Preparation and Performance Evaluation of Ionic Liquid Copolymer Shale Inhibitor for Drilling Fluid Gel System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PIL
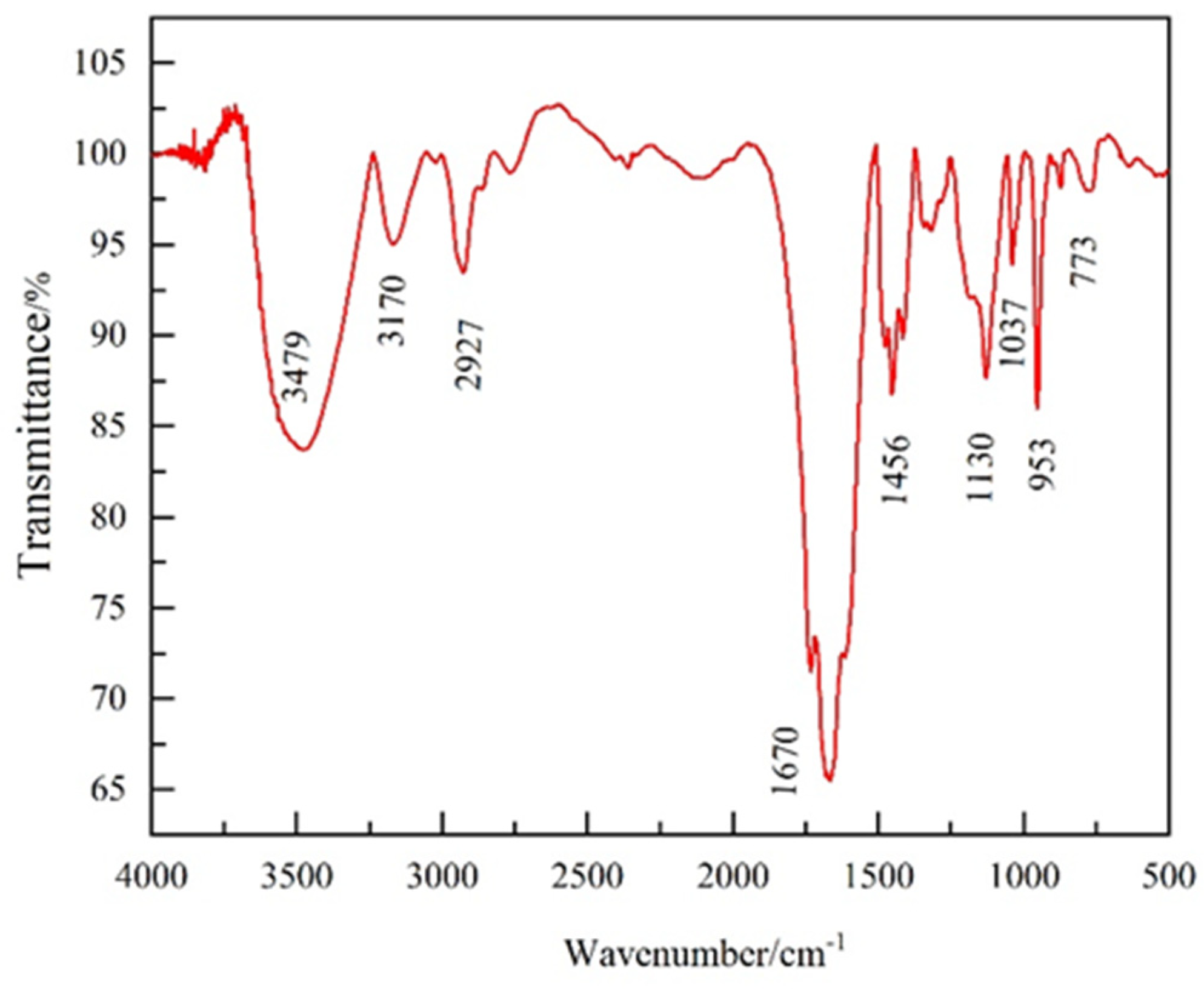
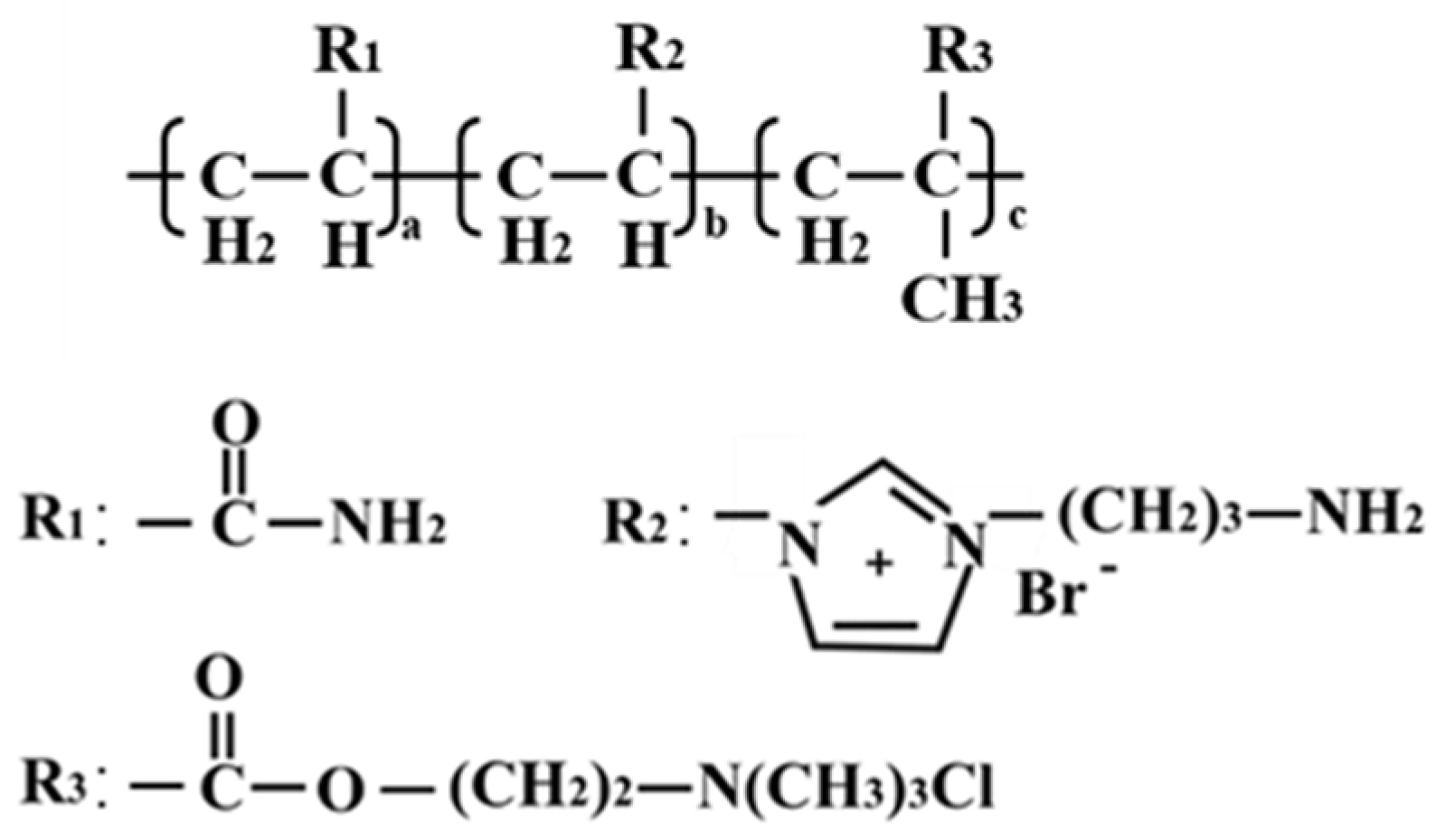
2.1.1. Chemical Structure
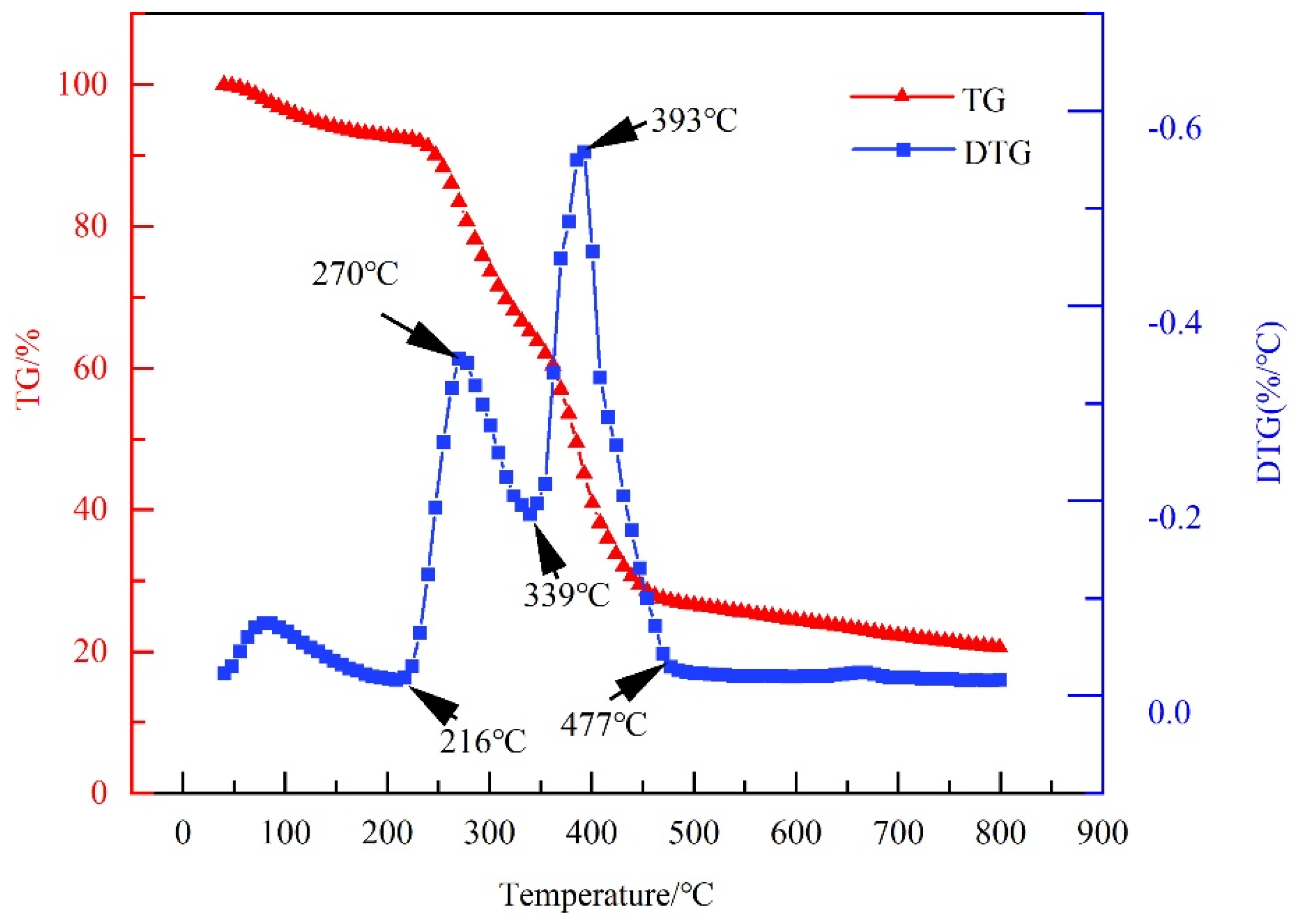
2.1.2. Thermal Stability
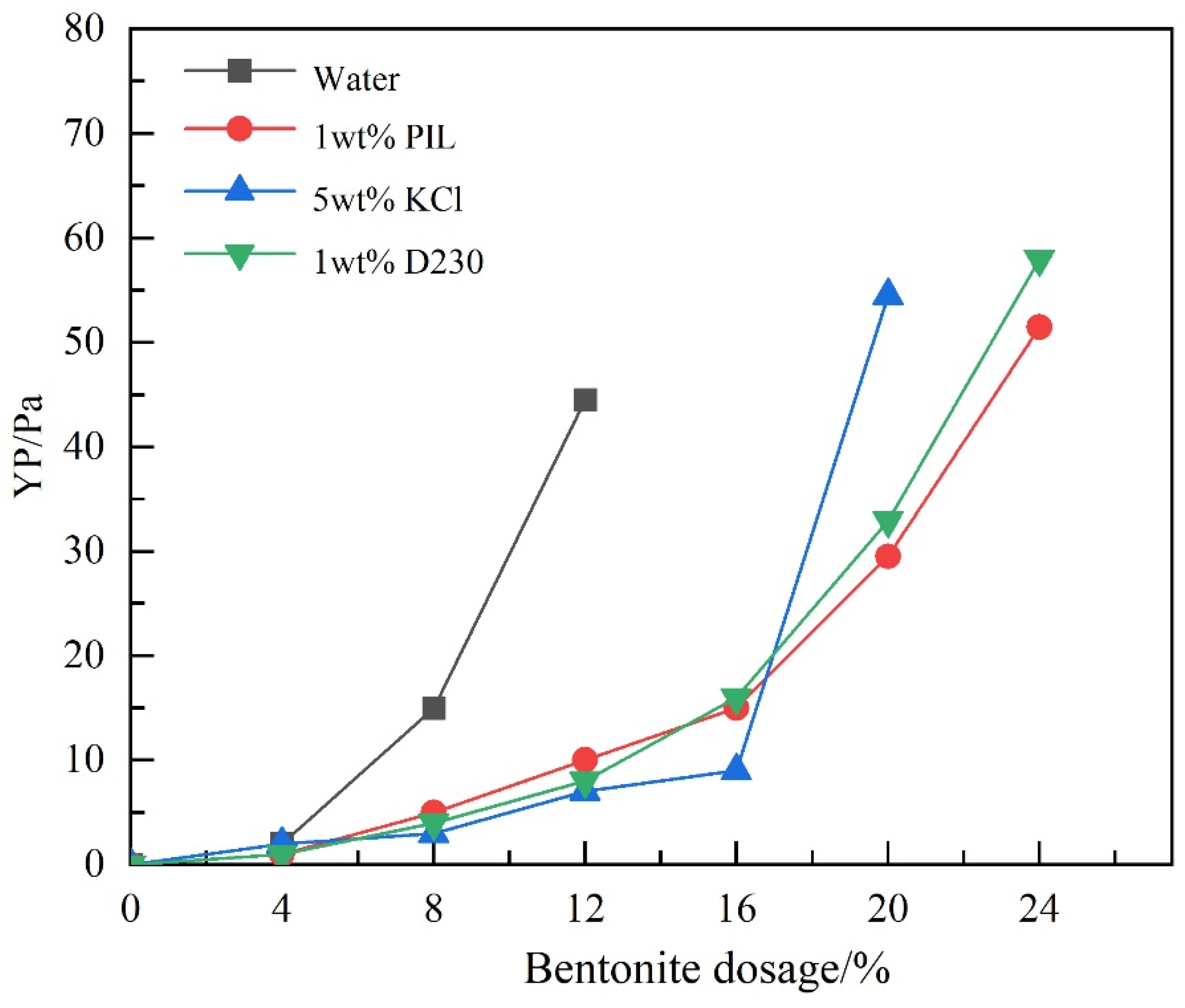
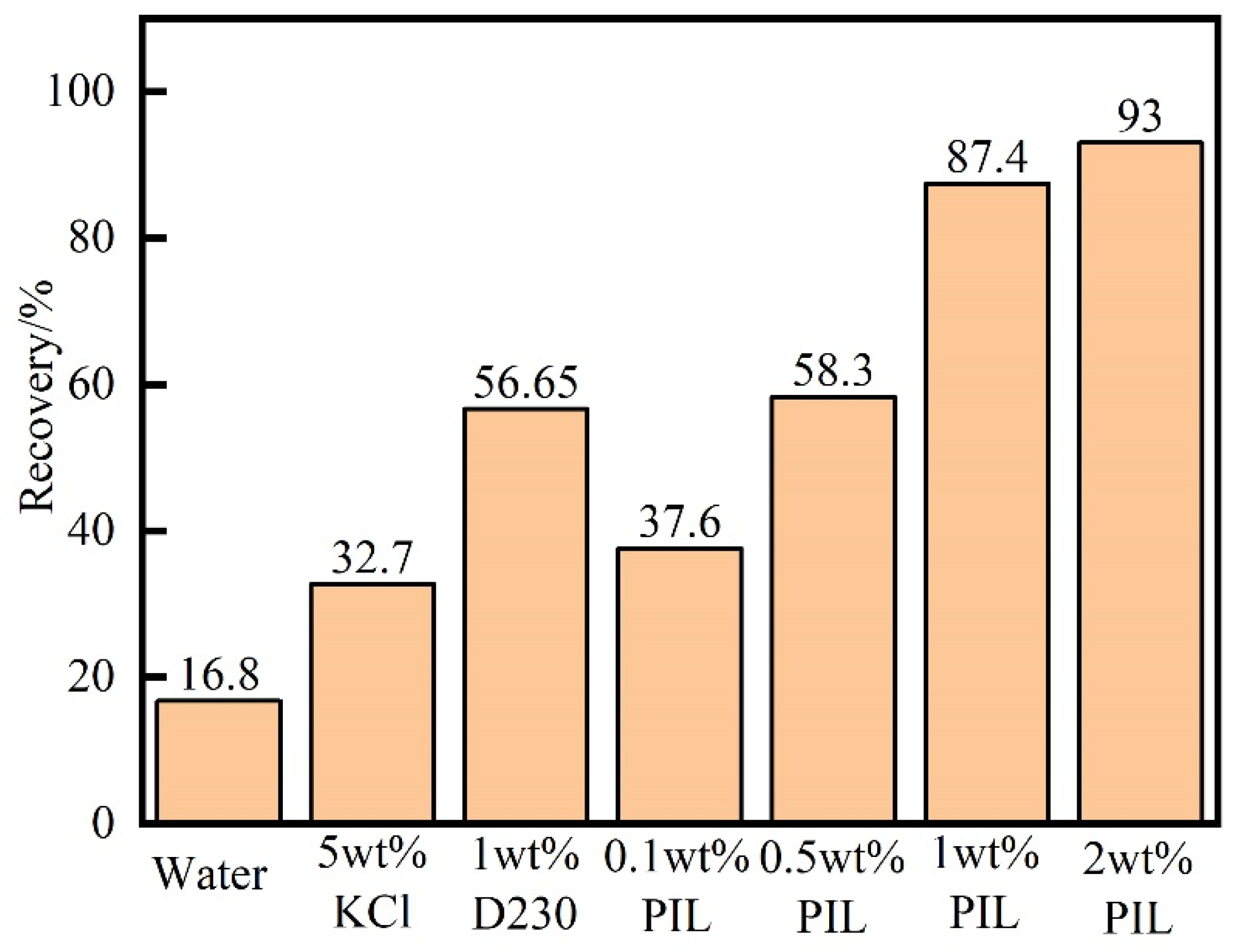
2.2. Inhibition Performance of PIL
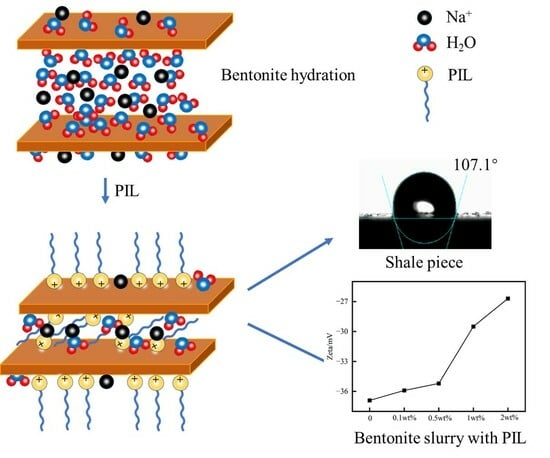
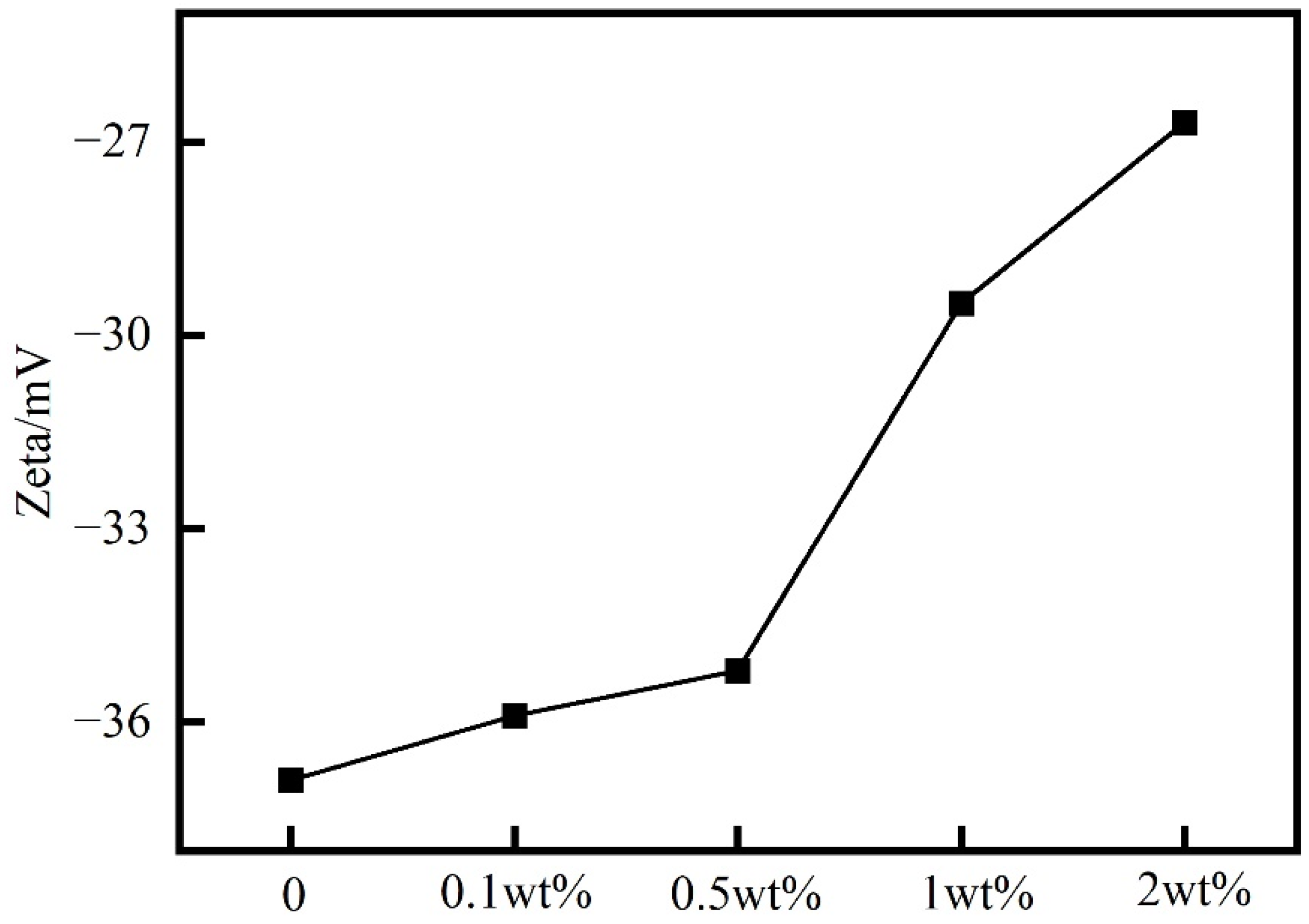
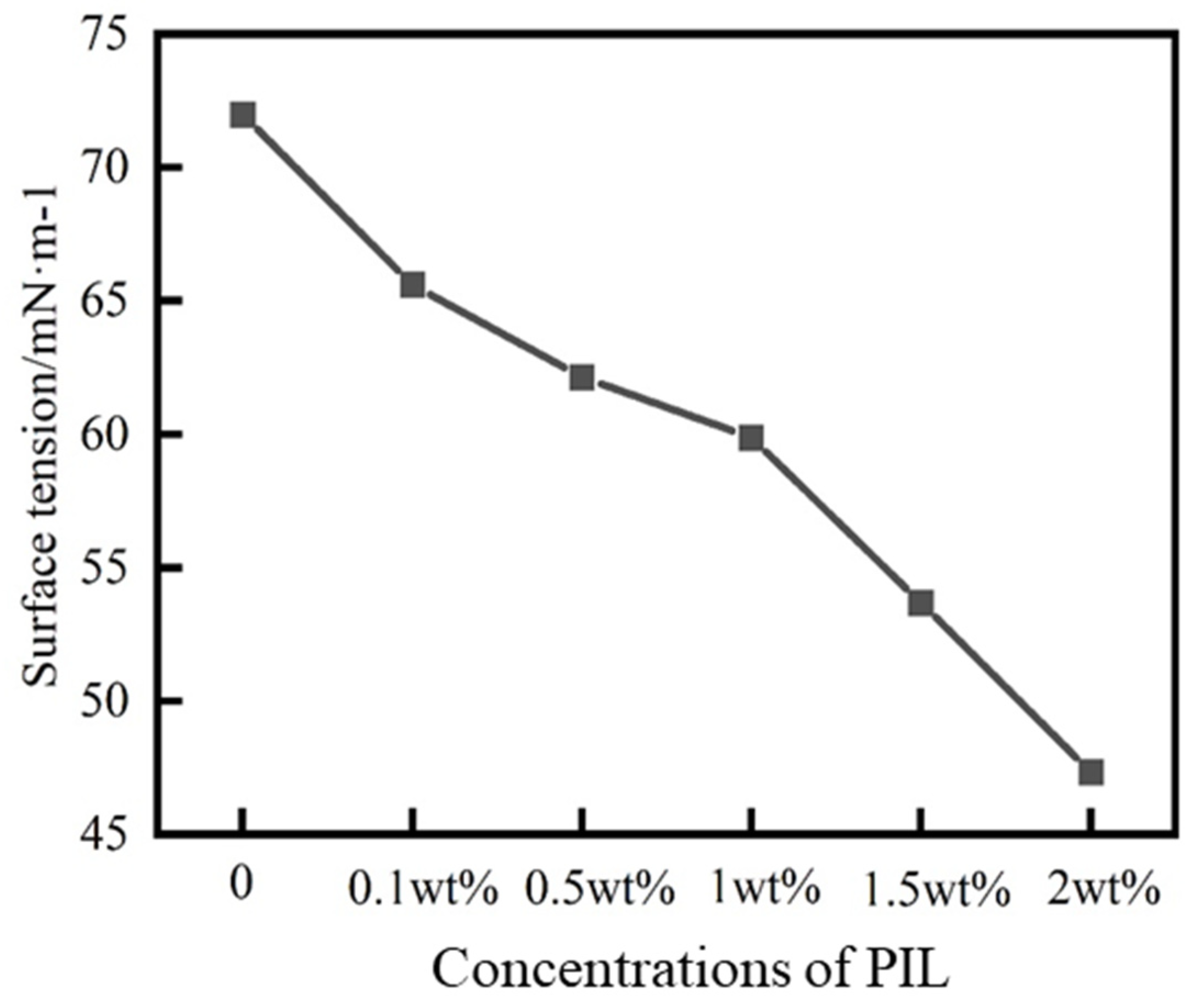
2.3. Analysis of PIL Inhibition Mechanism
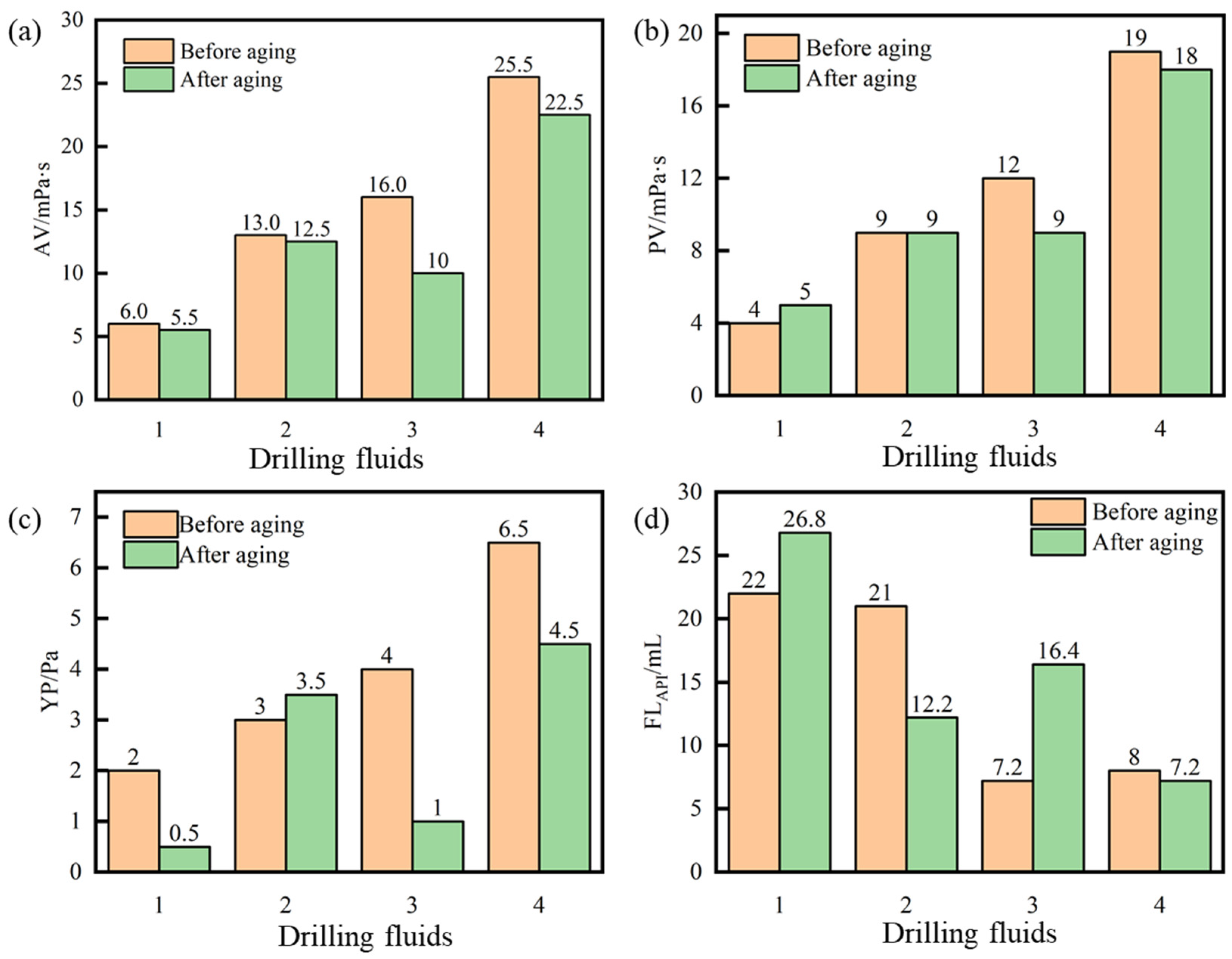
2.4. Compatibility with Drilling Fluid
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PIL
4.3. Characterization of PIL
4.3.1. FT-IR Analysis
4.3.2. H-NMR Spectra Analysis
4.3.3. Thermogravimetric Analysis
4.4. Performance Evaluation of PIL
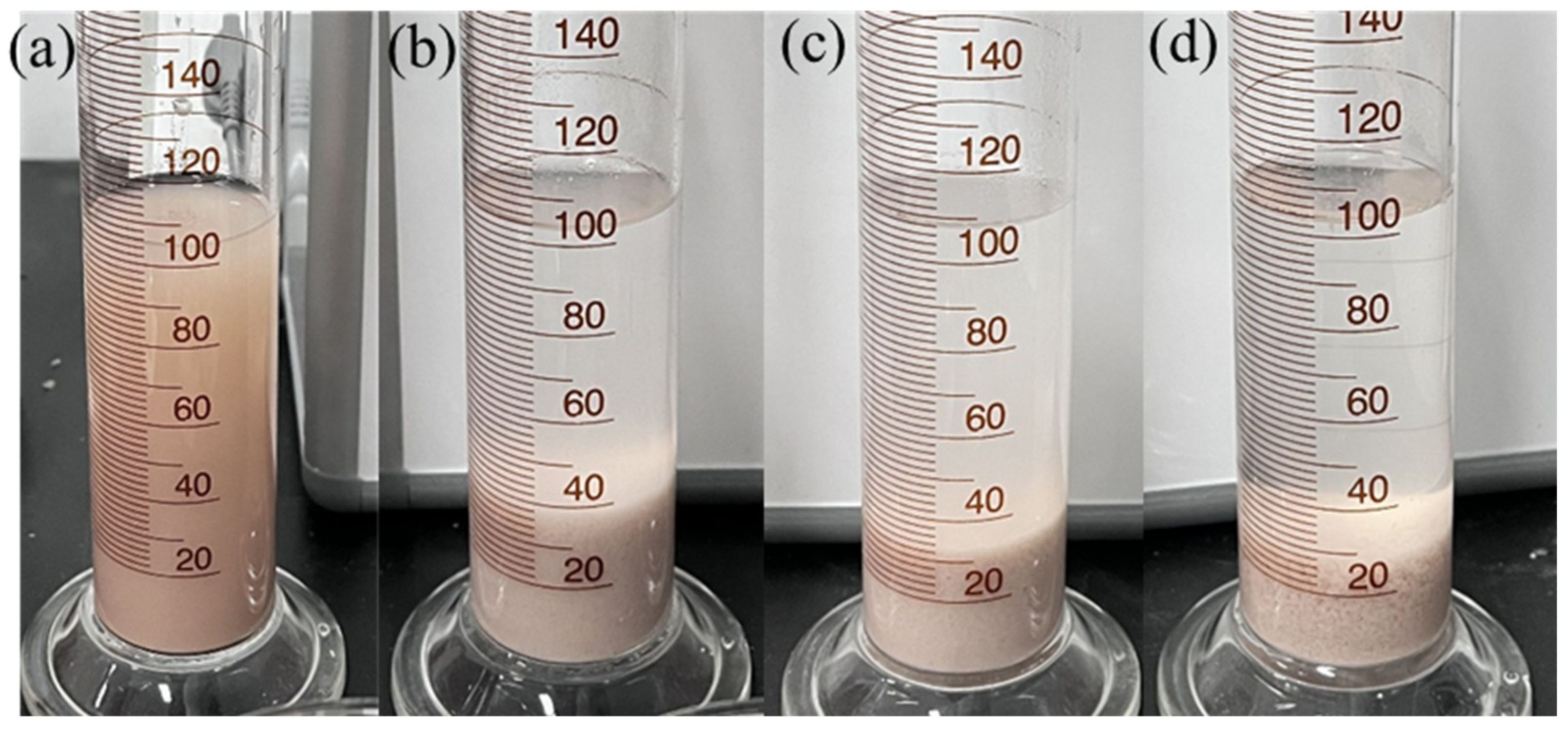
4.4.1. Bentonite Slurrying Test
4.4.2. Bentonite Flocculation Test
4.4.3. Linear Expansion Test
4.4.4. Rolling Recovery Test
4.5. Mechanism Analysis
4.5.1. Scanning Electron Microscope (SEM) and Surface Elemental Analysis
4.5.2. Zeta Potential Test
4.5.3. Particle Size Test
4.5.4. Contact Angle Test
4.5.5. Surface Tension Test
4.6. Compatibility with Drilling Fluid
