Botanicals and Phosphonate Show Potential to Replace Copper for Control of Potato Late Blight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolate and Inoculum Production for In Vitro Assays
2.2. Selection and Preparation of Antifungal Agents
2.3. In Vitro Experiments
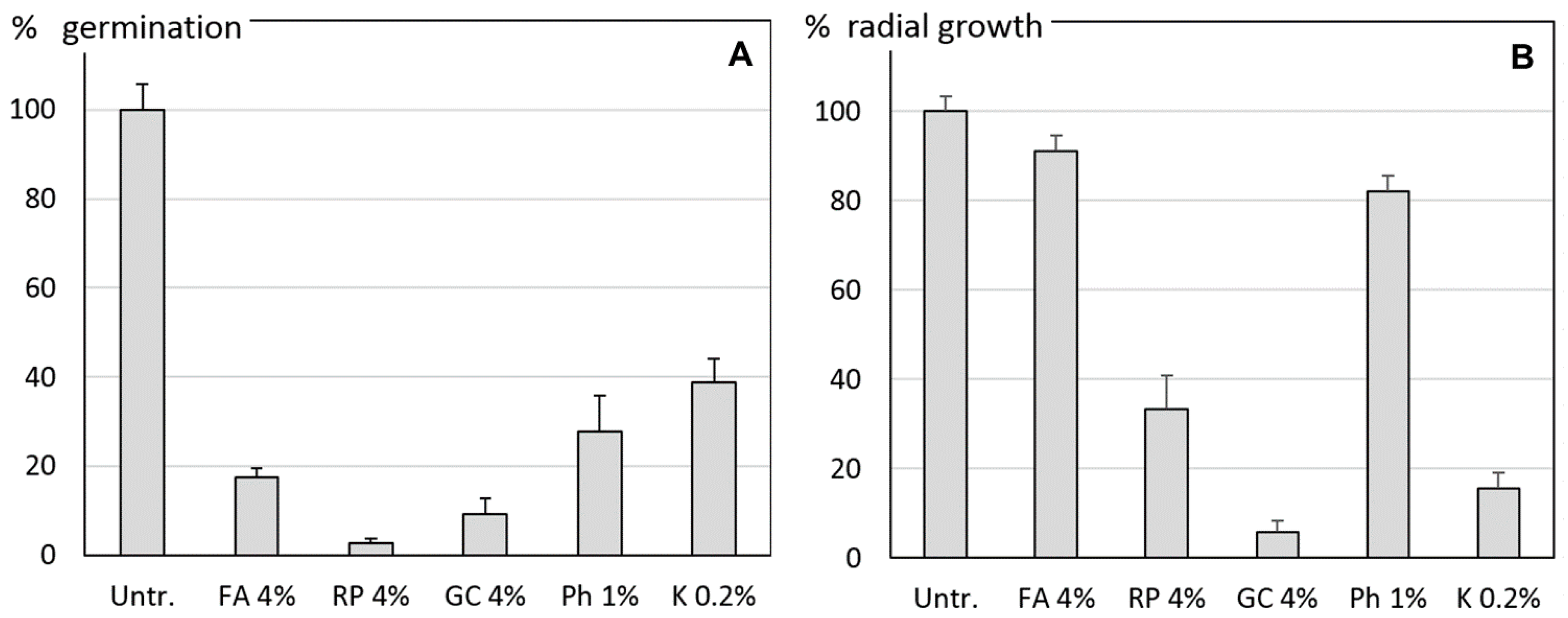
2.3.1. Sporangial Germination
2.3.2. Mycelial Growth
2.4. Field Experiments
2.5. Statistical Analysis
3. Results
3.1. In Vitro Experiments
3.2. Field Experiments
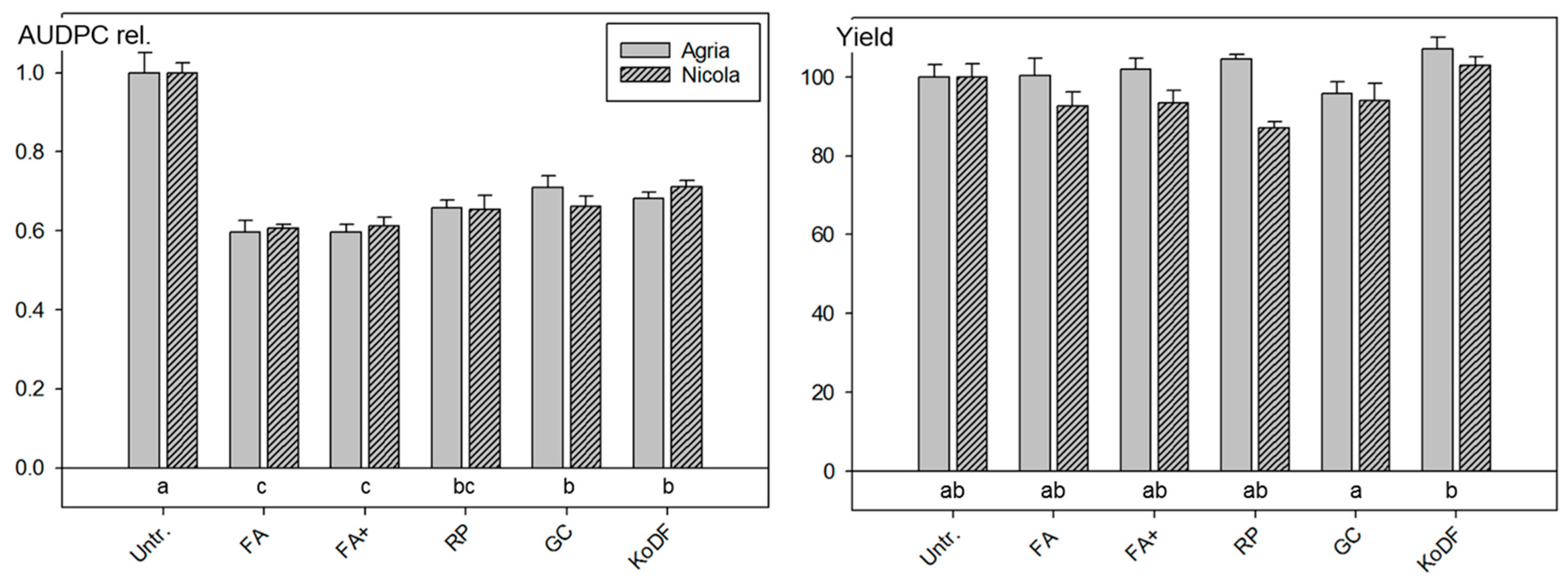
3.2.1. Field Experiment 2010
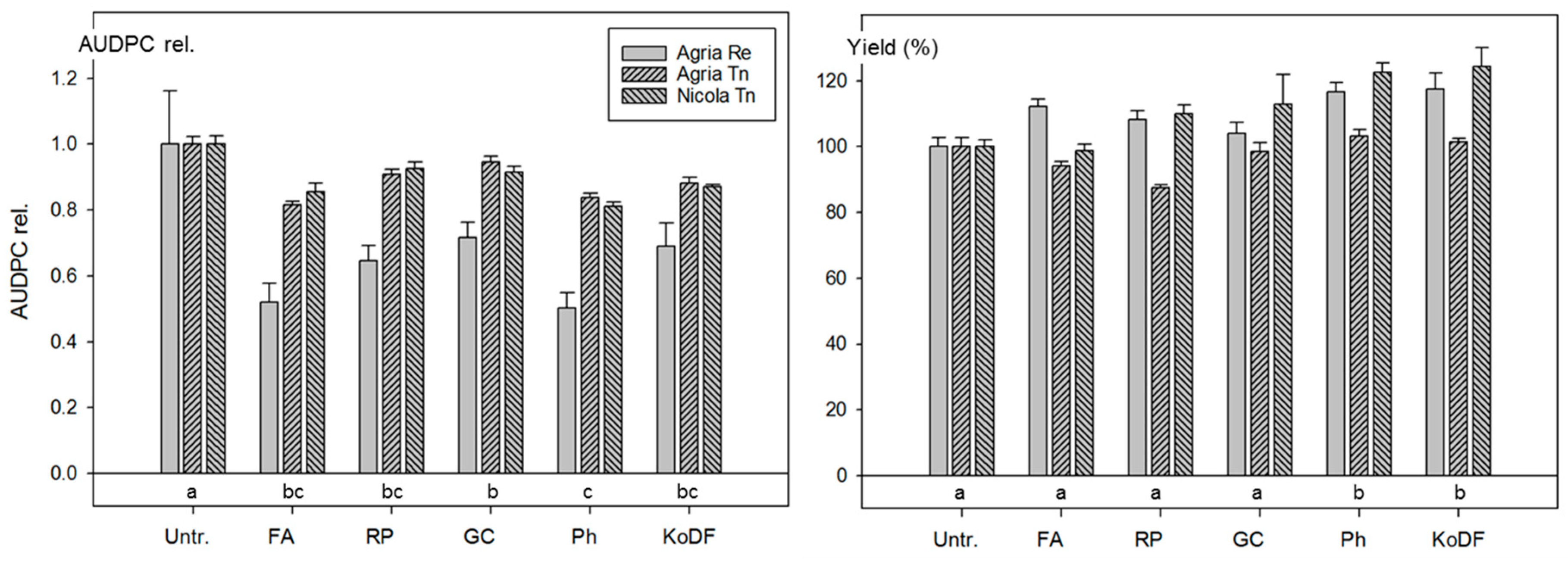
3.2.2. Field Experiments 2011
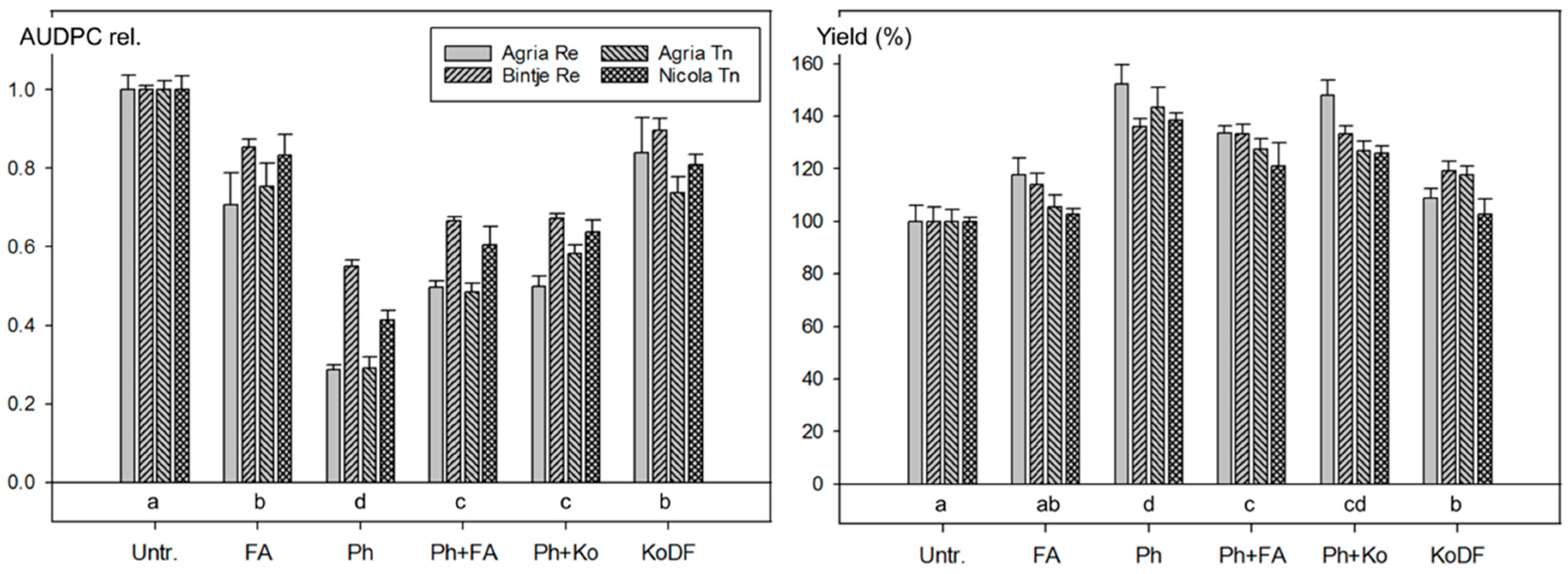
3.2.3. Field Experiments 2012
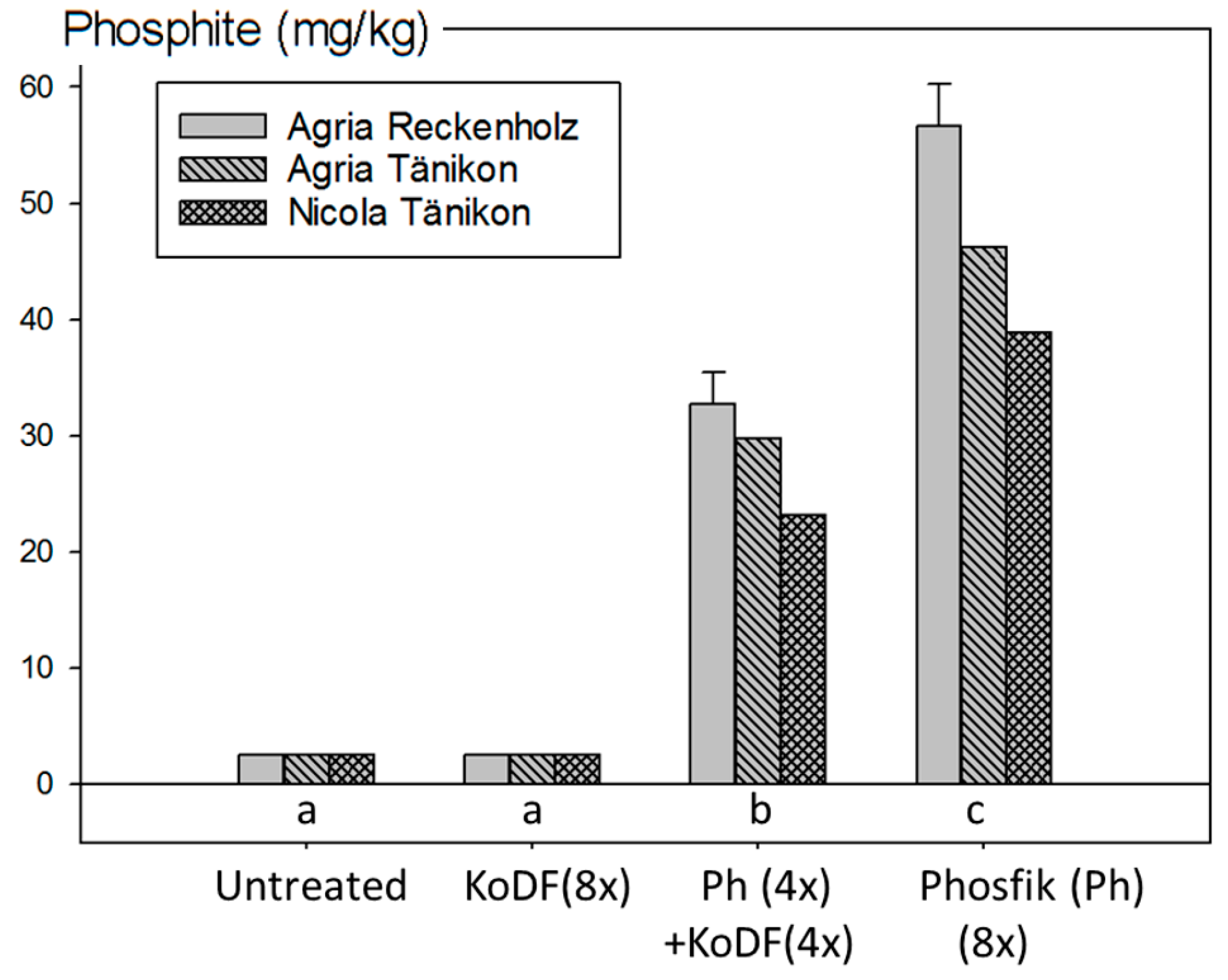
3.2.4. Phosphite Residues from the Field Experiments in 2013
4. Discussion
4.1. Disease Control with Botanicals
4.1.1. Disease Control
4.1.2. Effect on Yield
4.2. Disease Control with Phosfik®
Phosphite Residues in Potato Tubers
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Visser, R.G.F.; van der Vossen, E.A.G. Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Potato Res. 2008, 51, 47–57. [Google Scholar] [CrossRef]
