Preparation of a Novel Lignocellulose-Based Aerogel by Partially Dissolving Medulla Tetrapanacis via Ionic Liquid
Abstract
:1. Introduction
2. Results and Discussion
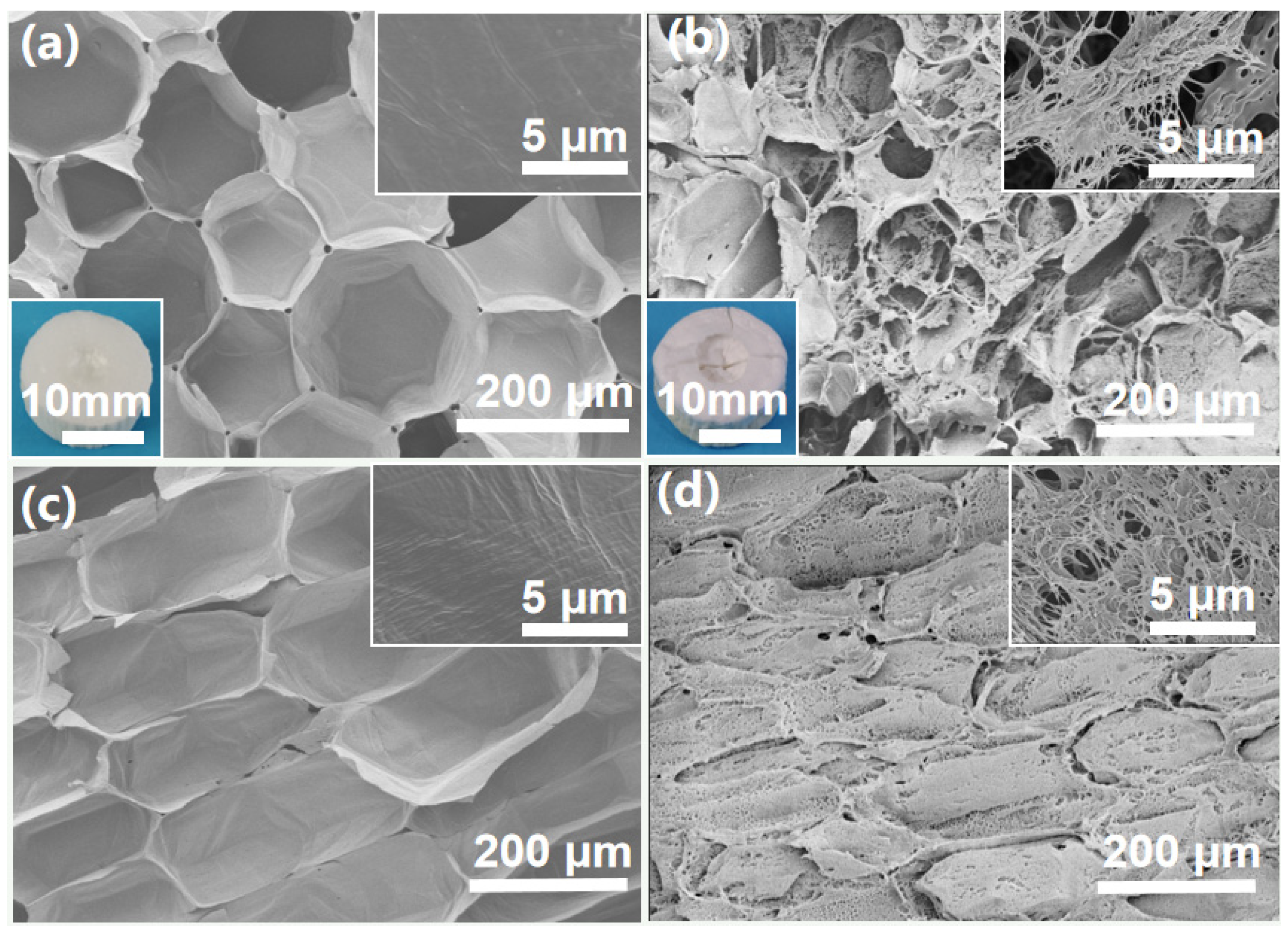
2.1. Morphological Features
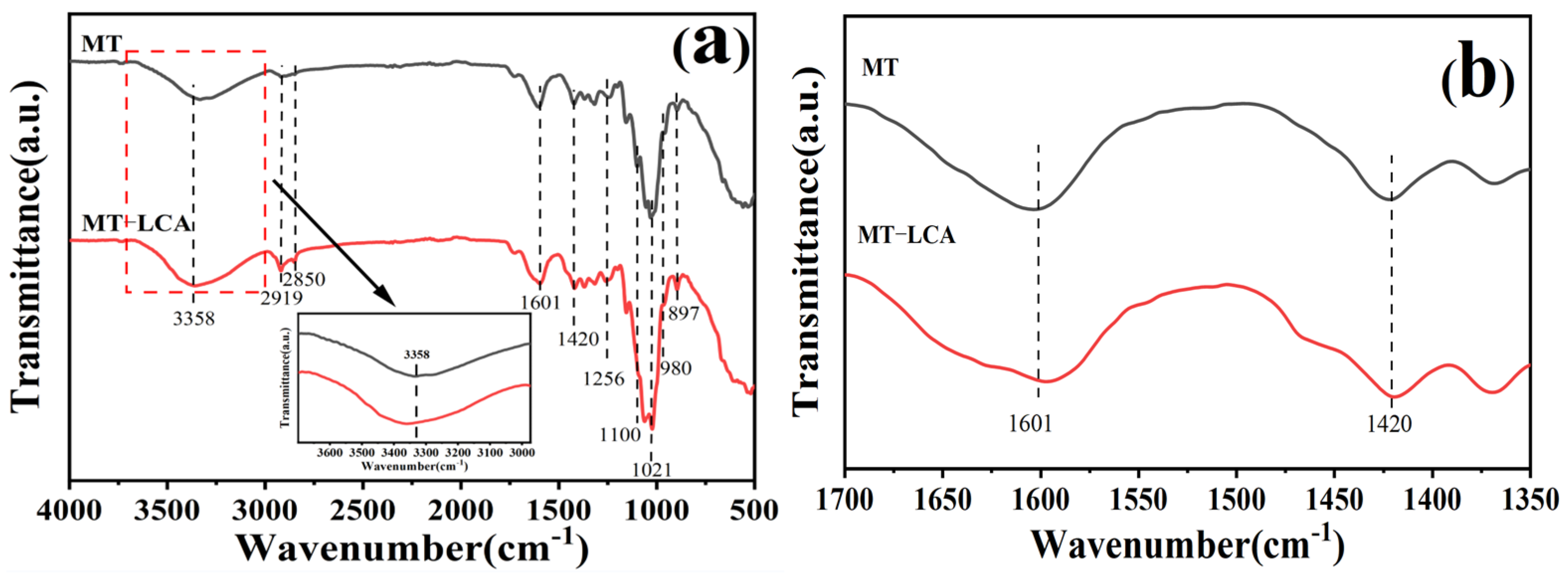
2.2. FTIR Analysis
2.3. XRD Analysis
2.4. Thermal Stability
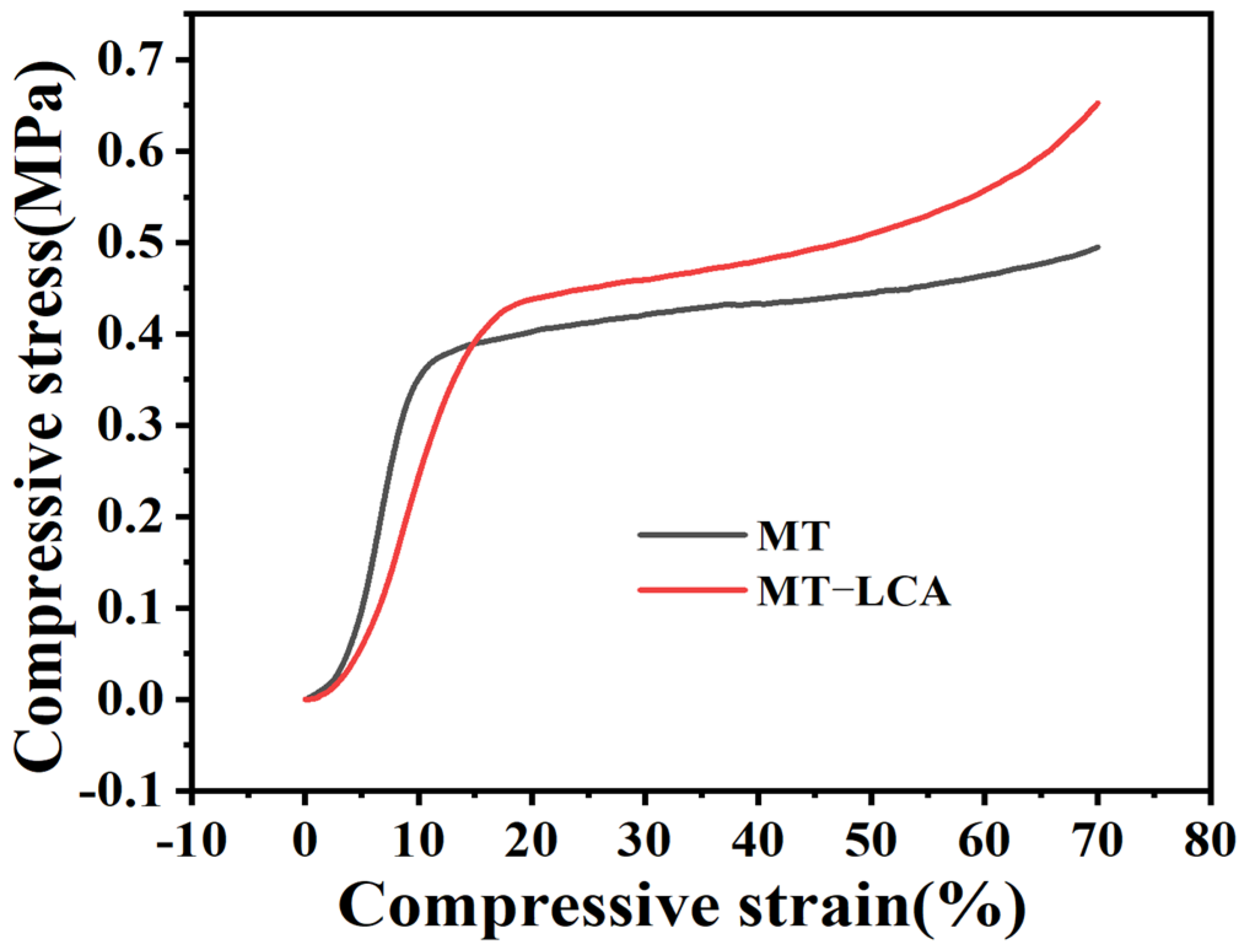
2.5. Compressive Tests
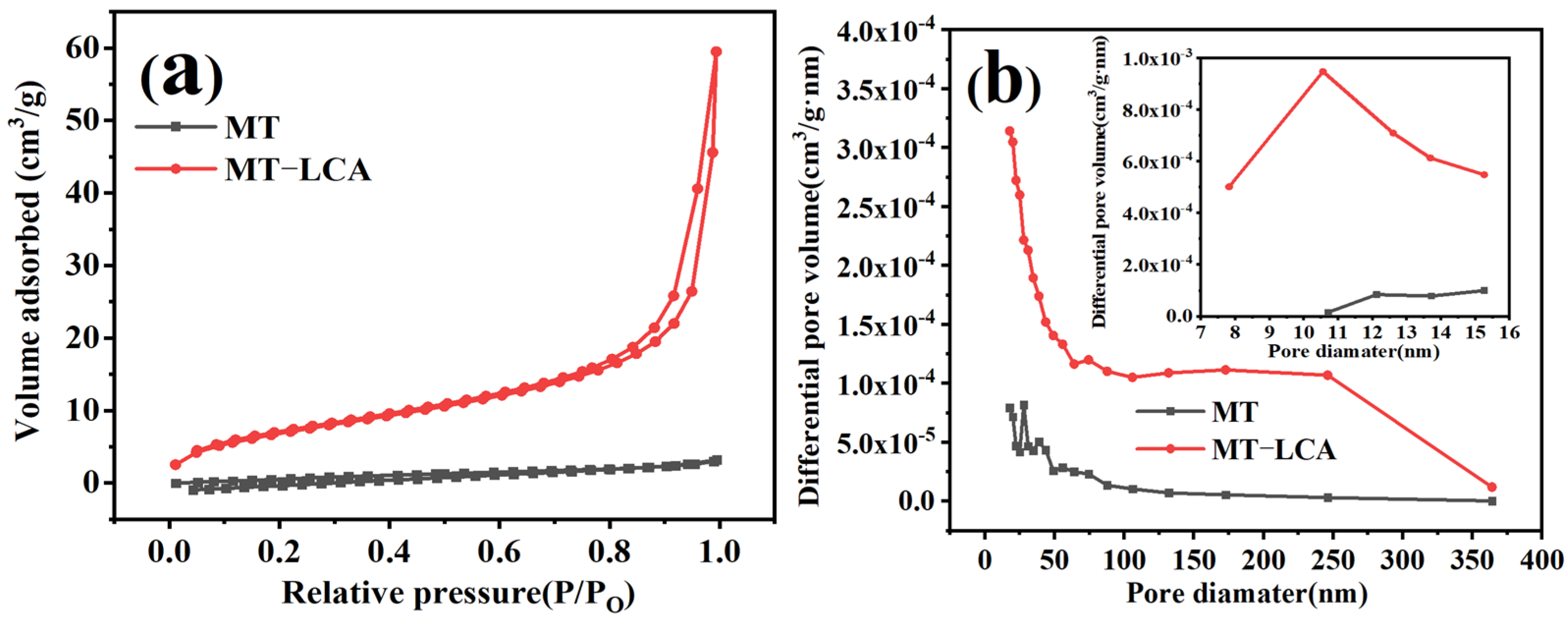
2.6. Porosity Analysis
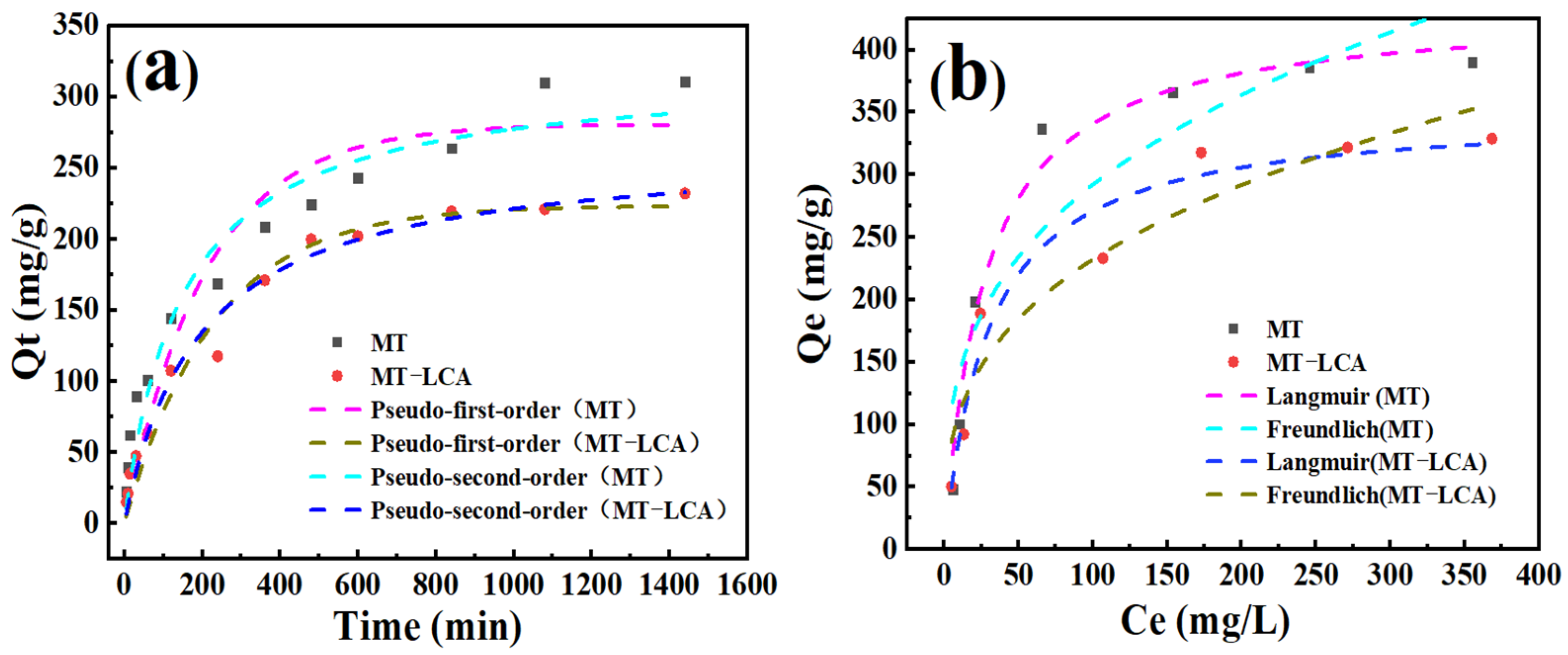
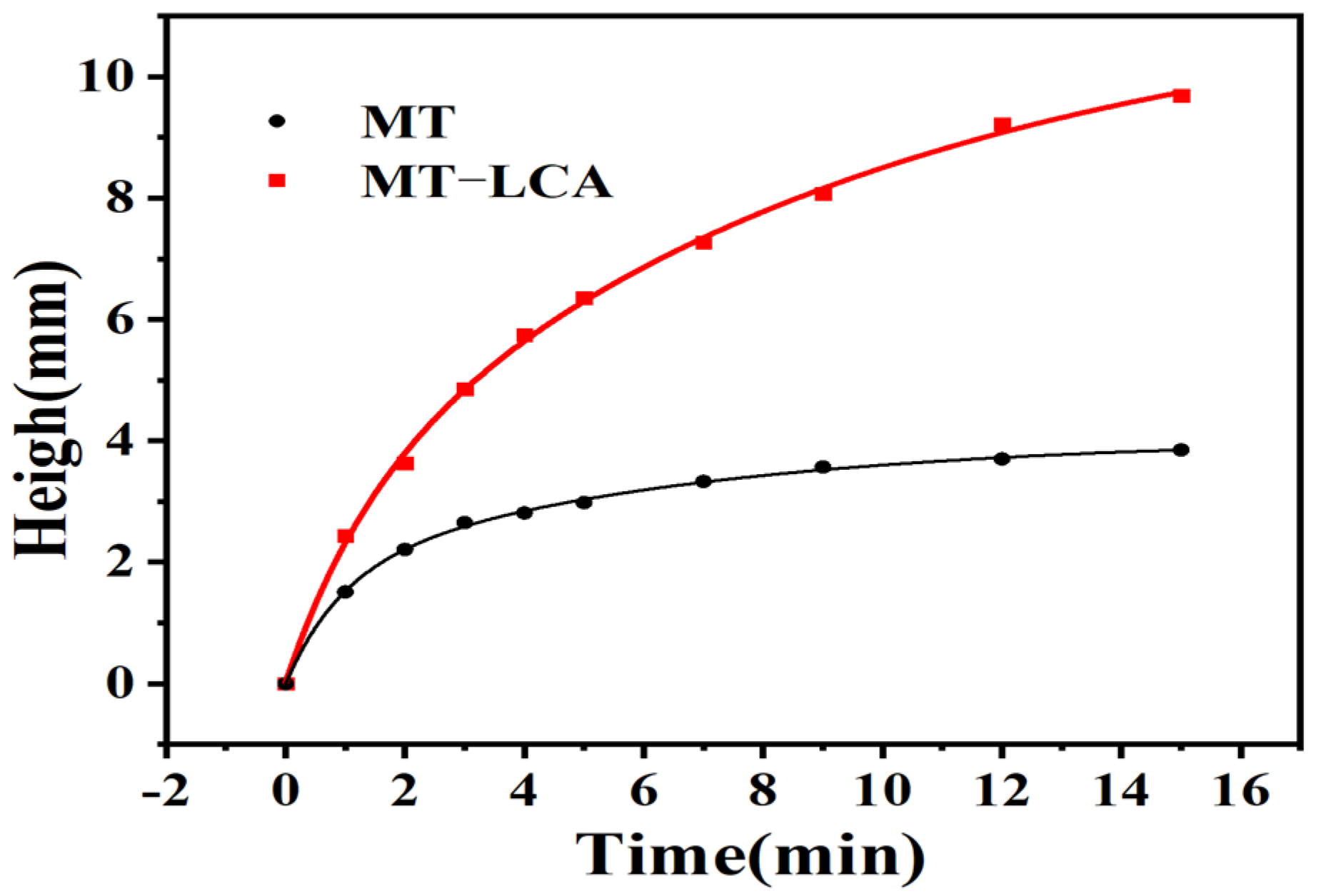
2.7. Absorption of Dye
2.8. Absorption Mechanism of Dye
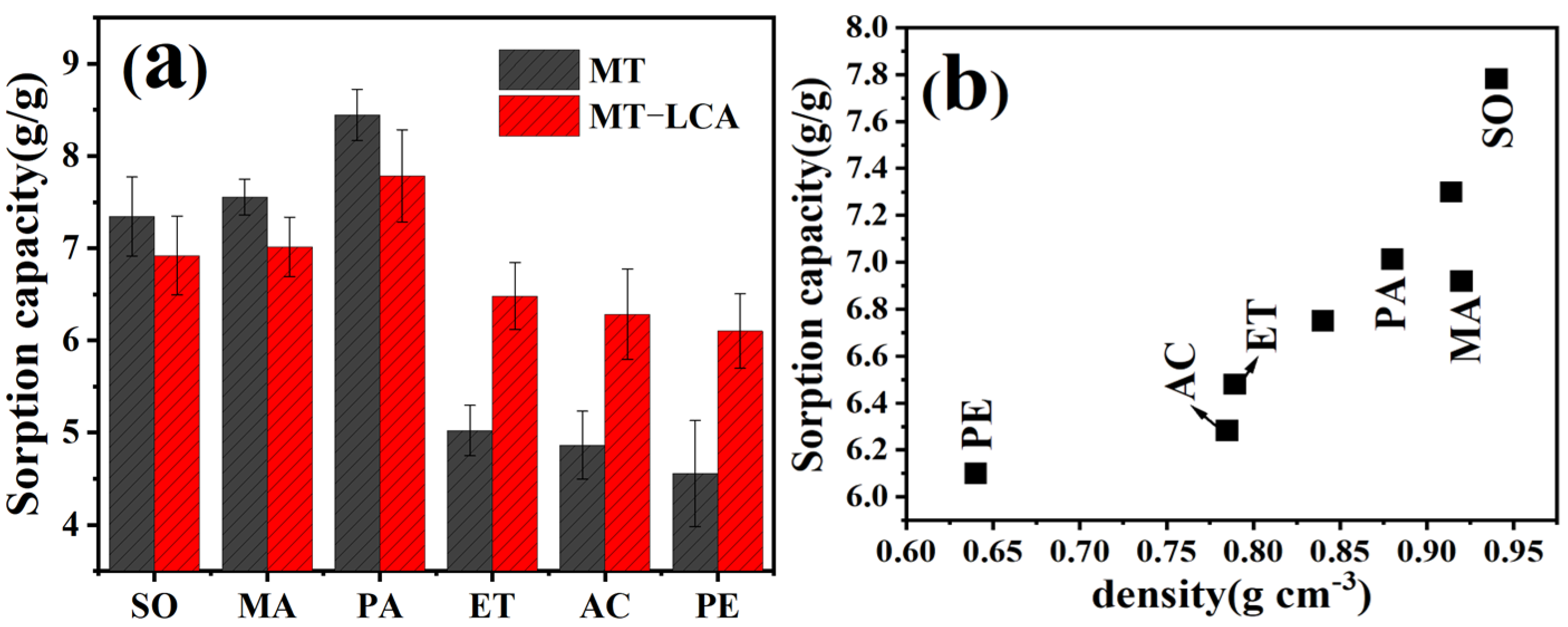
2.9. Absorption of Oil
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Lignocellulosic Aerogel
4.3. Characterizations
4.4. Density and Porosity
4.5. Adsorption of Dye
4.6. Adsorption Mechanism and Kinetics
4.7. Oil Sorption Capacity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Fu, H.; Liu, Y.; Dang, H.; Ye, L.; Conejio, A.N.; Xu, R. Review on biomass metallurgy: Pretreatment technology, metallurgical mechanism and process design. Int. J. Miner. Metall. Mater. 2022, 29, 1133–1149. [Google Scholar] [CrossRef]
- Deng, C.; Ma, F.; Xu, X.; Zhu, B.; Tao, J.; Li, Q. Allocation Patterns and Temporal Dynamics of Chinese Fir Biomass in Hunan Province, China. Forests 2023, 14, 286. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xu, F. Chemical Characteristics of Wood Cell Wall with an Emphasis on Ultrastructure: A Mini-Review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, H.; Liu, Z.; Zhang, L.; Wang, Z.; Guan, Y.; Gao, H. 3D Porous Structure-Inspired Lignocellulosic Biosorbent of Medulla tetrapanacis for Efficient Adsorption of Cationic Dyes. Molecules 2022, 27, 6228. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Cao, H.; Hu, D.; Chen, X.; Guan, Y.; Tang, J.; Gao, H. Ultra-efficient sorption of Cu2+ and Pb2+ ions by light biochar derived from Medulla tetrapanacis. Bioresour. Technol. 2019, 291, 121818. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.T.-K.; Chow, F.W.-N.; Cheung, K.Y.-C.; Zhang, X.-Y.; Mok, D.K.-W.; Kwan, Y.-W.; Chan, G.H.-H.; Leung, G.P.-H.; Cheung, K.-W.; Lee, S.M.-Y.; et al. Medulla Tetrapanacis water extract alleviates inflammation and infection by regulating macrophage polarization through MAPK signaling pathway. Inflammopharmacology 2023. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Yang, D.; Chen, H.; Li, H. Medulla tetrapanacis-derived O/N co-doped porous carbon materials for efficient oxygen reduction electrocatalysts and high-rate supercapacitors. Electrochim. Acta 2018, 272, 88–96. [Google Scholar] [CrossRef]
- Cai, X.; Shi, T.; Yu, C.; Liao, R.-P.; Ren, J. Sorption Characteristics of Methylene Blue on Medulla Tetrapanacis Biochar and its Activation Technology. Water Air Soil Pollut. 2023, 234, 223. [Google Scholar] [CrossRef]