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, Y.; Bai, J.; Li, X.; Chen, M.; You, L.; Li, X.; Li, Q.; Fang, D. Influence of water-rock interaction on stress sensitivity of organic-rich shales: A case study from Longmaxi formation in the southeast area of Chongqing. Reserv. Eval. Dev. 2019, 9, 54–62. [Google Scholar]
- Liu, X.; Zhuang, Y.; Liang, L.; Xiong, J. Investigation on the Influence of Water-Shale Interaction on Stress Sensitivity of Organic-Rich Shale. Geofluids 2019, 2019, 2598727. [Google Scholar] [CrossRef]
- Ding, W.L.; Li, C.; Li, C.Y.; Xu, C.C.; Jiu, K.; Zeng, W.T.; Wu, L.M. Fracture development in shale and its relationship to gas accumulation. Geosci. Front. 2012, 3, 97–105. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Geng, D.; Li, Z. A new technology of plugging and collapse prevention with oil-based drilling fluid in Chongqing shale gas wells. Reserv. Eval. Dev. 2021, 11, 527–535. [Google Scholar]
- Shen, Z.; Zhang, H.; Yu, X.; Wang, M.; Gao, C.; Li, S.; Zhang, H. Experimental optimization of high-temperature-resistant and low oil-Water ratio high-density oil-based drilling fluid. Processes 2023, 11, 1129. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M. Polymeric and low molecular weight shale inhibitors: A review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Aramendiz, J.; Imqam, A. Water-based drilling fluid formulation using silica and graphene nanoparticles for unconventional shale applications. J. Pet. Sci. Eng. 2019, 179, 742–749. [Google Scholar] [CrossRef]
- Hosseini, S.; Vaferi, B. Determination of methanol loss due to vaporization in gas hydrate inhibition process using intelligent connectionist paradigms. Arab. J. Sci. Eng. 2022, 47, 5811–5819. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zeng, J.; Leong, Y.-K.; Elsworth, D.; Tian, J. Water Liberating/Sealing effects on shale gas Extraction: A fully coupled multidomain and multiphysics model. Fuel 2022, 325, 124953. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, J.; Zhang, W.; Zhang, D. Nanopore structure and nanomechanical properties of organic-rich terrestrial shale: An insight into technical issues for hydrocarbon production. Nano Energy 2020, 69, 104426. [Google Scholar] [CrossRef]
- Naeimavi, M.; Khazali, F.; Abdideh, M.; Saadati, Z. Potassium sorbate left-to-right markas substitute for KCl to shale left-to-right markinhibition in water-base drilling fluids. Energy Sources Part A-Recovery Util. Environ. Eff. 2021, 43, 1691–1705. [Google Scholar] [CrossRef]