- Fry, E.W.; Goodwin, S.B. Re-emergence of Potato and Tomato Late Blight in the United States. Plant Dis. 1997, 81, 1349–1357. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide resistance in crop pathogens: How can it be managed? In FRAC Monograph 1, 2nd ed.; FRAC: Biel, Switzerland, 2007; p. 56. ISBN 90-72398-07-6. [Google Scholar]
- Speiser, B.; Tamm, L.; Amsler, T.; Lambion, J.; Bertrand, C.; Hermansen, A.; Ruissen, M.A.; Haaland, P.; Zarb, J.; Santos, J.; et al. Improvement of late blight management in organic potato production systems in Europe: Field tests with more resistant potato varieties and copper based fungicides. Biol. Agric. Hortic. 2006, 23, 393–412. [Google Scholar] [CrossRef]
- Buenemann, E.K.; Schwenke, G.D.; Van Zwieten, L. Impact of agricultural inputs on soil organisms—A review. Aust. J. Soil. Res. 2006, 44, 379–406. [Google Scholar] [CrossRef]
- Leifert, C.; Wilcockson, S.J. Blight-MOP: Development of a systems approach for the management of late blight (caused by Phytophthora infestans) in EU organic potato production. Biol. Agric. Hortic. 2005, 23, 393–412. [Google Scholar]
