Fabrication and Characterization of Taro (Colocasia esculenta)-Mucilage-Based Nanohydrogel for Shelf-Life Extension of Fresh-Cut Apples
Abstract
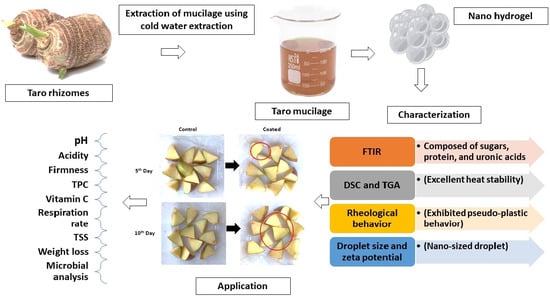
:1. Introduction
2. Results and Discussion
2.1. Taro Mucilage Yield
2.2. Characterization of Nanohydrogel
2.2.1. Functional Group Determination
2.2.2. Thermal Analysis
2.2.3. Rheological Behavior
2.2.4. Droplet Size and Zeta Potential
2.3. Physicochemical Properties of Fresh-Cut Apples
2.4. Microbial Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Methods
4.3.1. Extraction of Taro Mucilage
4.3.2. Development of Nanohydrogel
4.3.3. Characterization of Nanohydrogel
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
Thermal Analysis
Rheological Behavior
Droplet Size and Zeta Potential
4.4. Physicochemical Properties of Fresh-Cut Apples
4.4.1. Weight Loss and pH
4.4.2. TSS and Acidity
4.4.3. Firmness
4.4.4. Antioxidant Activity
4.4.5. Vitamin C
4.4.6. Respiration Rate
4.4.7. Total Phenolic Content
4.4.8. Microbial Analysis
4.4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Singla, G.; Chaturvedi, K.; Sandhu, P.P. Status and recent trends in fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 17–49. [Google Scholar] [CrossRef]
- Ghidelli, C.; Pérez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, P.K.; Chaturvedi, S.; Kumar, P.; Nannaware, A.D.; Kalra, A.; Rout, P.K. Biocatalyst for the synthesis of natural flavouring compounds as food additives: Bridging the gap for a more sustainable industrial future. Food Chem. 2024, 435, 137217. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.; Peterson, A.; Madden, J.; Wai, K.; Lesniauskas, R.; Garza, J.; Gere, A.; Amin, S.; Lammert, A. The Crick-Eatery: A Novel Approach to Evaluate Cricket (Acheta domesticus) Powder Replacement in Food Products through Product Eating Experience and Emotional Response. Foods 2022, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Paliya, B.S.; Sharma, V.K.; Sharma, M.; Diwan, D.; Nguyen, Q.D.; Aminabhavi, T.M.; Rajauria, G.; Singh, B.N.; Gupta, V.K. Protein-polysaccharide nanoconjugates: Potential tools for delivery of plant-derived nutraceuticals. Food Chem. 2023, 428, 136709. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N.; Hussain, T. Nano-technology platforms to increase the antibacterial drug suitability of essential oils: A drug prospective assessment. OpenNano 2022, 9, 100115. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Taha, A.; Abdel-Moneim, A.M.E.; Shehata, A.M.; Tan, C.; Kharazmi, M.S.; Li, Y.; Assadpour, E.; Castro-Muñoz, R.; et al. Bioactive-loaded nanodelivery systems for the feed and drugs of livestock: Purposes, techniques and applications. Adv. Colloid Interface Sci. 2022, 308, 102772. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Tamanadar, E.; Pour, M.M.; Thakur, V.K. Novel approaches for encapsulation of plant probiotic bacteria with sustainable polymer gums: Application in the management of pests and diseases. Adv. Polym. Technol. 2022, 2022, 4419409. [Google Scholar] [CrossRef]
- Dybka-Stępień, K.; Otlewska, A.; Góźdź, P.; Piotrowska, M. The renaissance of plant mucilage in health promotion and industrial applications: A review. Nutrients 2021, 13, 3354. [Google Scholar] [CrossRef]
- Lutz, T.M.; Kimna, C.; Casini, A.; Lieleg, O. Bio-based and bio-inspired adhesives from animals and plants for biomedical applications. Mater. Today Bio 2022, 13, 100203. [Google Scholar] [CrossRef]
- Kapoor, B.; Singh, S.; Kumar, P. Taro (Colocasia esculenta): Zero wastage orphan food crop for food and nutritional security. S. Afr. J. Bot. 2022, 145, 157–169. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Klepacka, J.; Bains, A.; Chawla, P.; Kumar, A.; Sharma, M.; Sridhar, K.; Gautam, S.P.; Kaushik, R. A concise review on taro mucilage: Extraction techniques, chemical composition, characterization, applications, and health attributes. Polymers 2022, 14, 1163. [Google Scholar] [CrossRef]
- Andrade, L.A.; de Oliveira Silva, D.A.; Nunes, C.A.; Pereira, J. Experimental techniques for the extraction of taro mucilage with enhanced emulsifier properties using chemical characterization. Food Chem. 2020, 327, 127095. [Google Scholar] [CrossRef] [PubMed]
- Kalegowda, P.; Chauhan, A.S.; Urs, S.M.N. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ayub, H. Fourier Transform Infrared Spectroscopy (FTIR) Technique for Food Analysis and Authentication. In Nondestructive Quality Assessment Techniques for Fresh Fruits and Vegetables; Springer Nature Singapore: Singapore, 2022; pp. 103–142. [Google Scholar] [CrossRef]
