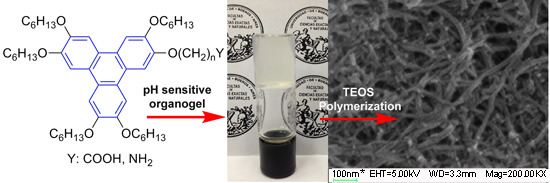
Supramolecular Assembly of pH-Sensitive Triphenylene Derived π-Gelators and Their Application as Molecular Template for the Preparation of Silica Nanotubes
Abstract
:1. Introduction
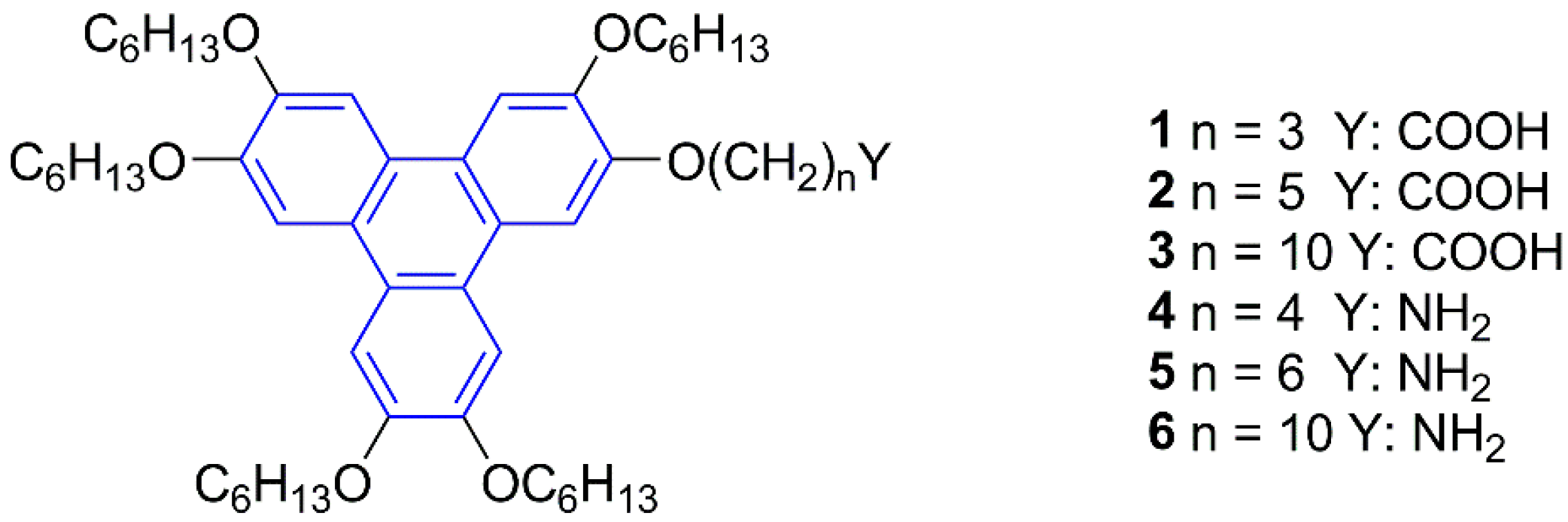
2. Results and Discussion
2.1. Gelation Scope and Thermal Stability
| Entry | Solvent | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1 | n-Hexane | I | I | I | I | I | G (0.25) |
| 2 | Toluene | S | S | S | S | S | G (0.35) |
| 3 | Dichloromethane | S | S | S | S | S | S |
| 4 | Ethylether | S | S | S | S | S | S |
| 5 | Ethylacetate | S | S | S | S | S | S |
| 6 | Methanol | TG * (0.1) | TG * (0.2) | TG * (0.2) | I | TG * (0.5) | TG * (1.0) |
| 7 | Water | I | I | I | I | I | I |
| Entry | Solvent | Tgel (°C) | ε * |
|---|---|---|---|
| 1 | Methanol | 31 | 33.6 |
| 2 | Ethanol | 17 | 25.2 |
| 3 | n-Butanol | 8 | 18.2 |
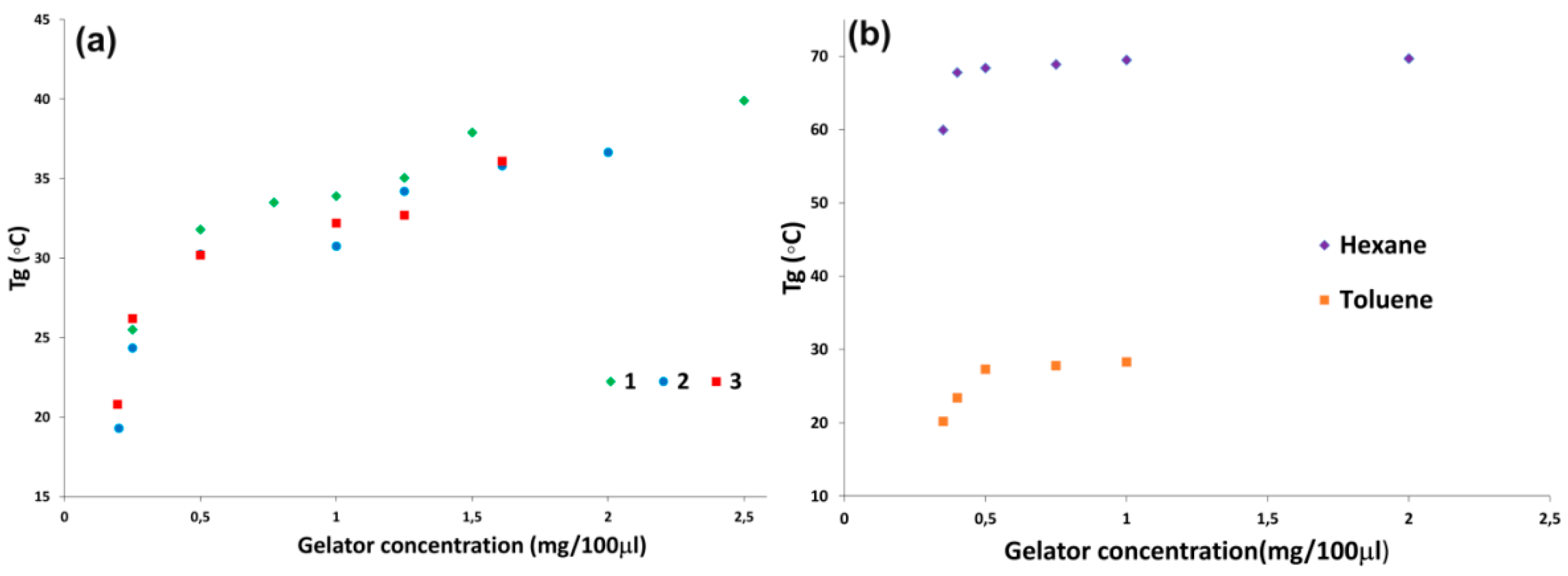
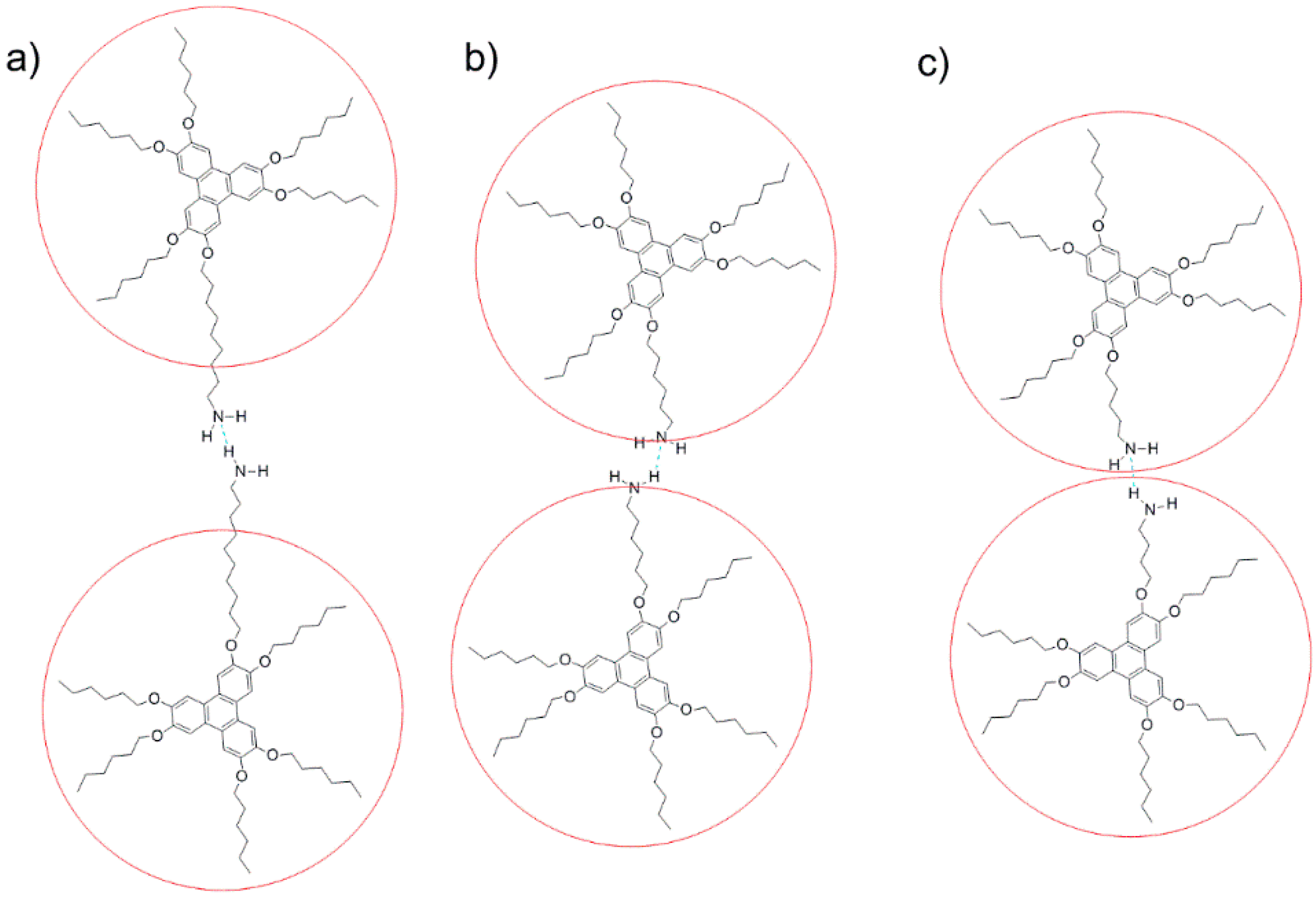
2.2. Morphology and Self-Assembly


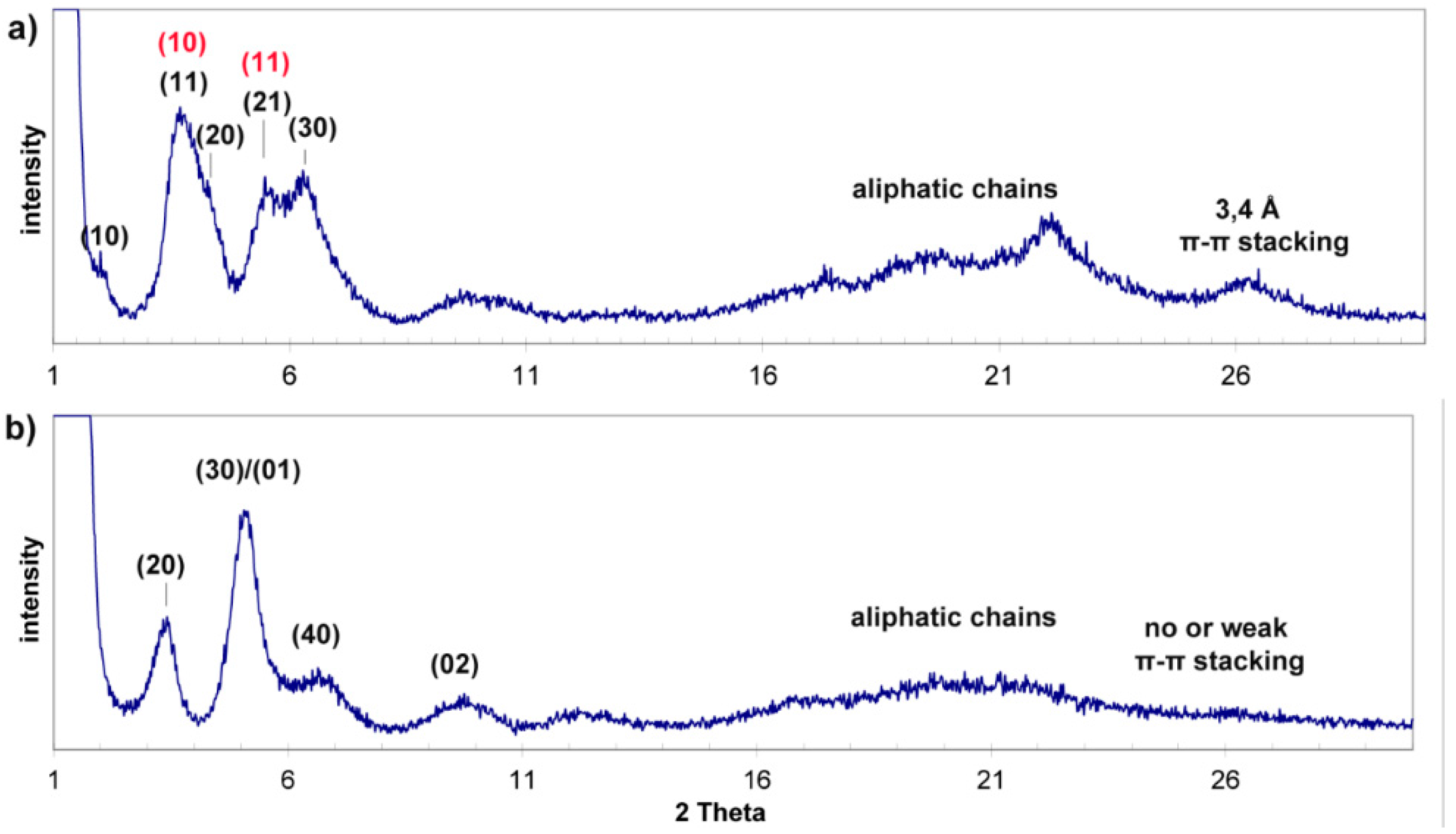
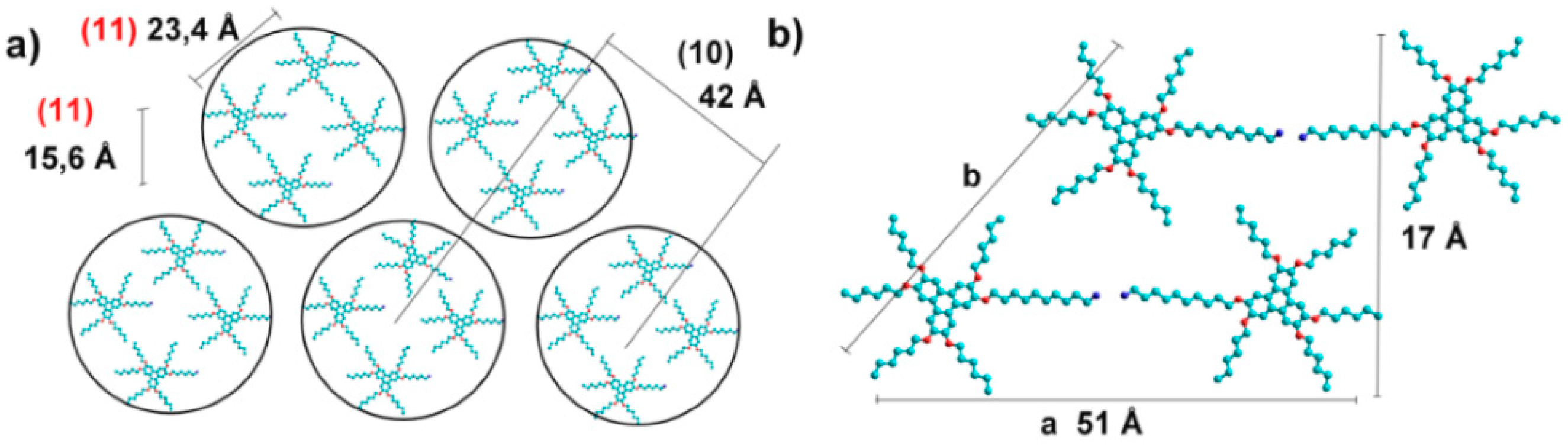
2.3. Effect of pH on Gelation
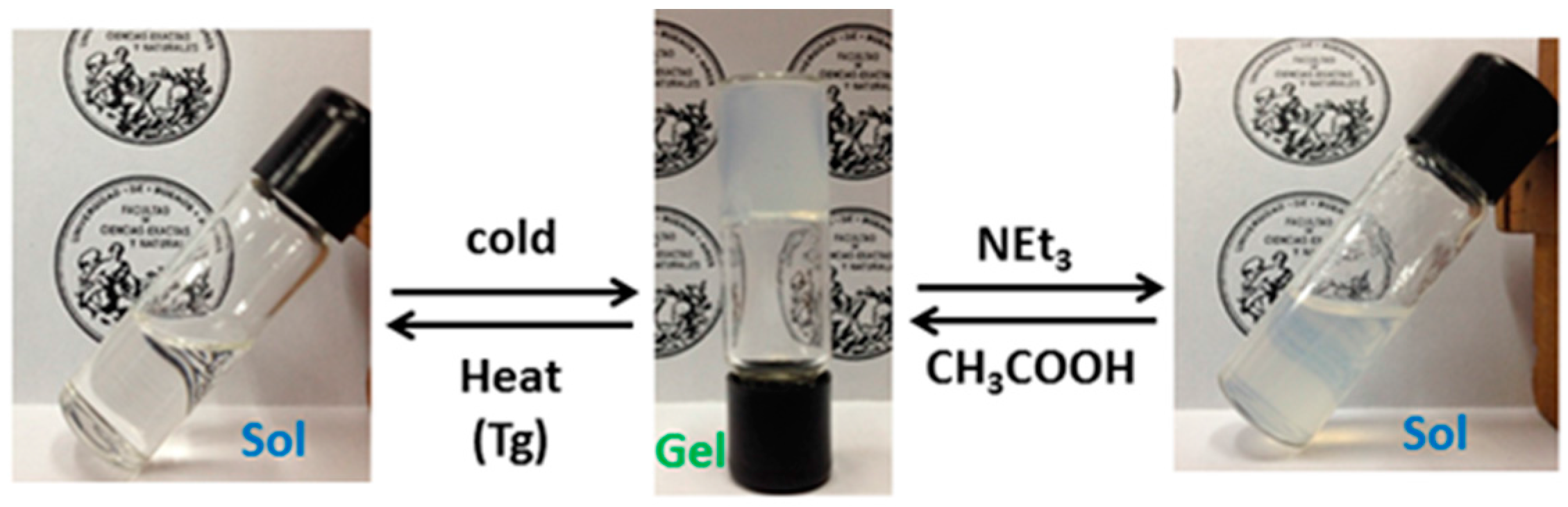
2.4. Self-Catalyzed Template Synthesis