- Stephan, D.; Schmitt, A.; Martins Carvalho, S.; Seddon, V.; Koch, E. Evaluation of biocontrol preparations and plant extracts for the control of Phytophthora infestans on potato leaves. Eur. J. Plant Pathol. 2005, 112, 235–246. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Leifert, C. Alternative treatments for late blight control in organic potato: Antagonistic micro-organisms and compost extracts for activity against Phytophthora infestans. Potato Res. 2005, 48, 181–189. [Google Scholar] [CrossRef]
- Dorn, B.; Musa, T.; Krebs, H.; Fried, P.M.; Forrer, H.R. Control of late blight in organic potato production: Evaluation of copper-free preparations under field, growth chamber and laboratory conditions. Eur. J. Plant Pathol. 2007, 119, 217–240. [Google Scholar] [CrossRef]
- Krebs, H.; Dorn, B.; Forrer, H.R. Control of late blight of potato with medicinal plant suspensions. Agrarforschung 2006, 13, 16–21. (In German) [Google Scholar]
- Gindro, K.G.; Godard, S.; De Groote, I.; Viret, O.; Forrer, H.R.; Dorn, B. Is it possible to induce grapevine defence mechanisms? A new method to evaluate the potential of elicitors. Rev. Suisse Vitic. Arboric. Hortic. 2007, 39, 377–383. (In French) [Google Scholar]
- Gessler, C.; Pertot, I.; Perazzoli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Dagostin, S.; Schaerer, H.J.; Pertot, I.; Tamm, L. Are there alternatives to copper for controlling grapevine downy mildew in organic viticulture? Crop Prot. 2011, 30, 776–788. [Google Scholar] [CrossRef]
