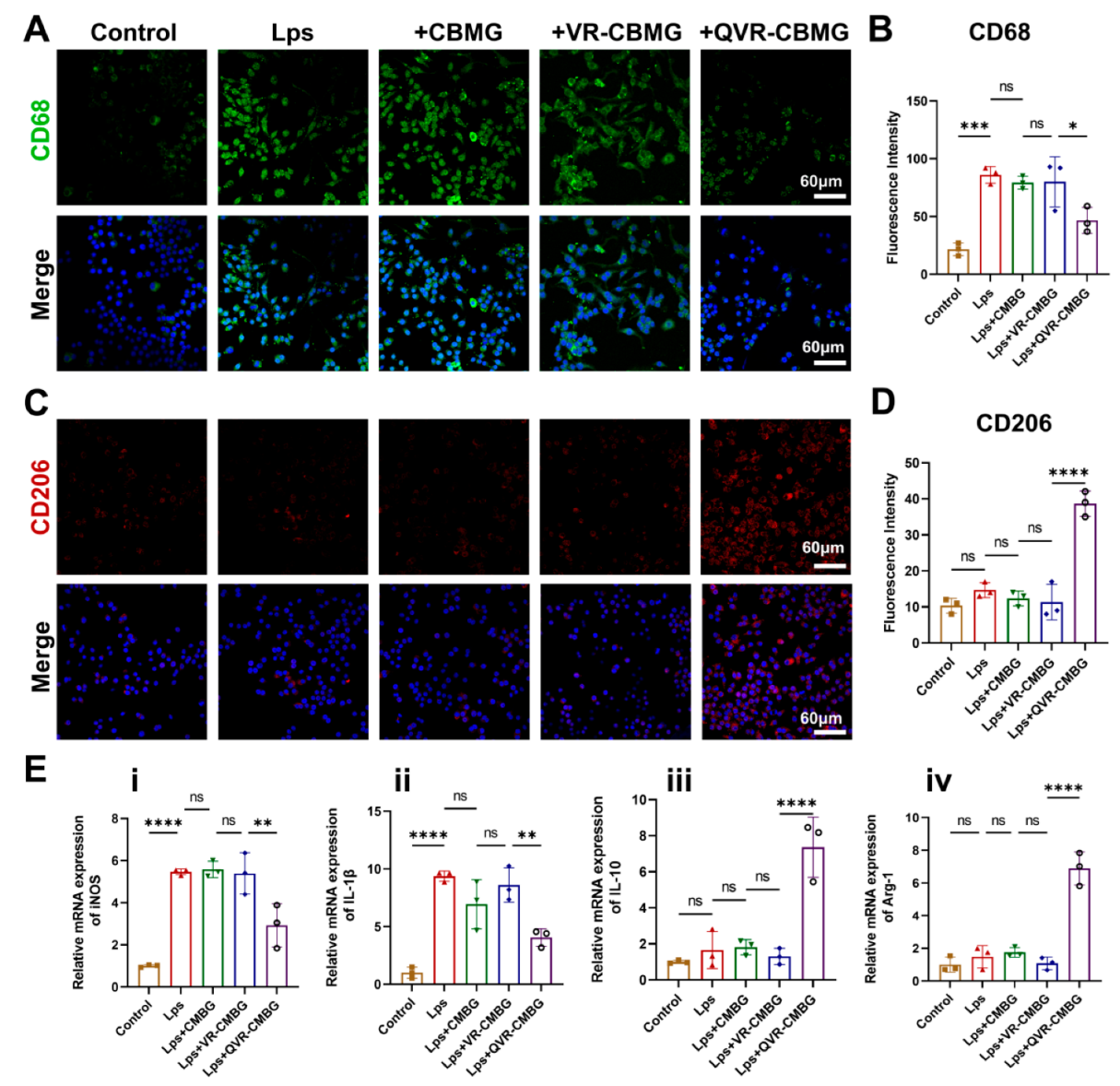
2.3. QVR-CBMG Hydrogel Affected the Polarization of Macrophages
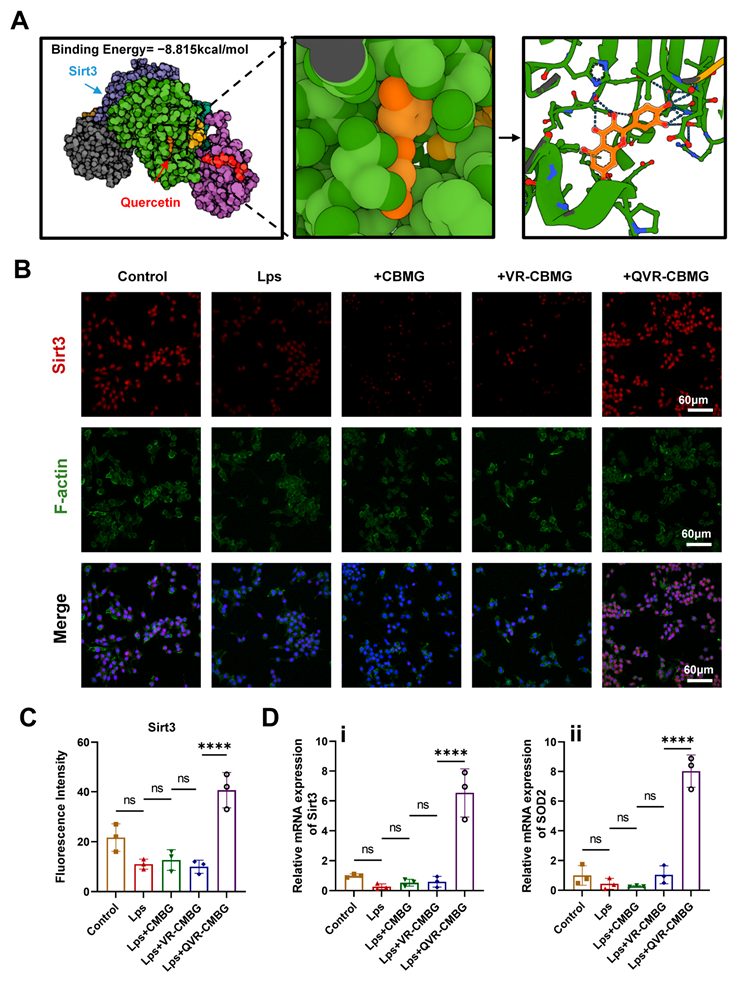
2.4. QVR-CBMG Hydrogel Activated the Sirtuin 3(SIRT3)/Superoxide Dismutase (SOD2) Pathway
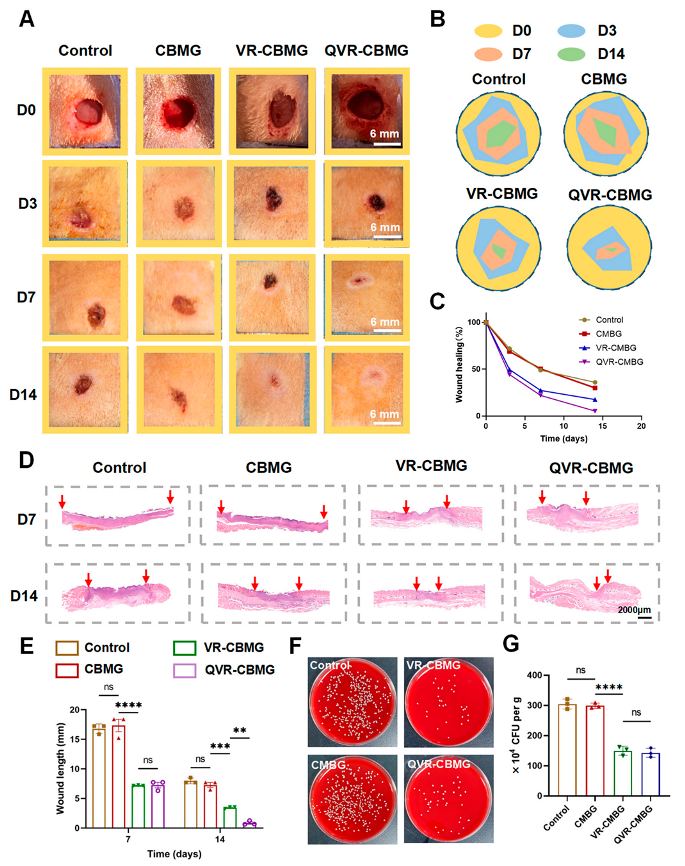
2.5. QVR-CBMG Hydrogel Accelerates Diabetic Infection Rat Wound Healing
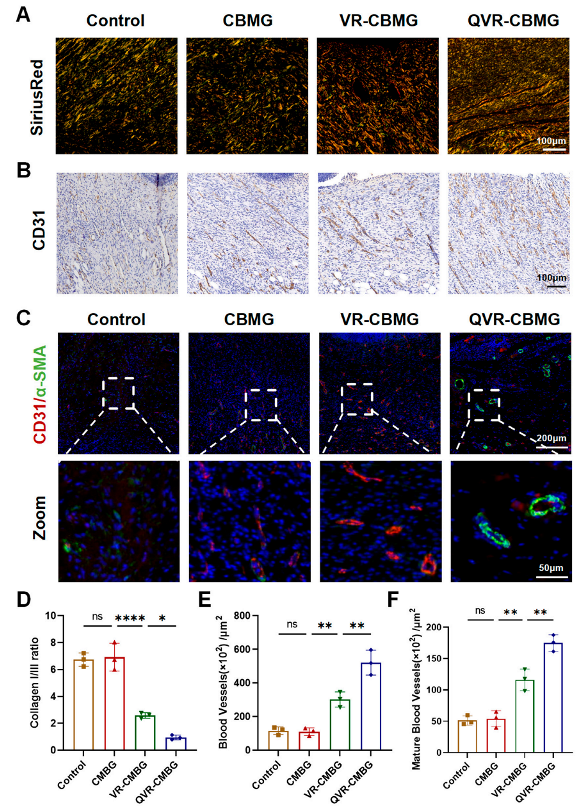
2.6. QVR-CBMG Hydrogel Promoted Collagen Repair and Angiogenesis In Vivo
2.7. QVR-CBMG Hydrogel Facilitates M2 Polarization of Macrophages In Vivo
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.3. Scanning Electron Microscope (SEM) Analysis
4.4. Rheological Experiment
4.5. Cell Biocompatibilitye
4.6. Macrophages Extraction and Culture
4.7. Immunofluorescence (IF) Staining
4.8. Western Blot (WB) Assay
4.9. Quantitative Polymerase Chain Reaction (qPCR)
4.10. Antimicrobial Activity Tests In Vitro
4.11. Rats Model and Treatment
4.12. Histological and Immunostaining Analyses
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, X.; Zhong, Y.; Yang, P.; Gao, P.; Wu, X.; Wang, X.; An, W. Exosomal ncRNAs: The pivotal players in diabetic wound healing. Front. Immunol. 2022, 13, 1005307. [Google Scholar] [CrossRef]
- Li, J.J.; Hu, Y.; Hu, B.; Wang, W.; Xu, H.; Hu, X.Y.; Ding, F.; Li, H.B.; Wang, K.R.; Zhang, X.; et al. Lactose azo calixarene drug delivery system for the treatment of multidrug-resistant pseudomonas aeruginosa infected diabetic ulcer. Nat. Commun. 2022, 13, 6279. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Jiang, L.; Xia, C.; Zeng, L.; Cai, X.; Jin, Z.; Qin, S.; Ding, W.; He, Q. Sonocatalytic hydrogen/hole-combined therapy for anti-biofilm and infected diabetic wound healing. Natl. Sci. Rev. 2023, 10, nwad063. [Google Scholar] [CrossRef]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, H.; Tang, T.; Liu, L.; Pan, B.; Chen, J.; Cheng, D.; Cai, X.; Sun, Y.; Zhu, F.; et al. Tetrahedral framework nucleic acids promote diabetic wound healing via the Wnt signalling pathway. Cell Prolif. 2022, 55, e13316. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Z.; Ren, X.; Jia, B.; Li, G.; Zhou, S.; Zhao, X.; Wang, W. High-strength hydrogels: Fabrication, rein-forcement mechanisms, and applications. Nano Res. 2023, 16, 3475–3515. [Google Scholar] [CrossRef]