- Rana, A.; Arfaj, M.K.; Saleh, T.A. Advanced developments in shale inhibitors for oil production with low environmental footprints—A review. Fuel 2019, 247, 237–249. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ibrahim, M.A. Advances in functionalized Nanoparticles based drilling inhibitors for oil production. Energy Rep. 2019, 5, 1293–1304. [Google Scholar] [CrossRef]
- Murtaza, M.; Khan, R.A.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M. Poly(Oxyethylene)-amidoamine Based Gemini Cationic Surfactants with Hydrophilic Spacers as Clay Stabilizers. Energy Fuels 2020, 34, 10619–10630. [Google Scholar] [CrossRef]
- Rana, A.; Saleh, T.A. An investigation of polymer-modified activated carbon as a potential shale inhibitor for water-based drilling muds. J. Pet. Sci. Eng. 2022, 216, 110763. [Google Scholar] [CrossRef]
- Alizadeh, M.; Nodehi, M.; Salmanpour, S.; Karimi, F.; Sanati, A.L.; Malekmohammadi, S.; Zakariae, N.; Esmaeili, R.; Jafari, H. Properties and recent advantages of N,N′-dialkylimidazolium-ion liquids application in electrochemistry. Curr. Anal. Chem. 2022, 18, 31–52. [Google Scholar] [CrossRef]
- Mallah, M.H.; Shemirani, F.; Maragheh, M.G.; Jamali, M.R. Evaluation of synergism in dispersive liquid-liquid microextraction for simultaneous preconcentration of some lanthanoids. J. Mol. Liq. 2010, 151, 122–124. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Catalytic conversion of carbohydrate biomass in Ionic liquids to 5-Hydroxymethyl furfural and levulinic acid: A Review. Bioenergy Res. 2020, 13, 693–736. [Google Scholar] [CrossRef]