- Magarey, P.A.; Wachtel, M.F.; Newton, M.R. Evaluation of phosphonate, fosetyl-AI and several phenylamide fungicides for post-infection control of grapevine downy mildew caused by Plasmopara viticola. Australas. Plant Pathol. 1991, 20, 34–40. [Google Scholar] [CrossRef]
- Bassin, S.; Forrer, H.R. Field screening of copper free fungicides against potato late blight. Agrarforschung 2001, 8, 124–129. (In German) [Google Scholar]
- Fact Sheet “Bundesverband Naturkost Naturwaren” Phosphonic Acid, Potassium Phosphonate (Potassium Salt of Phosphonic Acid), Fosetyl-Aluminium. Summary of Current Knowledge. May 2017. Available online: http://www.n-bnn.de/downloadbereich (accessed on 20 October 2017).
- Wieland, M.; Bauer, N.; Ströher Kolberg, D.I.; Wildgrube, C.; Anastassiades, M.; Scherbaum, E. Phosphonic Acid: Pesticide or “Foliar Fertilizer”? Residues in Organic and Conventional Samples from the German Market. European Pesticide Residue Workshop (EPRW): Dublin, Ireland, June/July 2014; Available online: http://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EPRW2014_Wieland_Phosponic-Acid-PesticideFoliarFertilizier_114.pdf (accessed on 25 October 2017).