- Melelli, A.; Jamme, F.; Beaugrand, J.; Bourmaud, A. Evolution of the ultrastructure and polysaccharide composition of flax fibres over time: When history meets science. Carbohydr. Polym. 2022, 291, 119584. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Xu, G.; Xu, Y.; Wang, F.; Shen, H. Ultralight, hydrophobic, sustainable, cost-effective and floating kapok/microfibrillated cellulose aerogels as speedy and recyclable oil superabsorbents. J. Hazard. Mater. 2021, 406, 124758. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Yang, Z.; Kuang, Y.; Li, T.; Li, Y.; Huang, H.; Kierzewski, I.; Liu, B.; He, S.; et al. Highly Compressible, Anisotropic Aerogel with Aligned Cellulose Nanofibers. ACS Nano 2018, 12, 140–147. [Google Scholar] [CrossRef]
- Gu, P.; Liu, W.; Hou, Q.; Ni, Y. Lignocellulose-derived hydrogel/aerogel-based flexible quasi-solid-state supercapacitors with high-performance: A review. J. Mater. Chem. A 2021, 9, 14233–14264. [Google Scholar] [CrossRef]
- Choi, H.-J. Assessment of sulfonation in lignocellulosic derived material for adsorption of methylene blue. Environ. Eng. Res. 2022, 27, 169–178. [Google Scholar] [CrossRef]
- Khakalo, A.; Tanaka, A.; Korpela, A.; Orelma, H. Delignification and Ionic Liquid Treatment of Wood toward Multifunctional High-Performance Structural Materials. ACS Appl. Mater. Interfaces 2020, 12, 23532–23542. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Cosby, T.; McFarland, J.; Durkin, D.P.; Trulove, P.C. Mesoporous xerogel cellulose composites from biorenewable natural cotton fibers. Carbohydr. Polym. 2022, 282, 119040. [Google Scholar] [CrossRef]
