Reasoning on Pore Terminology in 3D Bioprinting
Abstract
:1. Introduction
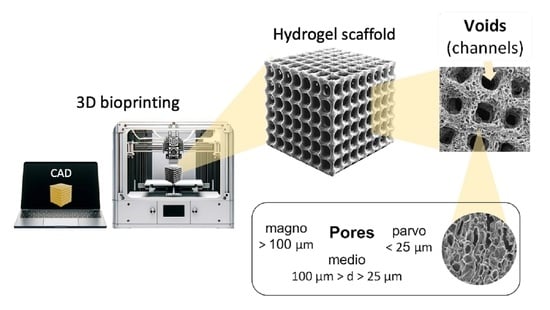
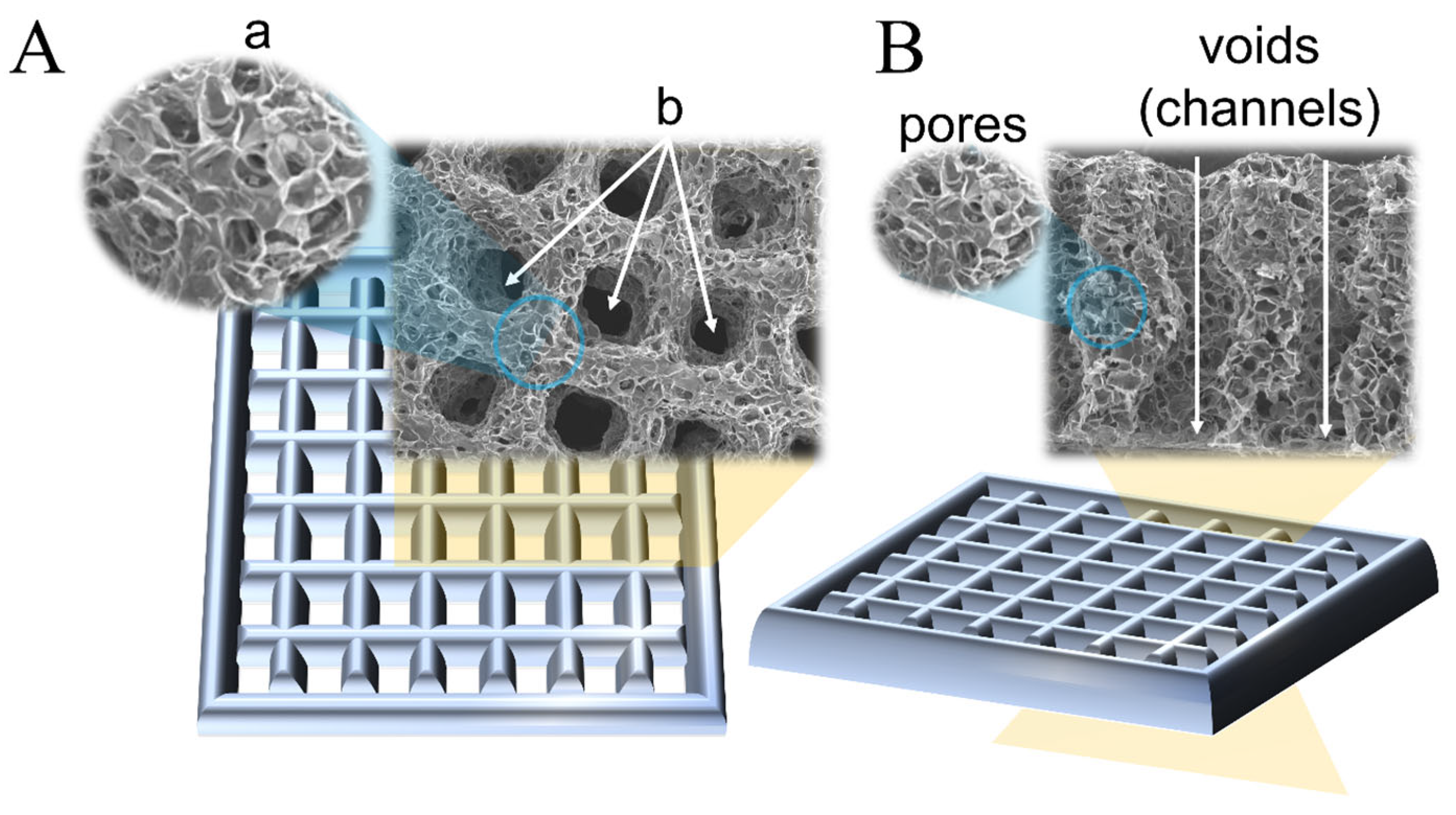
2. Pores in 3D-Printed Scaffolds
2.1. Pore Fabrication
2.2. Terminology
2.3. Suggested Nomenclature and Classification of Pore Sizes
3. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, e1701161. [Google Scholar] [CrossRef]
- Akilbekova, D.; Mektepbayeva, D. Patient Specific In Situ 3D Printing. In 3D Printing in Medicine; Kalaskar, D.M., Ed.; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the progress of hydrogel-based 3D printing: Correlating rheological properties with printing behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: Enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mater. Sci. 2015, 51, 271–310. [Google Scholar] [CrossRef]
- Smith, P.T.; Basu, A.; Saha, A.; Nelson, A. Chemical modification and printability of shear-thinning hydrogel inks for direct-write 3D printing. Polymer 2018, 152, 42–50. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, e2002931. [Google Scholar] [CrossRef]
- Moazzam, M.; Shehzad, A.; Sultanova, D.; Mukasheva, F.; Trifonov, A.; Berillo, D.; Akilbekova, D. Macroporous 3D printed structures for regenerative medicine applications. Bioprinting 2022, 28, e00254. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Chitin and chitosan composites for bone tissue regeneration. Handb. Chitin Chitosan 2020, 3, 499–553. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Kuipers, E.; Gawlitta, D.; Dhert, W.J.; Alblas, J. Scaffold Porosity and Oxygenation of Printed Hydrogel Constructs Affect Functionality of Embedded Osteogenic Progenitors. Tissue Eng. Part A 2011, 17, 2473–2486. [Google Scholar] [CrossRef] [PubMed]
- Podhorská, B.; Vetrík, M.; Chylíková-Krumbholcová, E.; Kománková, L.; Banafshehvaragh, N.R.; Šlouf, M.; Dušková-Smrčková, M.; Janoušková, O. Revealing the True Morphological Structure of Macroporous Soft Hydrogels for Tissue Engineering. Appl. Sci. 2020, 10, 6672. [Google Scholar] [CrossRef]
- Maksoud, F.J.; de la Paz, M.F.V.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Cheng, D.; Xu, S.; Du, C.; Xie, L.; Zhao, W. Applications of electrospun scaffolds with enlarged pores in tissue engineering. Biomater. Sci. 2022, 10, 1423–1447. [Google Scholar] [CrossRef]
- Farazin, A.; Zhang, C.; Gheisizadeh, A.; Shahbazi, A. 3D bio-printing for use as bone replacement tissues: A review of biomedical application. Biomed. Eng. Adv. 2023, 5, 100075. [Google Scholar] [CrossRef]
- Egan, P.F.; Gonella, V.C.; Engensperger, M.; Ferguson, S.J.; Shea, K. Computationally designed lattices with tuned properties for tissue engineering using 3D printing. PLoS ONE 2017, 12, e0182902. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wang, Z.; Wang, Y.; Dong, W.; Ma, W.; Zhao, S.; Sun, D. 3D-printed porous PEEK scaffold combined with CSMA/POSS bioactive surface: A strategy for enhancing osseointegration of PEEK implants. Compos. Part B Eng. 2021, 230, 109512. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D printed scaffolds with gradient porosity based on a cellulose nanocrystal hydrogel. Nanoscale 2018, 10, 4421–4431. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Chen, X.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef]