- Hasnul, M.H.; Mohd Zulkifli, N.W.; Hassan, M.; Zulkifli, S.A.; Mohd Yusoff, M.N.A.; Zakaria, M.Z. Synergistic behavior of graphene and ionic liquid as bio-based lubricant additive. Lubricants 2021, 9, 46. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef]
- Semnani, R.H.; Salehi, M.B.; Mokhtarani, B.; Sharifi, A.; Mirzaei, M.; Taghikhani, V. Evaluation of the interfacial activity of imidazolium-based ionic liquids and their application in enhanced oil recovery process. J. Mol. Liq. 2022, 362, 119735. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, G.; Shi, Y.; Yang, X. Application of ionic liquid and polymeric ionic liquid as shale hydration inhibitors. Energy Fuels 2017, 31, 4308–4317. [Google Scholar] [CrossRef]
- Rahman, M.T.; Negash, B.M.; Danso, D.K.; Idris, A.; Elryes, A.A.; Umar, I.A. Effects of imidazolium- and ammonium-based ionic liquids on clay swelling: Experimental and simulation approach. J. Pet. Explor. Prod. Technol. 2022, 12, 1841–1853. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M. Epsom Salt-based natural deep eutectic solvent as a drilling fluid additive: A game-changer for shale swelling inhibition. Molecules 2023, 28, 5784. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Dai, J.; Song, J.; Zhang, F.; Yan, H.; Lv, K. Study of a gemini surface active ionic liquid 1,2-bis(3-hexylimidazolium-1-yl) ethane bromide as a high performance shale inhibitor and inhibition mechanism. J. Mol. Liq. 2020, 301, 112401. [Google Scholar] [CrossRef]
- Ren, Y.; Zhai, Y.; Wu, L.; Zhou, W.; Qin, H.; Wang, P. Amine- and alcohol-functionalized ionic liquids: Inhibition difference and application in water-based drilling fluids for wellbore stability. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 609, 125678. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Wang, T.; Jiang, G.; Luckham, P.F.; Li, X.; Shi, H.; Luo, J. Effect of alkyl chain length on shale hydration inhibitive performance of vinylimidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2019, 58, 8565–8577. [Google Scholar] [CrossRef]
- Hosseinzadeh, F.; Mahkam, M.; Galehassadi, M. Synthesis and characterization of ionic liquid functionalized polymers for drug delivery of an anti-inflammatory drug. Des. Monomers Polym. 2012, 15, 379–388. [Google Scholar] [CrossRef]
- Sengupta, A.; Ethirajan, S.K.; Kamaz, M.; Jebur, M.; Wickramasinghe, R. Synthesis and characterization of antibacterial poly ionic liquid membranes with tunable performance. Sep. Purif. Technol. 2019, 212, 307–315. [Google Scholar] [CrossRef]