- Garemark, J.; Yang, X.; Sheng, X.; Cheung, O.; Sun, L.; Berglund, L.A.; Li, Y. Top-Down Approach Making Anisotropic Cellulose Aerogels as Universal Substrates for Multifunctionalization. ACS Nano 2020, 14, 7111–7120. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Y.; Zhou, J.; Yang, H. Filtration Capacity and Radiation Cooling of Cellulose Aerogel Derived from Natural Regenerated Cellulose Fibers. J. Nat. Fibers 2023, 20, 2181276. [Google Scholar] [CrossRef]
- Xing, H.; Fei, Y.; Cheng, J.; Wang, C.; Zhang, J.; Niu, C.; Fu, Q.; Cheng, J.; Lu, L. Green Preparation of Durian Rind-Based Cellulose Nanofiber and Its Application in Aerogel. Molecules 2022, 27, 6507. [Google Scholar] [CrossRef]
- Lei, E.; Gan, W.; Sun, J.; Wu, Z.; Ma, C.; Li, W.; Liu, S. High-Performance Supercapacitor Device with Ultrathick Electrodes Fabricated from All-Cellulose-Based Carbon Aerogel. Energy Fuels 2021, 35, 8295–8302. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Snari, R.M.; Al-Ahmed, Z.A.; Hossan, A.; Munshi, A.M.; Alfi, A.A.; El-Metwaly, N.M. Novel halochromic hydrazonal chromophore immobilized into rice-straw based cellulose aerogel for vapochromic detection of ammonia. J. Mol. Liq. 2022, 350, 118539. [Google Scholar] [CrossRef]
- Song, Y.; Wu, T.; Bao, J.; Xu, M.; Yang, Q.; Zhu, L.; Shi, Z.; Hu, G.-H.; Xiong, C. Porous cellulose composite aerogel films with super piezoelectric properties for energy harvesting. Carbohydr. Polym. 2022, 288, 119407. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef]