- Afsharipour, M.; Mahmoudi, S.; Raji, H.; Pourakbari, B.; Mamishi, S. Three-year evaluation of the nosocomial infections in pediatrics: Bacterial and fungal profile and antimicrobial resistance pattern. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 6. [Google Scholar] [CrossRef]
- Peek, J.; Lilic, M.; Montiel, D.; Milshteyn, A.; Woodworth, I.; Biggins, J.B.; Ternei, M.A.; Calle, P.Y.; Danziger, M.; Warrier, T.; et al. Rifamycin congeners kanglemycins are active against rifampicin-resistant bacteria via a distinct mechanism. Nat. Commun. 2018, 9, 4147. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, B.; Rahman, H.S.; Omer, K.M. Highly Luminescent and Biocompatible P and N Co-Doped Passivated Carbon Nanodots for the Sensitive and Selective Determination of Rifampicin Using the Inner Filter Effect. Materials 2020, 13, 2275. [Google Scholar] [CrossRef] [PubMed]
- Almasri, D.; Dahman, Y. Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option. Pharmaceutics 2023, 15, 1401. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fu, L.; Zou, H.; He, Y.; Pan, Y.; Ye, L.; Huang, Y.; Fan, W.; Zhang, J.; Ma, Y.; et al. Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnol. 2023, 21, 176. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Z.; Bu, P.; Yu, T.; Endo, Y.; Zhou, W.; Sun, Y.; Cao, F.; Dai, G.; Hu, Y.; et al. A Whole-Course-Repair System Based on Neurogenesis-Angiogenesis Crosstalk and Macrophage Reprogramming Promotes Diabetic Wound Healing. Adv. Mater. 2023, 35, e2212300. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. J. Lab. Clin. Med. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, K.; Yu, F.; Shen, L.; Wang, F.; Yang, J.; Su, C. Field application of nanoliposomes delivered quercetin by inhibiting specific hsp70 gene expression against plant virus disease. J. Nanobiotechnol. 2022, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Liu, Y.; Jiang, C.; Shen, Y.; Chu, G.; Liu, C.; Jiang, L.; Huang, G.; Qin, Y.; Zhang, Y.; et al. Arbutin-modified microspheres prevent osteoarthritis progression by mobilizing local anti-inflammatory and antioxidant responses. Mater. Today Bio 2022, 16, 100370. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chu, G.; Jin, J.; Liu, C.; Cheng, L.; Guo, Y.; Deng, Z.; Wang, Y. A Combined Cyanine/Carbomer Gel Enhanced Photodynamic Antimicrobial Activity and Wound Healing. Nanomaterials 2022, 12, 2173. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, G.; Zhang, Y.H.; Chen, F.Q.; Weng, H.F.; Xiao, A.F. Agarose Stearate-Carbomer(940) as Stabilizer and Rheology Modifier for Surfactant-Free Cosmetic Formulations. Mar. Drugs 2021, 19, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an antioxidant and hemostatic function for wound healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, S.; Zhang, J.; Zhang, S.; Huang, Y.; Zhu, H.; Li, Y.; Qi, B. Controlled release of curcumin from gelatin hydrogels by the molecular-weight modulation of an oxidized dextran cross-linker. Food Chem. 2023, 418, 135966. [Google Scholar] [CrossRef]
- Tang, X.; Wang, X.; Sun, Y.; Zhao, L.; Li, D.; Zhang, J.; Sun, H.; Yang, B. Magnesium Oxide-Assisted Dual-Cross-Linking Bio-Multifunctional Hydrogels for Wound Repair during Full-Thickness Skin Injuries. Adv. Funct. Mater. 2021, 31, 2105718. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.W.; Hu, X.K.; et al. Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef]
- Wang, T.; Liao, Q.; Wu, Y.; Wang, X.; Fu, C.; Geng, F.; Qu, Y.; Zhang, J. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int. J. Biol. Macromol. 2020, 164, 3846–3857. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, M.; Wang, C.; Xue, Y.; Huang, H.; Chen, Q.; Sun, W.; Zhou, X.; Xu, G.; Jiang, X. Dual-Cross-Linked Hydrogel Patch for Promoting Diabetic Wound Healing. Small 2022, 18, e2106172. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Shi, Y.F.; Wang, L.Y.; Zhao, Y.F.; Wang, R.K.; Li, K.; Zhang, S.T.; Zha, X.J.; Wang, W.; Zhao, X.; et al. All-Natural Immunomodulatory Bioadhesive Hydrogel Promotes Angiogenesis and Diabetic Wound Healing by Regulating Macrophage Heterogeneity. Adv. Sci. 2023, 10, e2206771. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Paschalidi, P.; Gkouveris, I.; Soundia, A.; Kalfarentzos, E.; Vardas, E.; Georgaki, M.; Kostakis, G.; Erovic, B.M.; Tetradis, S.; Perisanidis, C.; et al. The role of M1 and M2 macrophage polarization in progression of medication-related osteonecrosis of the jaw. Clin. Oral Investig. 2021, 25, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, H.; Wang, Z.; Zhang, C.; Bian, Y.; Liu, X.; Zhang, C.; Zhang, X.; Zhao, Y. Quercetin promotes in vitro maturation of oocytes from humans and aged mice. Cell Death Dis. 2020, 11, 965. [Google Scholar] [CrossRef]