- Reguieg, F.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal characterization by DSC and TGA analyses of PVA hydrogels with organic and sodium MMT. Polym. Bull. 2020, 77, 929–948. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Bajer, D.; Demirkesen, I.; Kumar, Y.; Su, C.; Wang, Y.; Nowacka, M.; Singha, P.; Falsafi, S.R. Starch modification through its combination with other molecules: Gums, mucilages, polyphenols and salts. Carbohydr. Polym. 2023, 314, 120905. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Abbasian Chaleshtari, Z.; Salimi-Kenari, H.; Foudazi, R. Interdroplet interactions and rheology of concentrated nanoemulsions for templating porous polymers. Langmuir 2020, 37, 76–89. [Google Scholar] [CrossRef]
- Behrouzian, F.; Razavi, S.M. Steady shear rheological properties of emerging hydrocolloids. In Emerging Natural Hydrocolloids: Rheology and Functions; Wiley: Hoboken, NJ, USA, 2019; pp. 81–100. [Google Scholar] [CrossRef]
- Pérez-Orozco, J.P.; Sánchez-Herrera, L.M.; Ortiz-Basurto, R.I. Effect of concentration, temperature, pH, co-solutes on the rheological properties of Hyptis suaveolens L. mucilage dispersions. Food Hydrocoll. 2019, 87, 297–306. [Google Scholar] [CrossRef]
- Nor, S.M.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Wang, H.; Zheng, Y.; Zhou, Q.; Duan, L.; Tang, Y.; Jiang, Y.; Li, X.; Jiang, Y. Harvest maturity stage affects watercore dissipation and postharvest quality deterioration of watercore ‘Fuji’ apples. Postharvest Biol. Technol. 2024, 210, 112736. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An extensive review of natural polymers used as coatings for postharvest shelf-life extension: Trends and challenges. Polymers 2021, 13, 3271. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the delivery of plant-derived (poly) phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Thakur, B.; Naushad, M.; Kumar, A.; Stadler, F.J.; Alfadul, S.M.; Mola, G.T. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ. Chem. Lett. 2018, 16, 113–146. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzon, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, A.K. Potential of engineered nanostructured biopolymer based coatings for perishable fruits with Coronavirus safety perspectives. Prog. Org. Coat. 2022, 163, 106632. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.A.; Maders, C.; Alexandre, B.; Pinilla, C.M.B.; Brandelli, A.; da Silva Malheiros, P. Carvacrol encapsulation into nanoparticles produced from chia and flaxseed mucilage: Characterization, stability and antimicrobial activity against Salmonella and Listeria monocytogenes. Food Microbiol. 2022, 108, 104116. [Google Scholar] [CrossRef]
- Khodaman, E.; Barzegar, H.; Jokar, A.; Jooyandeh, H. Production and evaluation of physicochemical, mechanical and antimicrobial properties of chia (Salvia hispanica L.) mucilage-gelatin based edible films incorporated with chitosan nanoparticles. J. Food Meas. Charact. 2022, 16, 3547–3556. [Google Scholar] [CrossRef]
- Thakur, S.; Bains, A.; Sridhar, K.; Kaushik, R.; Gupta, V.K.; Chawla, P.; Sharma, M. Gum Arabic/guar gum based biopolymeric nanohydrogel for shelf-life enhancement of grapes and photocatalytic dye reduction. Ind. Crops Prod. 2023, 203, 117114. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly (acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Nandane, A.S.; Dave, R.K.; Rao, T.R. Optimization of edible coating formulations for improving postharvest quality and shelf life of pear fruit using response surface methodology. J. Food Sci. Technol. 2017, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khorram, F.; Ramezanian, A.; Hosseini, S.M.H. Effect of different edible coatings on postharvest quality of ‘Kinnow’mandarin. J. Food Meas. Charact. 2017, 11, 1827–1833. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT—Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Valdes, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]







| Taro Mucilage Concentration (%) w/v | Droplet Size (nm) | Zeta Potential (mV) |
|---|---|---|
| 1 | 245.35 ± 0.58 | −24.36 ± 0.38 |
| 2 | 211.48 ± 1.24 | −15.79 ± 0.43 |
| 3 | 175.61 ± 0.92 | −30.25 ± 0.94 |
| 4 | 199.76 ± 0.75 | −12.81 ± 0.71 |
| 5 | 186.19 ± 0.37 | −19.46 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosif, M.M.; Bains, A.; Sridhar, K.; Inbaraj, B.S.; Ali, N.; Dikkala, P.K.; Kumar, A.; Chawla, P.; Sharma, M. Fabrication and Characterization of Taro (Colocasia esculenta)-Mucilage-Based Nanohydrogel for Shelf-Life Extension of Fresh-Cut Apples. Gels 2024, 10, 95. https://doi.org/10.3390/gels10020095
Tosif MM, Bains A, Sridhar K, Inbaraj BS, Ali N, Dikkala PK, Kumar A, Chawla P, Sharma M. Fabrication and Characterization of Taro (Colocasia esculenta)-Mucilage-Based Nanohydrogel for Shelf-Life Extension of Fresh-Cut Apples. Gels. 2024; 10(2):95. https://doi.org/10.3390/gels10020095
Chicago/Turabian StyleTosif, Mansuri M., Aarti Bains, Kandi Sridhar, Baskaran Stephen Inbaraj, Nemat Ali, Praveen Kumar Dikkala, Ankur Kumar, Prince Chawla, and Minaxi Sharma. 2024. "Fabrication and Characterization of Taro (Colocasia esculenta)-Mucilage-Based Nanohydrogel for Shelf-Life Extension of Fresh-Cut Apples" Gels 10, no. 2: 95. https://doi.org/10.3390/gels10020095