- Baysal, G.; Aydin, H.; Köytepe, S.; Seçkin, T. Comparison dielectric and thermal properties of polyurethane/organoclay nanocomposites. Thermochim. Acta 2013, 566, 305–313. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Wu, Z.; Liu, X.; Li, Q.; Zhu, W.; Jiang, Y.; Hu, L. Enhanced Mechanical Stability and Hydrophobicity of Cellulose Aerogels via Quantitative Doping of Nano-Lignin. Polymers 2023, 15, 1316. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, H.; Fauziyah, M.a.; Tomo, H.S.S.; Widiyastuti, W.; Nurtono, T. Fabrication of Hydrophobic Cellulose Aerogels from Renewable Biomass Coir Fibers for Oil Spillage Clean-Up. J. Polym. Environ. 2022, 30, 5228–5238. [Google Scholar] [CrossRef]
- Luo, M.; Wang, M.; Pang, H.; Zhang, R.; Huang, J.; Liang, K.; Chen, P.; Sun, P.; Kong, B. Super-assembled highly compressible and flexible cellulose aerogels for methylene blue removal from water. Chin. Chem. Lett. 2021, 32, 2091–2096. [Google Scholar] [CrossRef]
- Zhang, J.; Koubaa, A.; Xing, D.; Liu, W.; Wang, Q.; Wang, X.; Wang, H. Improving lignocellulose thermal stability by chemical modification with boric acid for incorporating into polyamide. Mater. Des. 2020, 191, 108589. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Liu, P. Hydrophobic Aerogel from Cotton Pulp: Reusable Adsorbents for Oil/Organic Solvent-Water Separation. J. Polym. Environ. 2023, 31, 2380–2387. [Google Scholar] [CrossRef]