- Chen, W.J.; Cheng, Y.; Li, W.; Dong, X.K.; Wei, J.L.; Yang, C.H.; Jiang, Y.H. Quercetin Attenuates Cardiac Hypertrophy by Inhibiting Mitochondrial Dysfunction through SIRT3/PARP-1 Pathway. Front. Pharmacol. 2021, 12, 739615. [Google Scholar] [CrossRef]
- Scher, M.B.; Vaquero, A.; Reinberg, D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007, 21, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. Yi Chuan Xue Bao 2022, 49, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kurundkar, D.; Kurundkar, A.R.; Bone, N.B.; Becker, E.J., Jr.; Liu, W.; Chacko, B.; Darley-Usmar, V.; Zmijewski, J.W.; Thannickal, V.J. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 2019, 4, e120722. [Google Scholar] [CrossRef]
- Klimova, N.; Fearnow, A.; Long, A.; Kristian, T. NAD(+) precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Exp. Neurol. 2020, 325, 113144. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.M.; denDekker, A.D.; Davis, F.M.; Joshi, A.; Kimball, A.S.; Schaller, M.; Allen, R.; Bermick, J.; Nycz, D.; Skinner, M.E.; et al. SIRT3 Regulates Macrophage-Mediated Inflammation in Diabetic Wound Repair. J. Investig. Dermatol. 2019, 139, 2528–2537.e2. [Google Scholar] [CrossRef]
- Yang, S.; Xu, M.; Meng, G.; Lu, Y. SIRT3 deficiency delays diabetic skin wound healing via oxidative stress and necroptosis enhancement. J. Cell Mol. Med. 2020, 24, 4415–4427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell Mol. Med. 2020, 24, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chang, L.; Hu, Y.; Zhao, X.; Huang, S.; Chen, Z.; Ren, X.; Mei, X. Tea polyphenol modified, photothermal responsive and ROS generative black phosphorus quantum dots as nanoplatforms for promoting MRSA infected wounds healing in diabetic rats. J. Nanobiotechnol. 2021, 19, 362. [Google Scholar] [CrossRef]
