Effect of Spray-Type Alginate Hydrogel Dressing on Burn Wounds
Abstract
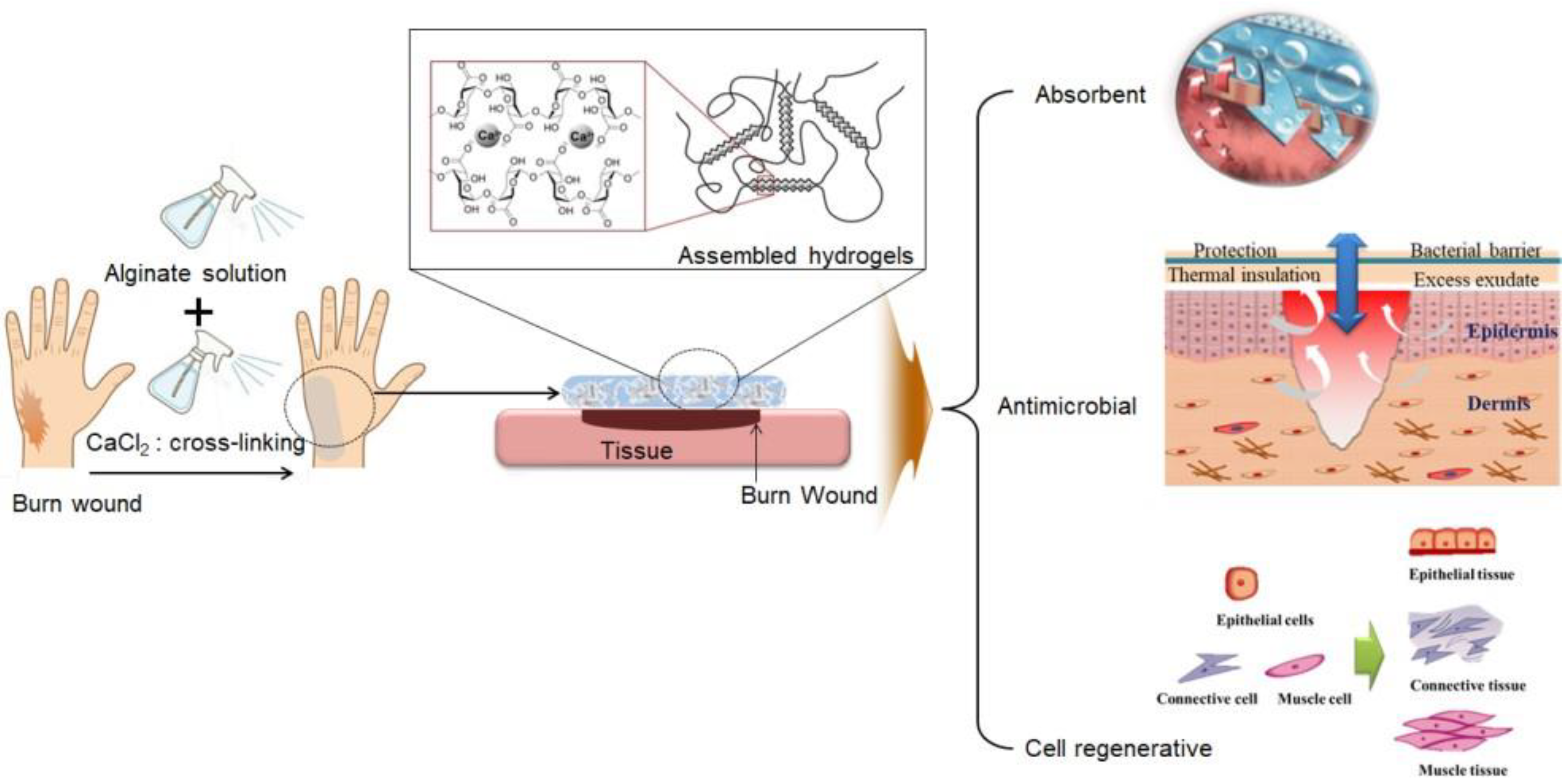
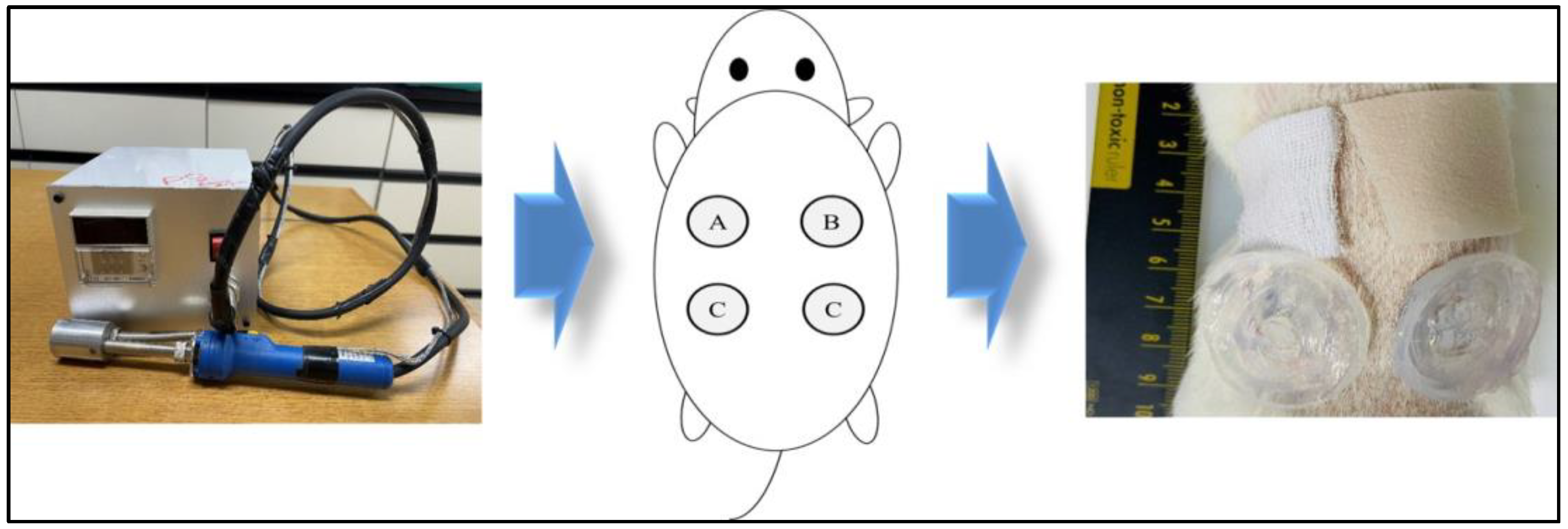
:1. Introduction
2. Results and Discussion
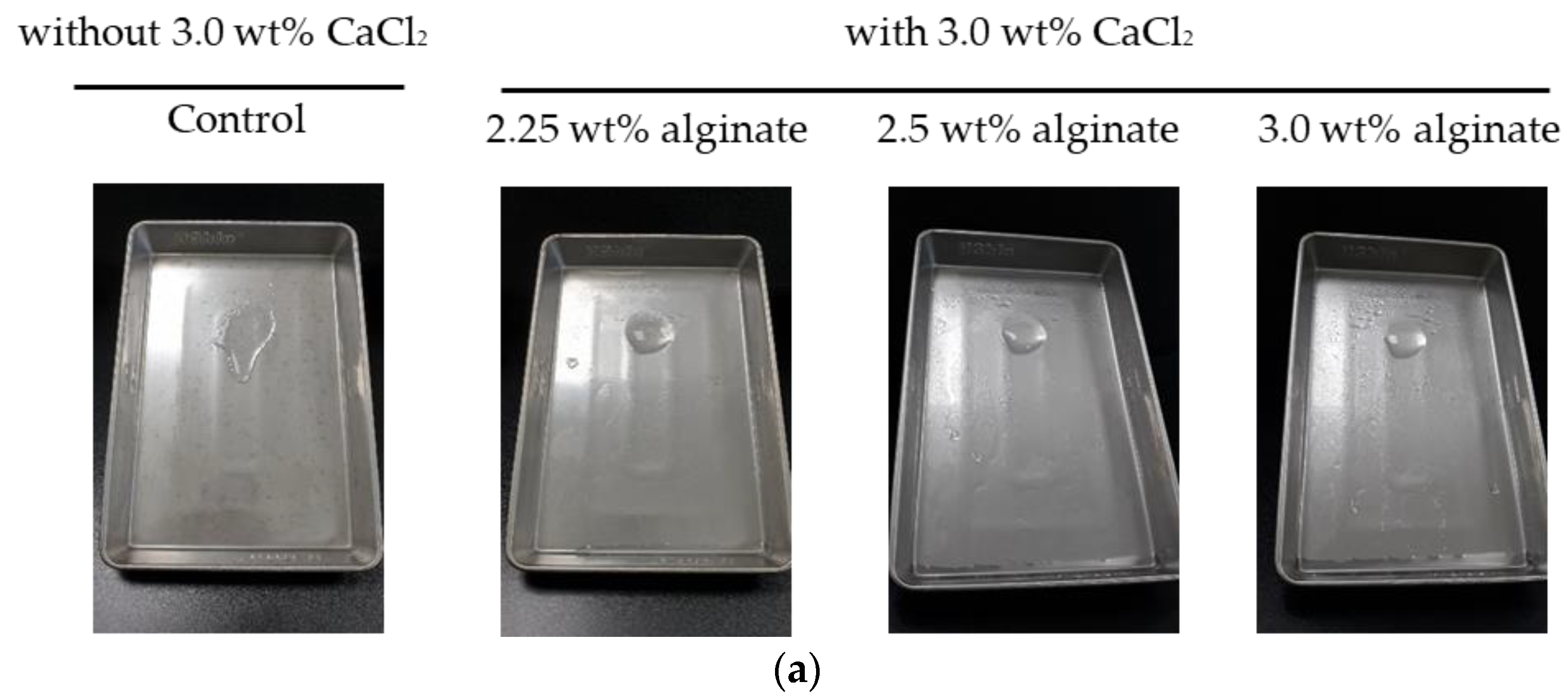
2.1. Evaluation of the Stability of a Gel Formulation
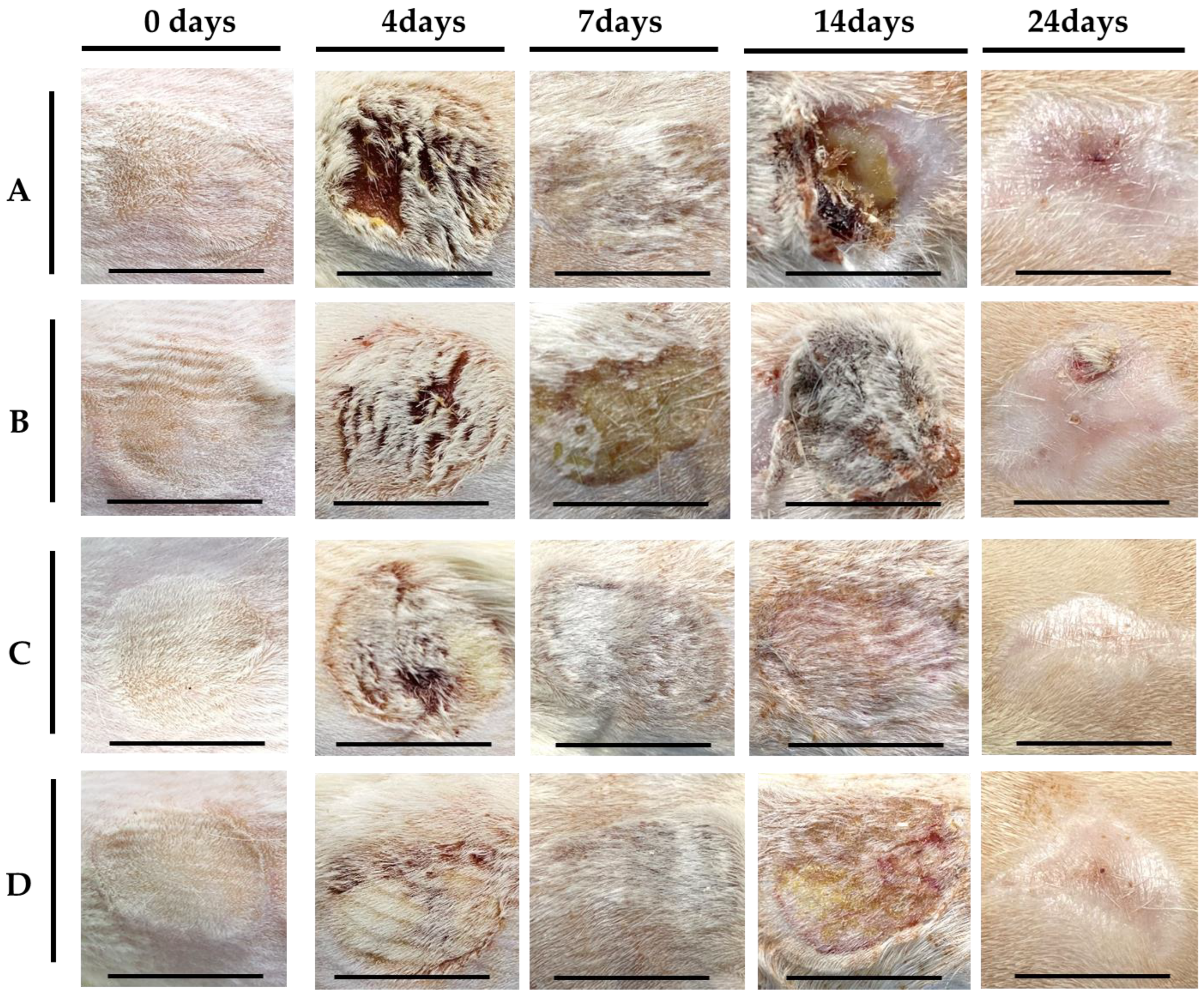
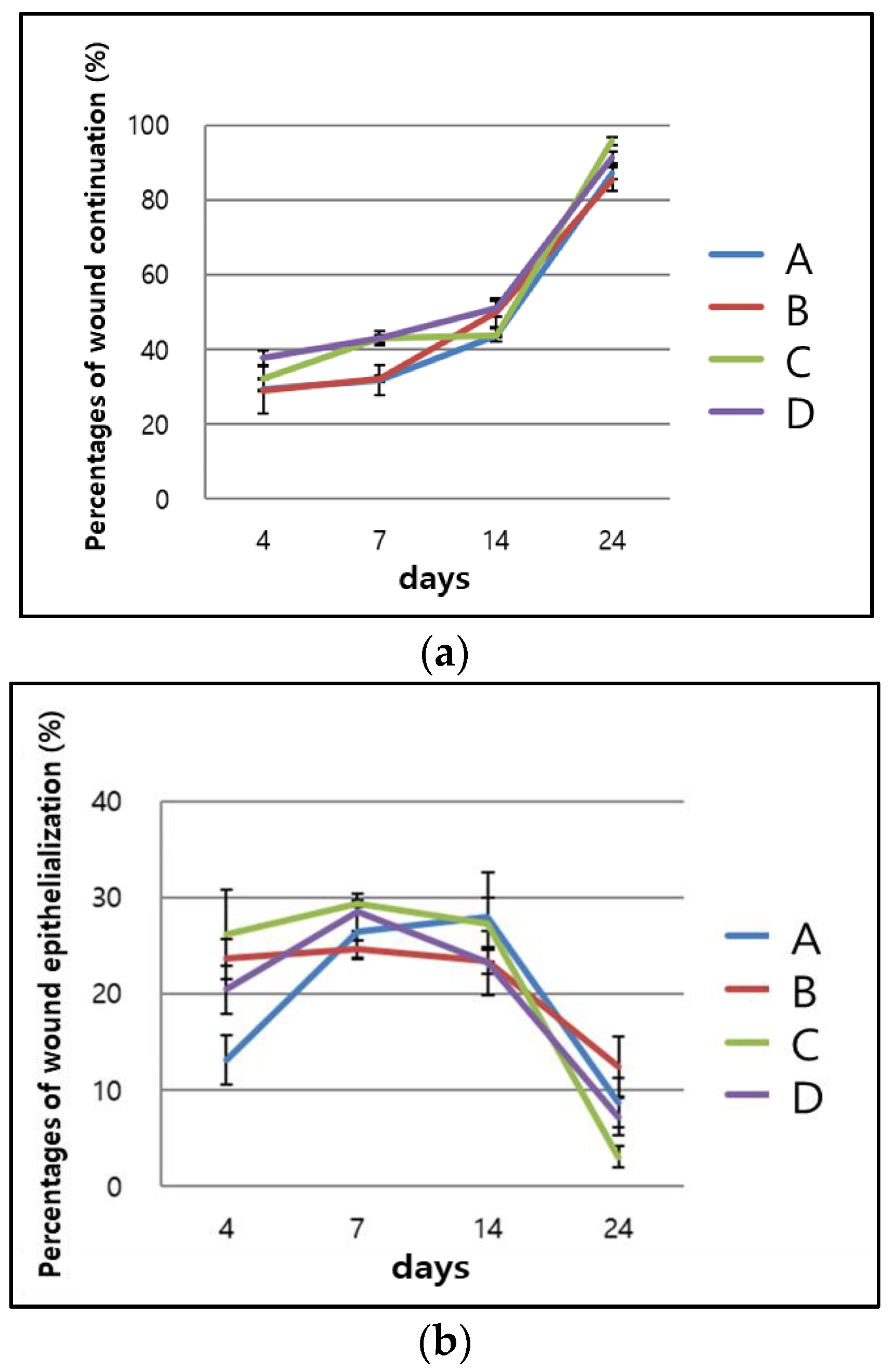
2.2. In Vivo Evaluation
2.2.1. Visual Evaluation
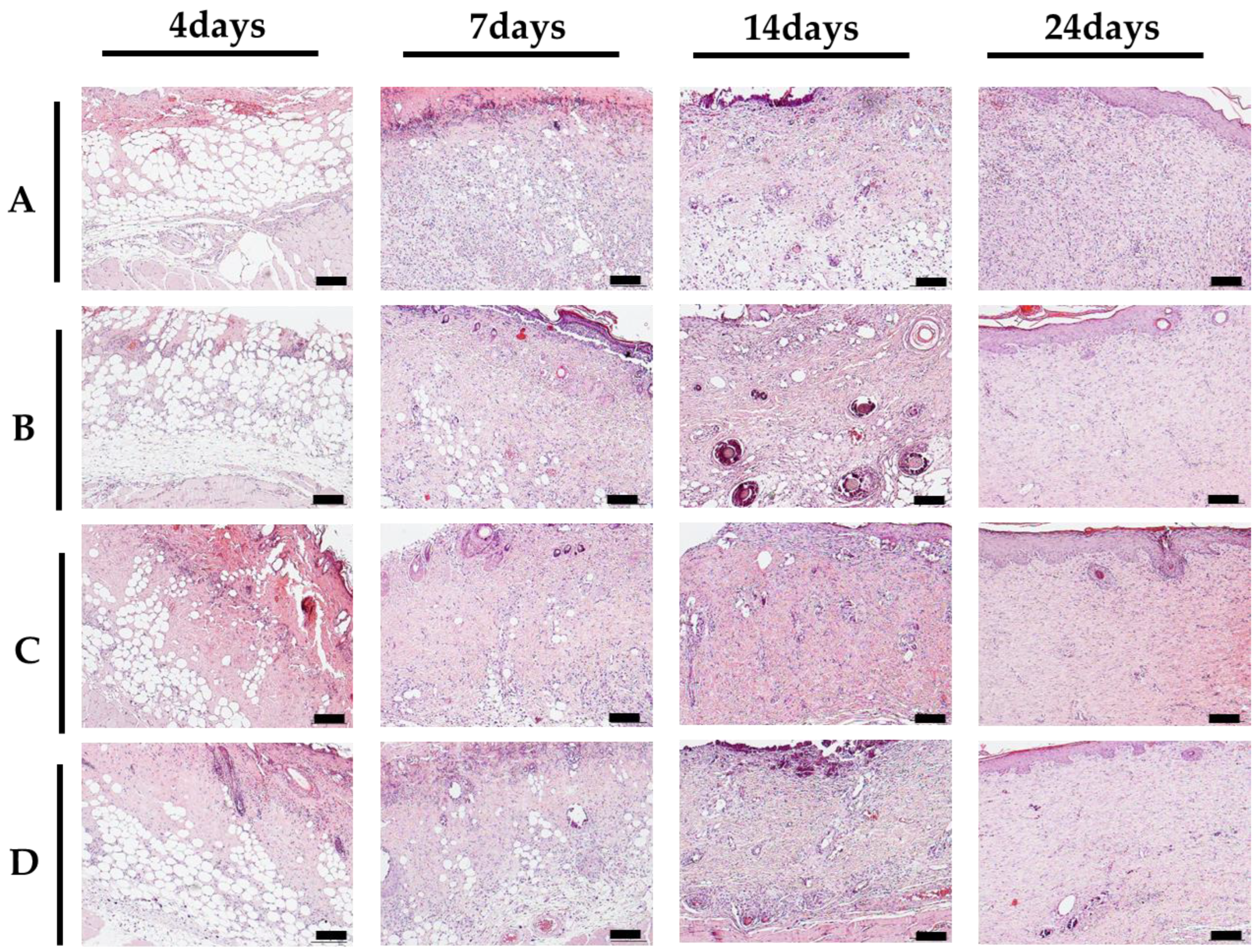
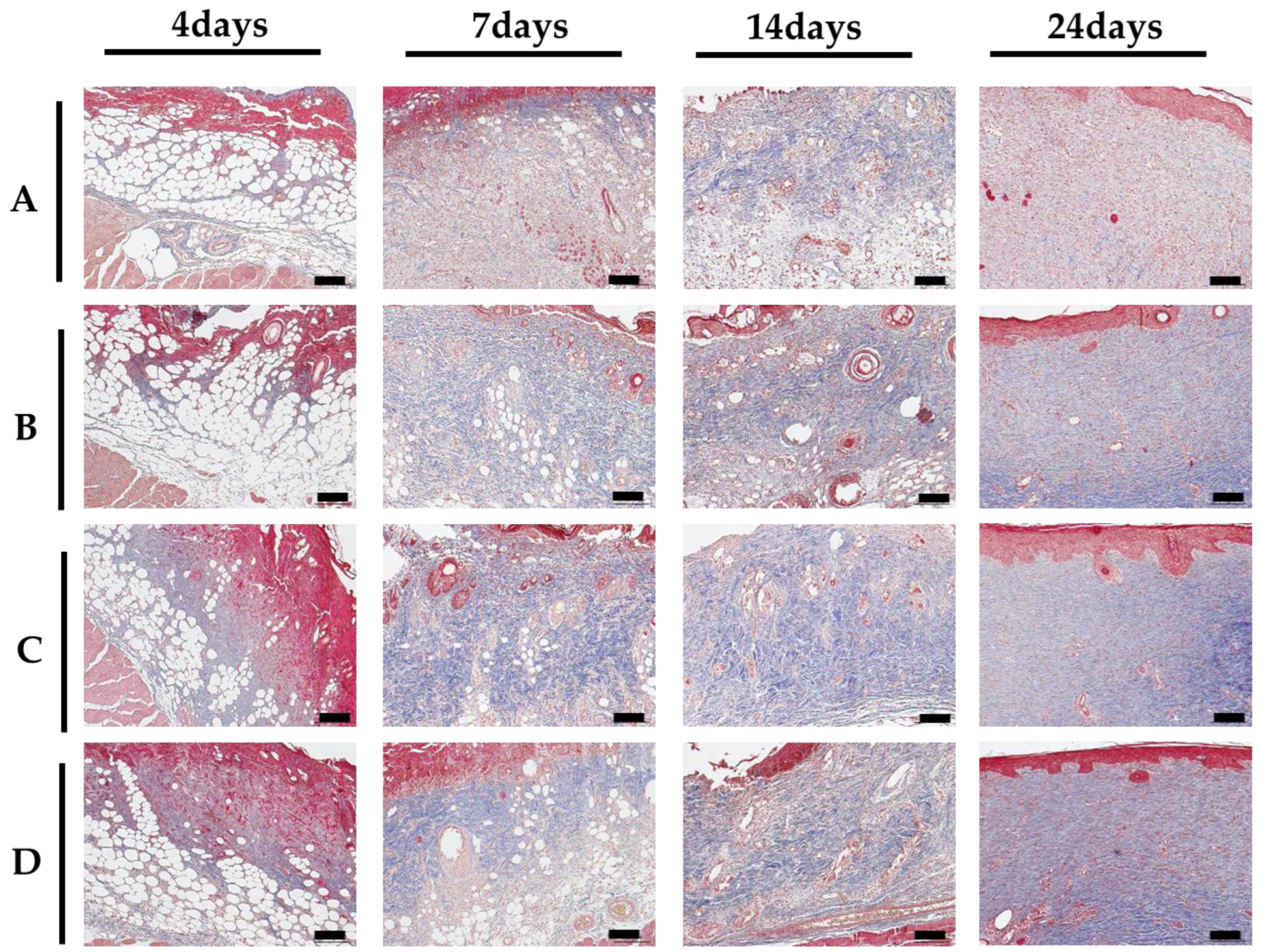
2.2.2. Histological Evaluation
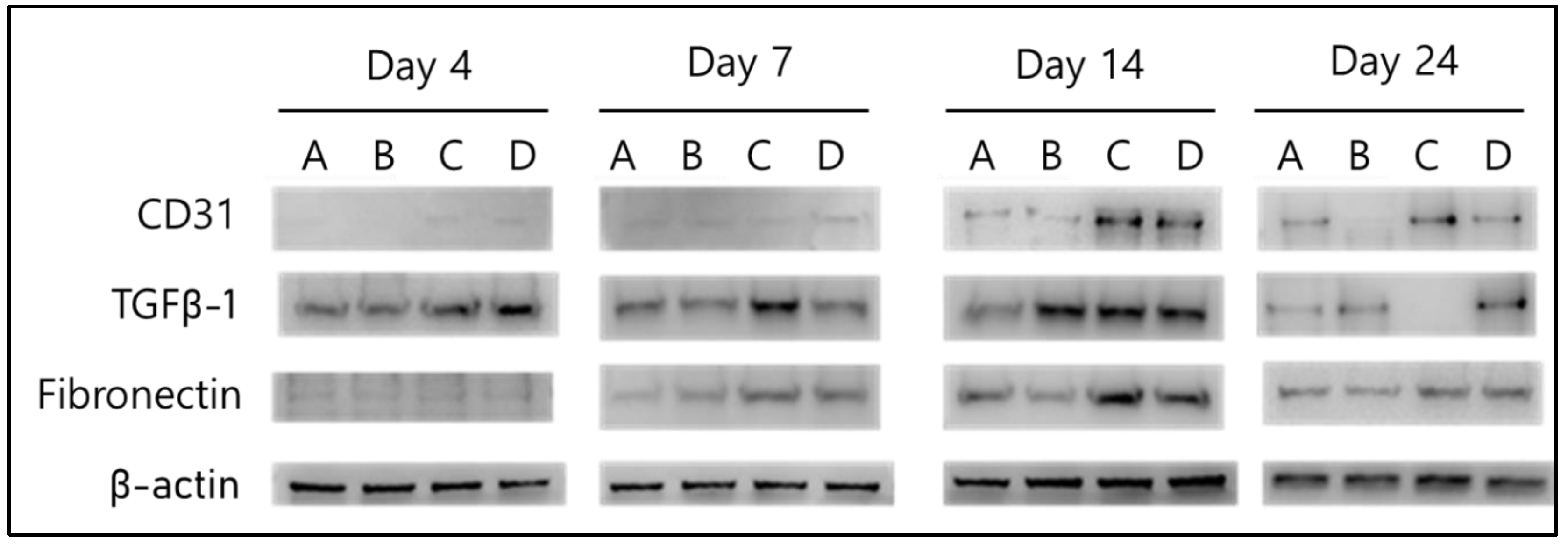
2.2.3. Biochemical Evaluation
3. Conclusions
4. Materials and Methods
4.1. Preparation of Reagents
4.2. Analysis of Stability of a Gel Formulation
4.3. In Vivo Model
4.4. Visual Analysis