- Baoqing, Z.; Jinwen, Q. Synthesis and characterization of star poly(acrylamide-methacryloyloxyethyl trimethyl ammonium chloride)copolymer and its application in flocculation. J. Zhejiang Univ. Sci. Ed. 2007, 34, 443–447. [Google Scholar]
- Nellepalli, P.; Tome, L.C.; Vijayakrishna, K.; Marrucho, I.M. Imidazolium-based copoly(ionic liquid) membranes for CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 2017–2026. [Google Scholar] [CrossRef]
- Da Camara, P.C.F.; Madruga, L.Y.C.; Marques, N.d.N.; Balaban, R.C. Evaluation of polymer/bentonite synergy on the properties of aqueous drilling fluids for high-temperature and high-pressure oil wells. J. Mol. Liq. 2021, 327, 114808. [Google Scholar] [CrossRef]
- Siddiqui, M.A.Q.; Ali, S.; Fei, H.X.; Roshan, H. Current understanding of shale wettability: A review on contact angle measurements. Earth-Sci. Rev. 2018, 181, 1–11. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, Z.; Zhao, X.; Zhang, Y.; Li, G.; Huang, W. Application of Nano-Polymer Emulsion for Inhibiting Shale Self-Imbibition in Water-Based Drilling Fluids. J. Surfactants Deterg. 2018, 21, 155–164. [Google Scholar] [CrossRef]