- Serex, L.; Braschler, T.; Filippova, A.; Rochat, A.; Béduer, A.; Bertsch, A.; Renaud, P. Pore Size Manipulation in 3D Printed Cryogels Enables Selective Cell Seeding. Adv. Mater. Technol. 2018, 3, 1700340. [Google Scholar] [CrossRef]
- Qi, D.; Wu, S.; Kuss, M.A.; Shi, W.; Chung, S.; Deegan, P.T.; Kamenskiy, A.; He, Y.; Duan, B. Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering. Acta Biomater. 2018, 74, 131–142. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Parra-Cantu, C.; Tang, G.; Zhang, J.; Wang, H.; Chen, S.; Huang, N.; Xie, J.; Zhang, Y.S. Bioprinted Injectable Hierarchically Porous Gelatin Methacryloyl Hydrogel Constructs with Shape-Memory Properties. Adv. Funct. Mater. 2020, 30, 2003740. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef]
- Elhattab, K.; Sikder, P.; Walker, J.M.; Bottino, M.; Bhaduri, S. Fabrication and evaluation of 3-D printed PEEK scaffolds containing Macropores by design. Mater. Lett. 2020, 263, 127227. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Cao, Q.; Wu, Y.; Wang, Y.; Yuan, B.; Li, X.; Zhou, Y.; Chen, X.; Zhu, X.; et al. Fabrication and biological evaluation of 3D-printed calcium phosphate ceramic scaffolds with distinct macroporous geometries through digital light processing technology. Regen. Biomater. 2022, 9, rbac005. [Google Scholar] [CrossRef] [PubMed]
- Kessel, B.; Lee, M.; Bonato, A.; Tinguely, Y.; Tosoratti, E.; Zenobi-Wong, M. 3D Bioprinting of Macroporous Materials Based on Entangled Hydrogel Microstrands. Adv. Sci. 2020, 7, 2001419. [Google Scholar] [CrossRef] [PubMed]
- Seymour, A.J.; Shin, S.; Heilshorn, S.C. 3D Printing of Microgel Scaffolds with Tunable Void Fraction to Promote Cell Infiltration. Adv. Healthc. Mater. 2021, 10, 2100644. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology, (the “Gold Book”), 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.K.; Chen, Q.; Lin, Y.; Stevens, M.M. Void-Free 3D Bioprinting for In Situ Endothelialization and Microfluidic Perfusion. Adv. Funct. Mater. 2020, 30, 1908349. [Google Scholar] [CrossRef]
- Lai, J.; Ye, X.; Liu, J.; Wang, C.; Li, J.; Wang, X.; Ma, M.; Wang, M. 4D printing of highly printable and shape morphing hydrogels composed of alginate and methylcellulose. Mater. Des. 2021, 205, 109699. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, C. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17–21. [Google Scholar] [CrossRef]
- Ebrahimi, M. Porosity parameters in biomaterial science: Definition, impact, and challenges in tissue engineering. Front. Mater. Sci. 2021, 15, 352–373. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, Y.; MacEwan, M.R.; Xia, Y. Neovascularization in Biodegradable Inverse Opal Scaffolds with Uniform and Precisely Controlled Pore Sizes. Adv. Healthc. Mater. 2013, 2, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. C 2020, 117, 111245. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, T.; Malda, J.; de Wijn, J.; Péters, F.; Riesle, J.; van Blitterswijk, C. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Goh, M.H.; Yeong, W.Y.; Naing, M.W. Applying macromolecular crowding to 3D bioprinting: Fabrication of 3D hierarchical porous collagen-based hydrogel constructs. Biomater. Sci. 2018, 6, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 629270. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Eichholz, K.F.; Hoey, D.A. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; García, A.; González-Jiménez, A.; Vallet-Regí, M. Antibacterial effect of 3D printed mesoporous bioactive glass scaffolds doped with metallic silver nanoparticles. Acta Biomater. 2022, 155, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Gao, Q.; Xie, C.; Fu, J.; Xiang, M.; Liu, Z.; Xiang, L.; He, Y. Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Bio-Design Manuf. 2020, 3, 30–39. [Google Scholar] [CrossRef]
- Farzadi, A.; Waran, V.; Solati-Hashjin, M.; Rahman, Z.A.A.; Asadi, M.; Abu Osman, N.A. Effect of layer printing delay on mechanical properties and dimensional accuracy of 3D printed porous prototypes in bone tissue engineering. Ceram. Int. 2015, 41, 8320–8330. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Guan, L.X.; Chen, J.L.; Duan, L.; Jia, Z.F.; Huang, J.H.; Li, W.C.; Liu, J.Q.; Xiong, J.Y.; et al. A 3D-Printed PLCL Scaffold Coated with Collagen Type I and Its Biocompatibility. BioMed Res. Int. 2018, 2018, 5147156. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.; Silva-Correia, J.; Ribeiro, V.P.; da Silva Morais, A.; Oliveira, J.M.; Reis, R.L. Engineering patient-specific bioprinted constructs for treatment of degenerated intervertebral disc. Mater. Today Commun. 2019, 19, 506–512. [Google Scholar] [CrossRef]
- Mohan, T.; Štiglic, A.D.; Beaumont, M.; Konnerth, J.; Gürer, F.; Makuc, D.; Maver, U.; Gradišnik, L.; Plavec, J.; Kargl, R.; et al. Generic Method for Designing Self-Standing and Dual Porous 3D Bioscaffolds from Cellulosic Nanomaterials for Tissue Engineering Applications. ACS Appl. Bio Mater. 2020, 3, 1197–1209. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, X.; Zhong, L.; Peng, D.; Lu, J.; He, J.; Shen, X.; Xiong, C.; Xu, T.; Niu, W. Doping polyvinyl alcohol can improve the injectability of biological ceramics in 3D printing and influence the adhesion of cells to the scaffolds after sintering. Ceram. Int. 2021, 47, 25363–25372. [Google Scholar] [CrossRef]
- Liu, R.; Ma, L.; Liu, H.; Xu, B.; Feng, C.; He, R. Effects of pore size on the mechanical and biological properties of stereolithographic 3D printed HAp bioceramic scaffold. Ceram. Int. 2021, 47, 28924–28931. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Zhang, L.; Zhou, Q.; Luo, L. Automated quantitative assessment of three-dimensional bioprinted hydrogel scaffolds using optical coherence tomography. Biomed. Opt. Express 2016, 7, 894–910. [Google Scholar] [CrossRef]
- Nyberg, E.; O’sullivan, A.; Grayson, W. scafSLICR: A MATLAB-based slicing algorithm to enable 3D-printing of tissue engineering scaffolds with heterogeneous porous microarchitecture. PLoS ONE 2019, 14, e0225007. [Google Scholar] [CrossRef]