4.5. Histological Analysis
4.6. Biochemical Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Paulino, L.C.; Tseng, C.H.; Strober, B.E.; Blaser, M.J. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J. Clin. Microbiol. 2006, 44, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-term persistence of the pathophysiologic response to severe burn injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef] [PubMed]
- Stanojcic, M.; Abdullahi, A.; Rehou, S.; Parousis, A.; Jeschke, M.G. Pathophysiological response to burn injury in adults. Ann. Surg. 2018, 267, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef]
- Venter, T.H.J.; Karpelowsky, J.S.; Rode, H. Cooling of the burn wound: The ideal temperature of the coolant. Burns 2007, 33, 917–922. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Choucair, M.; Phillips, T. A review of wound healing and dressings material. Ski. Aging 1998, 6, 37–43. [Google Scholar]
- Turner, T.D. Development of Wound Management Products in Chronic Wound Care. In Chronic Wound Care: A Clinical Source Book for Healthcare Professionals, 3rd ed.; Krasner, D., Rodeheaver, G., Sibbald, R.G., Eds.; HMP Communications: Wayne, PA, USA, 2001. [Google Scholar]
- Zarrintaj, P.; Ghorbani, S.; Barani, M.; Chauhan, N.P.S.; Yazdi, M.K.; Saeb, M.R.; Ramsey, J.D.; Hamblin, M.R.; Mozafari, M.; Mostafavi, E. Polylysine for skin regeneration: A review of recent advances and future perspectives. Bioeng. Transl. Med. 2022, 7, e10261. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hong, G.S.; Kwon, T.; Lim, J.O. Fabrication of oxygen releasing scaffold by embedding H2O2-PLGA microspheres into alginate-based hydrogel sponge and its application for wound healing. Appl. Sci. 2018, 8, 1492. [Google Scholar] [CrossRef]
- Hong, G.S.; Choi, J.; Choi, J.H. Evaluation of physical properties and biocompatibility of HA-Dex fusion hydrogel patch for atopic healing ability. J. Soc. Cosmet. Sci. Korea 2020, 46, 219–229. [Google Scholar]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Burdick, J.A.; Stevens, M.M. Biomedical hydrogels, biomaterials, artificial organs and tissue engineering. Woodhead Publ. Ser. Biomater. 2005, 107–115. [Google Scholar]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2007, 122, 34–47. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.; Li, D. Polysaccharide-based hydrogels for wound dressing: Design considerations and clinical applications. Bioeng. Biotechnol. 2022, 10, 8457350. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Joo, Y.J.; Jeon, H.K.; Choi, J.Y.; Hong, G.S. Development of Alginate-based Spray-type Hydrogel and Evaluation of Technical Compatibility for Container Application. J. Biomed. Eng. Res. 2023, 44, 64–72. [Google Scholar]
- Dabrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Martin, P. Wound Healing-Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [PubMed]
- Ohno, N.; Miura, N.N.; Chiba, N.; Adachi, Y.; Yadomae, T. Comparison of the Immunopharmacological Activities of Triple and