3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Gelation Test
4.3. FT-IR Spectra
4.4. Xerogel Preparation
4.5. SEM Microscopy
4.6. In Situ Sol-Gel Polymerization Experiments
4.7. Fluorescence Experiments
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Segarra-Maset, M.D.; Nebot, V.J.; Miravet, J.F.; Escuder, B. Control of molecular gelation by chemical stimuli. Chem. Soc. Rev. 2013, 42, 7086–7098. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, Q.; He, T.; Li, Z.; Zhang, Y.; Yi, L. Multistimuli-Responsive Supramolecular Gels: Design Rationale, Recent Advances, and Perspectives. ChemPhysChem 2014, 12, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, R.; Díaz Díaz, D. Photophysical and photochemical Processes in 3D Self-assembled Gels as Confined Microenvironments. Soft Matter 2015, 11, 5180–5187. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, E.L.; Edelsztein, V.C.; Menéndez, G.O.; Samaniego López, C.; Spagnuolo, C.C.; Di Chenna, P.H. Versatile Supramolecular Organogel with Outstanding Stability toward Aqueous Interfaces. ACS Appl. Mater. Interfaces 2014, 6, 8933–8936. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Zhanga, M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: Towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef] [PubMed]
- Edelsztein, V.C.; Mac Cormack, A.S.; Ciarlantini, M.; Di Chenna, P.H. Self-assembly of 2,3-dihydroxycholestane steroids into supramolecular organogels as a soft template for the in-situ generation of silicate nanomaterials. Beilstein J. Org. Chem. 2013, 9, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Santhosh Babu, S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Müllen, K.; Scherf, U. Organic Light Emitting Devices; VCH: Weinheim, Germany, 2006. [Google Scholar]
- Mas-Torrent, M.; Rovira, C. Role of Molecular Order and Solid-State Structure in Organic Field-Effect Transistors. Chem. Rev. 2011, 111, 4833–4856. [Google Scholar] [CrossRef] [PubMed]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular semiconductors in organic photovoltaic cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pal, S.; Setia, S.; Avinash, B.S.; Kumar, S. Triphenylene-based discotic liquid crystals: Recent advances. Liq. Cryst. 2013, 40, 1769–1816. [Google Scholar]
- Funahashi, M. Development of Liquid-Crystalline Semiconductors with High Carrier Mobilities and Their Application to Thin-film Transistors. Polym. J. 2009, 41, 459–469. [Google Scholar] [CrossRef]
- Wöhrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J.C.; Staffeld, P.; Baro, A.; Giesselmann, F.; et al. Discotic Liquid Crystals. Chem. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mizoshita, N.; Monobe, H.; Inoue, M.; Ukon, M.; Watanabe, T.; Shimizu, Y.; Hanabusa, K.; Kato, T. The positive effect on hole transport behaviour in anisotropic gels consisting of discotic liquid crystals and hydrogen-bonded fibers. Chem. Commun. 2002, 5, 428–429. [Google Scholar] [CrossRef]
- Kato, T.; Hirai, Y.; Nakaso, S.; Moriyama, M. Liquid-crystalline physical gels. Chem. Soc. Rev. 2007, 36, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Zhao, K.Q.; Hu, P.; Wang, B.Q.; Shimizu, Y. Synthesis of Amide Group Containing Triphenylene Derivatives as Discotic Liquid Crystals and Organic Gelators. Mol. Cryst. Liq. Cryst. 2009, 509, 60[802]–76[818]. [Google Scholar] [CrossRef]
- Ikeda, M.; Takeuchi, M.; Shinkai, S. Unusual emission properties of a triphenylene-based organogel system. Chem. Commun. 2003, 12, 1354–1355. [Google Scholar] [CrossRef]
- Kimura, M.; Hatanaka, T.; Nomoto, H.; Takizawa, J.; Fukawa, T.; Tatewaki, Y.; Shirai, H. Self-Assembled Helical Nanofibers Made of Achiral Molecular Disks Having Molecular Adapter. Chem. Mater. 2010, 22, 5732–5738. [Google Scholar] [CrossRef]