- Ataie, Z.; Kheirabadi, S.; Zhang, J.W.; Kedzierski, A.; Petrosky, C.; Jiang, R.; Vollberg, C.; Sheikhi, A. Nanoengineered Granular Hydrogel Bioinks with Preserved Interconnected Microporosity for Extrusion Bioprinting. Small 2022, 18, e2202390. [Google Scholar] [CrossRef]
- Zopf, D.A.; Flanagan, C.L.; Mitsak, A.G.; Brennan, J.R.; Hollister, S.J. Pore architecture effects on chondrogenic potential of patient-specific 3-dimensionally printed porous tissue bioscaffolds for auricular tissue engineering. Int. J. Pediatr. Otorhinolaryngol. 2018, 114, 170–174. [Google Scholar] [CrossRef]
- Gupta, D.; Vashisth, P.; Bellare, J. Multiscale porosity in a 3D printed gellan–gelatin composite for bone tissue engineering. Biomed. Mater. 2021, 16, 034103. [Google Scholar] [CrossRef]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair. Polymers 2019, 11, 1924. [Google Scholar] [CrossRef]
- Zhang, J.; Eyisoylu, H.; Qin, X.-H.; Rubert, M.; Müller, R. 3D bioprinting of graphene oxide-incorporated cell-laden bone mimicking scaffolds for promoting scaffold fidelity, osteogenic differentiation and mineralization. Acta Biomater. 2021, 121, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K. Determination of pore size and pore size distribution: 1. Adsorbents and catalysts. J. Membr. Sci. 1994, 96, 59–89. [Google Scholar] [CrossRef]
- Mehta, K.P.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Kodikara, J.; Barbour, S.L.; Fredlund, D.G. Changes in clay structure and behaviour due to wetting and drying. In Consolidating knowledge. Proceedings of the 8th Australia New Zealand Conference on Geomechanics, Hobart, Australia, February 1999; Vitharana, N., Colman, R., Vitharana, N., Colman, R., Eds.; Australian Geomechanics Society (AGS): The Gap, Australia, 1999; pp. 179–185. [Google Scholar]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Cheremskoj, P.G. Metodi Izsleddovania Poresti Tvurdi Tel; Energoatomizdat: Singapore, 1985. [Google Scholar]
- Lu, G.M.; Zhao, X.S. (Eds.) Nanoporous Materials: Science and Engineering; World Scientific: Singapore, 2004; Volume 4. [Google Scholar]
- Huang, Y.; Zeng, M.; Feng, Z.; Yin, D.; Xu, Q.; Fan, L. Graphene oxide-based composite hydrogels with self-assembled macroporous structures. RSC Adv. 2016, 6, 3561–3570. [Google Scholar] [CrossRef]
- Ko, H.; Ratri, M.C.; Kim, K.; Jung, Y.; Tae, G.; Shin, K. Formulation of Sugar/Hydrogel Inks for Rapid Thermal Response 4D Architectures with Sugar-derived Macropores. Sci. Rep. 2020, 10, 7527. [Google Scholar] [CrossRef] [PubMed]
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Biocompatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef]