- Rostamitabar, M.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Cellulose Aerogel Fibers for Wound Dressing Applications. Tissue Eng. Part A 2022, 28, S373. [Google Scholar]
- Zhuang, J.; Pan, M.; Zhang, Y.; Liu, F.; Xu, Z. Rapid adsorption of directional cellulose nanofibers/3-glycidoxypropyltrimethoxysilane/polyethyleneimine aerogels on microplastics in water. Int. J. Biol. Macromol. 2023, 235, 123884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, L.; Wang, Y.; Zhang, X.-F.; Yao, J. Construction of a hybrid graphene oxide/nanofibrillated cellulose aerogel used for the efficient removal of methylene blue and tetracycline. J. Phys. Chem. Solids 2021, 150, 109839. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef] [PubMed]
- Amaly, N.; El-Moghazy, A.Y.; Nitin, N.; Sun, G.; Pandey, P.K. Synergistic adsorption-photocatalytic degradation of tetracycline by microcrystalline cellulose composite aerogel dopped with montmorillonite hosted methylene blue. Chem. Eng. J. 2022, 430, 133077. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, H.; Zeng, F.; Li, X.; Sun, J.; Li, C.; Lin, H.; Su, Z. HKUST-1 modified ultrastability cellulose/chitosan composite aerogel for highly efficient removal of methylene blue. Carbohydr. Polym. 2021, 255, 117402. [Google Scholar] [CrossRef]
- Ruan, C.; Ma, Y.; Shi, G.; He, C.; Du, C.; Jin, X.; Liu, X.; He, S.; Huang, Y. Self-assembly cellulose nanocrystals/SiO2 composite aerogel under freeze-drying: Adsorption towards dye contaminant. Appl. Surf. Sci. 2022, 592, 153280. [Google Scholar] [CrossRef]