- Kotlewski, A.; Norder, B.; Jager, W.F.; Pickena, S.J.; Mendes, E. Can morphological transitions in fibrils drive stiffness of gels formed by discotic liquid crystal organogelators? Soft Matter 2009, 5, 4905–4913. [Google Scholar] [CrossRef]
- Boden, N.; Bushby, R.J.; Cammidge, A.N.; El-Mansoury, A.; Martina, P.S.; Lu, Z. The creation of long-lasting glassy columnar discotic liquid crystals using ”dimeric” discogens. J. Mat. Chem. 1999, 9, 1391–1402. [Google Scholar] [CrossRef]
- Cecchi, F. Functionalized Mesogenic Triphenylenes: Structural Organizers of Polymeric Materials. PhD. Thesis, University of Buenos Aires, Buenos Aires, Argentine, 15 March 2012. [Google Scholar]
- Kong, X.; He, Z.; Zhang, Y.; Mu, L.; Liang, C.; Chen, B.; Jing, X.; Cammidge, A.N. A Mesogenic Triphenylene-Perylene-Triphenylene Triad. Org. Lett. 2011, 13, 764–767. [Google Scholar] [CrossRef]
- Gregory, A.P.; Clarke, R.N. Traceable measurements of the static permittivity of dielectric reference liquids over the temperature range 5–50 °C. Meas. Sci. Technol. 2005, 16, 1506–1516. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Monobe, H.; Wan, W.; Terasawa, N.; Kiyohara, K.; Nakamura, N.; Shimizu, Y. Infrared Spectral Studies of Triphenylene Mesogens Possessing Terminal Functional Groups in the Peripheral Chains for Hydrogen-Bond Interaction. Mol. Cryst. Liq. Cryst. 2004, 412, 1619–1628. [Google Scholar] [CrossRef]
- Miao, J.; Zhu, L. Topology Controlled Supramolecular Self-Assembly of Octa Triphenylene-Substituted Polyhedral Oligomeric Silsesquioxane Hybrid Supermolecules. J. Phys. Chem. B 2010, 114, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Llusar, M.; Sanchez, C. Inorganic and Hybrid Nanofibrous Materials Templated with Organogelators. Chem. Mater. 2008, 20, 782–820. [Google Scholar] [CrossRef]
- Ono, Y.; Kanekijo, Y.; Inoue, K.; Hojo, J.; Shinkai, S. Evidence for the Importance oa a Cationic Charge in the Formation of Hollow Silica from an Organic Gel System. Chem. Lett. 1999, 28, 23–24. [Google Scholar] [CrossRef]
- Edelsztein, V.C.; Burton, G.; Di Chenna, P.H. Self-assembly of a silylated steroid-based organogelator and its use as template for the in situ sol-gel polymerization of tetraethyl orthosilicate. Tetrahedron 2010, 66, 2162–2167. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Cipriano, B.H. Gel formation: Phase Diagrams using table top Rheology and Calorimetry. In Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Weiss, R.G., Terech, P., Eds.; Springer Verlag: Berlin, Germany, 2006. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz Resta, I.; Manzano, V.E.; Cecchi, F.; Spagnuolo, C.C.; Cukiernik, F.D.; Di Chenna, P.H. Supramolecular Assembly of pH-Sensitive Triphenylene Derived π-Gelators and Their Application as Molecular Template for the Preparation of Silica Nanotubes. Gels 2016, 2, 7. https://doi.org/10.3390/gels2010007
Muñoz Resta I, Manzano VE, Cecchi F, Spagnuolo CC, Cukiernik FD, Di Chenna PH. Supramolecular Assembly of pH-Sensitive Triphenylene Derived π-Gelators and Their Application as Molecular Template for the Preparation of Silica Nanotubes. Gels. 2016; 2(1):7. https://doi.org/10.3390/gels2010007
Chicago/Turabian StyleMuñoz Resta, Ignacio, Verónica E. Manzano, Florencia Cecchi, Carla C. Spagnuolo, Fabio D. Cukiernik, and Pablo H. Di Chenna. 2016. "Supramolecular Assembly of pH-Sensitive Triphenylene Derived π-Gelators and Their Application as Molecular Template for the Preparation of Silica Nanotubes" Gels 2, no. 1: 7. https://doi.org/10.3390/gels2010007