- Jin, J.F.; Lv, K.H.; Sun, J.S.; Zhang, J.; Hou, Q.L.; Guo, X.; Liu, K.S. Robust superhydrophobic TiO2@carbon nanotubes inhibitor with bombax structure for strengthening wellbore in water-based drilling fluid. J. Mol. Liq. 2023, 370, 120946. [Google Scholar] [CrossRef]






| Element | Region 1 | Region 2 |
|---|---|---|
| Oxygen | 42.70% | 41.10% |
| Silicon | 41.21% | 26.99% |
| Aluminum | 9.94% | 7.40% |
| Calcium | 2.82% | 1.02% |
| Iron | 2.56% | 3.33% |
| Potassium | 0.68% | 1.89% |
| Chlorine | 0.09% | 0.39% |
| Magnesium | 0.01% | 0.11% |
| Carbon | 0 | 17.78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Sun, J.; Xiu, Z.; Huang, X.; Lv, K.; Liu, J.; Sun, Y.; Dong, X. Preparation and Performance Evaluation of Ionic Liquid Copolymer Shale Inhibitor for Drilling Fluid Gel System. Gels 2024, 10, 96. https://doi.org/10.3390/gels10020096
Dai Z, Sun J, Xiu Z, Huang X, Lv K, Liu J, Sun Y, Dong X. Preparation and Performance Evaluation of Ionic Liquid Copolymer Shale Inhibitor for Drilling Fluid Gel System. Gels. 2024; 10(2):96. https://doi.org/10.3390/gels10020096
Chicago/Turabian StyleDai, Zhiwen, Jinsheng Sun, Zhuoyang Xiu, Xianbin Huang, Kaihe Lv, Jingping Liu, Yuanwei Sun, and Xiaodong Dong. 2024. "Preparation and Performance Evaluation of Ionic Liquid Copolymer Shale Inhibitor for Drilling Fluid Gel System" Gels 10, no. 2: 96. https://doi.org/10.3390/gels10020096