- Zhang, Y.; Sam, E.K.; Liu, J.; Lv, X. Biomass-Based/Derived Value-Added Porous Absorbents for Oil/Water Separation. Waste Biomass Valorization 2023, 14, 3147–3168. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, W.; Li, A.; Wang, B.; Jiang, R.; Song, Z.; Zhang, Y.; Li, Z. Graphene oxide and polyethyleneimine cooperative construct ionic imprinted cellulose nanocrystal aerogel for selective adsorption of Dy(III). Cellulose 2022, 29, 469–481. [Google Scholar] [CrossRef]
- Padmavathy, K.S.; Madhu, G.; Haseena, P.V. A study on Effects of pH, Adsorbent Dosage, Time, Initial Concentration and Adsorption Isotherm Study for the Removal of Hexavalent Chromium (Cr (VI)) from Wastewater by Magnetite Nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Imran, M.; Islam, A.; Farooq, M.U.; Ye, J.; Zhang, P. Characterization and adsorption capacity of modified 3D porous aerogel from grapefruit peels for removal of oils and organic solvents. Environ. Sci. Pollut. Res. 2020, 27, 43493–43504. [Google Scholar] [CrossRef] [PubMed]

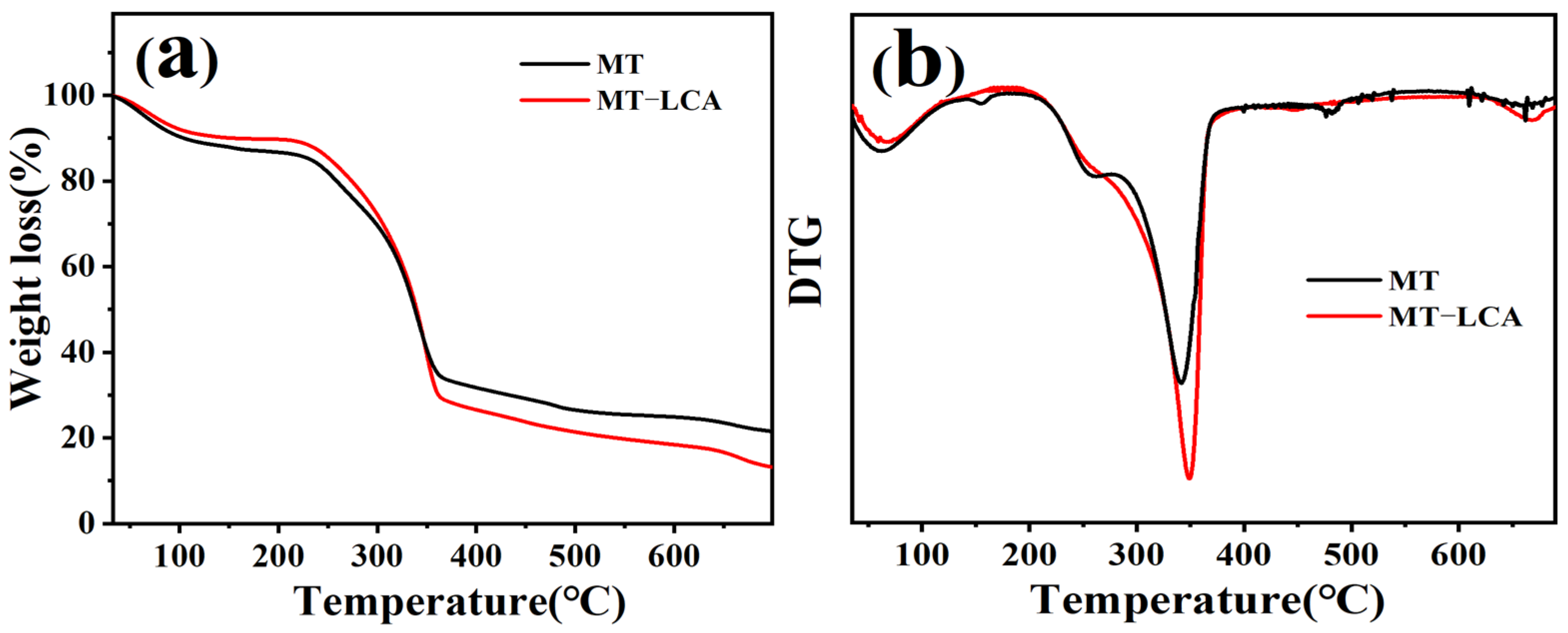


| Samples | Td5%/°C | TMax/°C |
|---|---|---|
| MT | 64 | 166/364 |
| MT-LCA | 93 | 135/364 |
| Samples | Density (g/cm3) | Compressive Modulus (MPa) | Compressive Strength (MPa) |
|---|---|---|---|
| MT | 0.050 ± 0.006 | 5.223 ± 0.121 | 0.411 ± 0.011 |
| MT-LCA | 0.084 ± 0.008 | 2.484 ± 0.081 | 0.472 ± 0.051 |