- Sung, H.; Labazzo, K.S.; Bolikal, D.; Weiner, M.; Zimnisky, R.; Kohn, J. Angiogenic competency of biodegradable hydrogels fabricated from polyethylene glycol-crosslinked tyrosine-derived polycarbonates. Eur. Cells Mater. 2008, 15, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Xu, X.; Sun, D.; Hong, Y.; Wen, C.; Xie, Y.; Yan, B.; Zhang, H.; Ge, Q.; Li, W.; et al. Biomimetic macroporous hydrogel with a triple-network structure for full-thickness skin regeneration. Appl. Mater. Today 2022, 27, 101442. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Tang, Y.; Yang, G.; Wu, X.; Lin, S.; Liu, X.; Cao, H.; Jiang, X. Preservation of alveolar ridge height through mechanical memory: A novel dental implant design. Bioact. Mater. 2021, 6, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, D.; Yao, D.; Guo, R.; Wang, W.; Dong, A.; Kong, D.; Zhang, J. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017, 64, 334–345. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Phan, V.G.; Lee, D.S.; Thambi, T.; Huynh, D.P. Bioresorbable pH- and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin. Polym. Degrad. Stab. 2019, 162, 36–46. [Google Scholar] [CrossRef]
- Boffito, M.; Laurano, R.; Giasafaki, D.; Steriotis, T.; Papadopoulos, A.; Tonda-Turo, C.; Cassino, C.; Charalambopoulou, G.; Ciardelli, G. Embedding Ordered Mesoporous Carbons into Thermosensitive Hydrogels: A Cutting-Edge Strategy to Vehiculate a Cargo and Control Its Release Profile. Nanomaterials 2020, 10, 2165. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF within Multilayered Polydopamine-Coated Scaffolds and the Resulting Osteogenic and Angiogenic Synergy of Co-Cultured Human Mesenchymal Stem Cells and Human Endothelial Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Jose, G.M.; Kurup, M. PEG-penetrated chitosan–alginate co-polysaccharide-based partially and fully cross-linked hydrogels as ECM mimic for tissue engineering applications. Prog. Biomater. 2015, 4, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for Tissue Repair and Organ Three-Dimensional (3D) Bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ma, Z.; Gao, C.; Wang, W.; Shen, J. Specially elaborated thermally induced phase separation to fabricate poly(L-lactic acid) scaffolds with ultra large pores and good interconnectivity. J. Appl. Polym. Sci. 2006, 101, 3336–3342. [Google Scholar] [CrossRef]
- Weigel, T.; Schinkel, G.; Lendlein, A. Expert Review of Medical Devices Design and preparation of polymeric scaffolds for tissue engineering Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev. Med. Devices 2006, 3, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Whang, K.; Healy, K.; Elenz, D.; Nam, E.; Tsai, D.; Thomas, C.; Nuber, G.; Glorieux, F.; Travers, R.; Sprague, S. Engineering Bone Regeneration with Bioabsorbable Scaffolds with Novel Microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef]
- O’brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]

| Source | Specific Term Used for Designed Voids (µm) | Specific Term Used for Interconnected Pores (µm) | Method |
|---|---|---|---|
| Godoy-Gallardo et al., 2020 [39] | n/a | Small, large, and extra large pores (0–60) (60–80) (300–550) | Two-step depressurization approach |
| Woodfield et al., 2004 [40] | Very large pores (1640) Very small pores (150) | n/a | 3D printing |
| Ng et al., 2018 [41] | Microstructure with pores (0.50 ± 0.13) Bigger Pores (1.67 ± 0.26) | n/a | 3D printing, drop-on-demand |