Single-helical Schizophyllan in Mice. Biol. Pharm. Bull. 1995, 18, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, K.M.; Chang, I.S.; Kang, H.H.; Sim, Y.C. β-(1,6)-branched β-(1,3)-glucan in Skin Care. Allured’s Cosmet. Tolletries 2000, 115, 79–86. [Google Scholar]
- Jacob, G.H.; David, S.Z.; Jennifer, L.R.; Adam, J.M. Evaluating polymeric biomaterials to improve next generation wound dressing design. Biomater. Res. 2022, 26, 50. [Google Scholar]
- Kim, J.T.; Lim, S.; Kim, B.S.; Lee, D.Y.; Choi, J.H. Fabrication and Characterization of 3-D Porous Collagen Scaffold. J. Biomed. Eng. Res. 2014, 35, 192–196. [Google Scholar] [CrossRef]
- Hhauflaire-Uhoda, E.; Paquet, P.; Pierard, G.E. Dew point effect of cooled hydrogel pads on human stratum conreum biosurface. Dermatology 2008, 216, 37–39. [Google Scholar] [CrossRef]
- Soucy, J.R.; Shirzaei Sani, E.; Portillo Lara, R.; Diaz, D.; Dias, F.; Weiss, A.S.; Koppes, A.N.; Koppes, R.A.; Annabi, N. Photocross linkable Gelatin/Tropoelastin Hydrogel Adhesives for Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Holbert, M.D.; Kimble, R.M.; Chatfield, M.; Griffin, B.R. Effectiveness of a hydrogel dressing as an analgesic adjunct to first aid for the treatment of acute paediatric burn injuries: A prospective randomised controlled trial. BMJ Open 2021, 11, e039981. [Google Scholar] [CrossRef]
- Wiegand, C.; Heinze, T.; Hilper, U.C. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair. Regen. 2009, 17, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Masatoshi, O.; Toshiji, K.; Keisuke, K. Gelation rates of poly(vinyl alcohol) solution. Polymer 1992, 33, 5044–5048. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Joo, Y.-J.; Kang, R.J.; Jeon, H.K.; Hong, G.S. Effect of Spray-Type Alginate Hydrogel Dressing on Burn Wounds. Gels 2024, 10, 152. https://doi.org/10.3390/gels10020152
Choi JY, Joo Y-J, Kang RJ, Jeon HK, Hong GS. Effect of Spray-Type Alginate Hydrogel Dressing on Burn Wounds. Gels. 2024; 10(2):152. https://doi.org/10.3390/gels10020152
Chicago/Turabian StyleChoi, Jeong Yeon, Yong-Joon Joo, Ri Jin Kang, Hee Kyung Jeon, and Gyeong Sik Hong. 2024. "Effect of Spray-Type Alginate Hydrogel Dressing on Burn Wounds" Gels 10, no. 2: 152. https://doi.org/10.3390/gels10020152