- Carmona, G.A.; Hoffmeyer, P.; Herrmann, F.R.; Vaucher, J.; Tschopp, O.; Lacraz, A.; Vischer, U.M. Major lower limb amputations in the elderly observed over ten years: The role of diabetes and peripheral arterial disease. Diabetes Metab. 2005, 31, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Madsen, D.H.; Hansen, M.; Schmidt, H.; Svane, I.M.; Karsdal, M.A.; Willumsen, N. Non-invasive biomarkers derived from the extracellular matrix associate with response to immune checkpoint blockade (anti-CTLA-4) in metastatic melanoma patients. J. Immunother. Cancer 2018, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, G.; Huang, H.; Wang, K.; Wang, H.; Lang, M.; Gao, H.; Zhao, S. Sequential Release of Small Extracellular Vesicles from Bilayered Thiolated Alginate/Polyethylene Glycol Diacrylate Hydrogels for Scarless Wound Healing. ACS Nano 2021, 15, 6352–6368. [Google Scholar] [CrossRef] [PubMed]
- Cakin, M.C.; Ozdemir, B.; Kaya-Dagistanli, F.; Arkan, H.; Bahtiyar, N.; Anapali, M.; Akbas, F.; Onaran, I. Evaluation of the in vivo wound healing potential of the lipid fraction from activated platelet-rich plasma. Platelets 2020, 31, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Zhou, Y.; He, Y.; Lin, S.; Zeng, Y.; Zhou, Y.; Li, W.; He, Z.; Zhao, Q.; et al. Antioxidant-enriched autologous biogel promoted diabetic wound healing by remodeling inherent posttraumatic inflammatory patterning and restoring compromised microenvironment homeostasis. Regen. Biomater. 2022, 9, rbac023. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, L.; Liu, B.; Jiang, N.; Huang, H.; Luo, J.; Wang, L.; Zeng, J.; Huang, F.; Huang, M.; et al. The Emerging Role of Immune Cells and Targeted Therapeutic Strategies in Diabetic Wounds Healing. J. Inflamm. Res. 2022, 15, 4119–4138. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Jin, J.; Zhang, C.; Gong, F.; Hu, B.; Wu, X.; Guan, M.; Xia, D. Multifunctional Drugs-Loaded Carbomol Hydrogel Promotes Diabetic Wound Healing via Antimicrobial and Immunoregulation. Gels 2023, 9, 761. https://doi.org/10.3390/gels9090761
Wang H, Jin J, Zhang C, Gong F, Hu B, Wu X, Guan M, Xia D. Multifunctional Drugs-Loaded Carbomol Hydrogel Promotes Diabetic Wound Healing via Antimicrobial and Immunoregulation. Gels. 2023; 9(9):761. https://doi.org/10.3390/gels9090761
Chicago/Turabian StyleWang, Hehui, Jiale Jin, Chi Zhang, Fangyi Gong, Baiwen Hu, Xiaochuan Wu, Ming Guan, and Dongdong Xia. 2023. "Multifunctional Drugs-Loaded Carbomol Hydrogel Promotes Diabetic Wound Healing via Antimicrobial and Immunoregulation" Gels 9, no. 9: 761. https://doi.org/10.3390/gels9090761