| Han et al., 2021 [42] | Pore (50–400) | n/a | Melt Electrowritten 3D Printing |
| Brennan et al., 2019 [43] | Pore (100–300) | n/a | Melt Electrowritten 3D Printing |
| Sanchez-Salcedo et al., 2022 [44] | Ultra-large pores (450) | Macropores (20–60) Mesopores (0.0037–0.0044) | Rapid prototyping technique |
| Zhang et al., 2014 [45] | Macropores (400) | Mesopores (0.003–0.004) | 3D Printing |
| Shao et al., 2020 [46] | n/a | Mesoscale pores (100–1000) | 3D Bioprinting |
| Farzadi et al., 2015 [47] | Macropores (700) | Micropores (10–30) | 3D Inkjet Printing |
| He et al., 2018 [48] | Macropores (445 ± 141) | Micropores (7.0 ± 1.5) | Low-Temperature Deposition Manufacturing 3D Printing |
| Costa et al., 2019 [49] | Macropores (400–500) | Micropores (70–100) | 3D Bioplotting |
| Mohan et al., 2020 [50] | Macropores (>200) | Micropores (<100) | 3D printing |
| Lin et al., 2021 [51] | Macropores (456.1 ± 11.2) | Micropores (4.44 ± 0.7) | 3D printing |
| Liu et al., 2021 [52] | Macropores (200–800) | Micropores * | Stereolithographic 3D printing |
| Liu et al., 2022 [21] | Unit pores/pores/Macropores (700) | Micropores/small pores/pores (1.15 ± 0.50) | 3D printing |
| Cox et al., 2015 [20] | Pore channels (1460–1750) | Micropores (10–60) | 3D printing |
| Xu et al., 2016 [53] | Void region/isolated channels/pores (341.2 ± 34.2) | Micropores (40) | 3D bioprinting |
| Egan et al., 2017 [19] | Larger pores (800) | Smaller pores (200) | 3D printing |
| Nyberg et al., 2019 [54] | Pores/microarchitecture (200–1000) | n/a | 3D printing |
| Ouyang et al., 2020 [34] | Interconnected void spaces/Pores/channels (450) | n/a | Void-Free 3D Printing |
| Seymour et al., 2021 [33] | Windows * | Interconnected voids/voids * | Microgel extrusion bioprinting |
| Ataie et al., 2022 [55] | Windows * | Microporosity/microscale pores (20–25) | Nanoengineered granular bioink bioprinting |
| Zopf et al., 2018 [56] | Micropores * | n/a | 3D printing |
| Gupta et al., 2021 [57] | Macropores (919 ± 89) | Micropores (20–250) | Cryogenic 3D printing |
| Wu et al., 2019 [58] | Interconnected pores/ Macroporous (~500) | n/a | 3D Bioprinting |
| Sultan et al., 2018 [22] | Pore/Void (500–2000) | n/a | 3D printing |
| Kessel et al., 2020 [30] | Pore/Aperture/Mesh/Void/Macroporous (40 and 100) | n/a | 3D Bioprinting |
| Ying et al., 2020 [26] | Macropores * | Micropores (60) Nanopores * | 3D Bioprinting |
| Zhang et al., 2021 [59] | Macropores (~500) | n/a | 3D Bioprinting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifonov, A.; Shehzad, A.; Mukasheva, F.; Moazzam, M.; Akilbekova, D. Reasoning on Pore Terminology in 3D Bioprinting. Gels 2024, 10, 153. https://doi.org/10.3390/gels10020153
Trifonov A, Shehzad A, Mukasheva F, Moazzam M, Akilbekova D. Reasoning on Pore Terminology in 3D Bioprinting. Gels. 2024; 10(2):153. https://doi.org/10.3390/gels10020153
Chicago/Turabian StyleTrifonov, Alexander, Ahmer Shehzad, Fariza Mukasheva, Muhammad Moazzam, and Dana Akilbekova. 2024. "Reasoning on Pore Terminology in 3D Bioprinting" Gels 10, no. 2: 153. https://doi.org/10.3390/gels10020153