| Samples | Porosity (%) | Total Pore Volume (cm3/g) | Average Pore Size (nm) | Specific Surface Area (m2/g) |
|---|---|---|---|---|
| MT | 38 | 0.001 | 36.71 | 0.669 |
| MT-LCA | 51 | 0.041 | 22.17 | 36.576 |
| Models | Parameters | MT | MT-LCA | |
|---|---|---|---|---|
| Kinetic adsorption fitting curves | Quasi-first-order kinetic models | Qe | 280.68 | 223.56 |
| K1 | 0.0048 | 0.0044 | ||
| R2 | 0.90 | 0.96 | ||
| Quasi-second-order kinetic models | Qe | 318.35 | 263.78 | |
| K2 | 0.000024 | 0.000019 | ||
| R2 | 0.95 | 0.99 | ||
| Adsorption isotherms | Langmuir | Qmax | 425.86 | 408.15 |
| KL | 0.040 | 0.038 | ||
| R2 | 0.98 | 0.95 | ||
| Freundlich | KF | 67.56 | 63.89 | |
| n | 3.15 | 3.10 | ||
| R2 | 0.84 | 0.90 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, L.; Shi, X.; Zhang, J.; Shu, Z.; Zhou, L. Preparation of a Novel Lignocellulose-Based Aerogel by Partially Dissolving Medulla Tetrapanacis via Ionic Liquid. Gels 2024, 10, 138. https://doi.org/10.3390/gels10020138
Quan L, Shi X, Zhang J, Shu Z, Zhou L. Preparation of a Novel Lignocellulose-Based Aerogel by Partially Dissolving Medulla Tetrapanacis via Ionic Liquid. Gels. 2024; 10(2):138. https://doi.org/10.3390/gels10020138
Chicago/Turabian StyleQuan, Long, Xueqian Shi, Jie Zhang, Zhuju Shu, and Liang Zhou. 2024. "Preparation of a Novel Lignocellulose-Based Aerogel by Partially Dissolving Medulla Tetrapanacis via Ionic Liquid" Gels 10, no. 2: 138. https://doi.org/10.3390/gels10020138