- Kühne, S.; Friedrich, B. 14th Expert Discussion: Plant Protection in Organic Farming—Problems and Solutions—Phosphonates. Berlin-Dahlem, Germany. 9 November 2010. Available online: http://orgprints.org/18618/1/kuehne_2010_fachgespr-pflanzensch_phosphonate.pdf (accessed on 20 October 2017).
- Palm, G.; Kruse, P. Phosphonates for apple production? Mitt. OVR 2014, 69, 77–87. (In German) [Google Scholar]
- Thao, H.T.B.; Yamaka, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Musa-Steenblock, T.; Forrer, H.R. Bio-PhytoPRE—A decision support system for late blight control in organic potato production in Switzerland. In Beiträge zur 8. Wissenschaftstagung Ökologischer Landbau: Ende der Nische, Kassel, Germany, 1–4 March 2005; Heß, J., Rahmann, G., Eds.; University Press GmbH: Kassel, Germany, 2005. (In German) [Google Scholar]
- Hansen, J.G.; Andersson, B.; Bain, R.; Ritchie, F.; Gulbis, G.; Kildea, S.; Cooke, L.; Dubois, L.; Chatot, C.; Filippov, A.; et al. The development and control of Late Blight (Phytophthora infestans) in Europe in 2010 and 2011. PPO Spec. Rep. 2012, 15, 11–31. [Google Scholar]
- Krebs, H. Agroscope, Zurich, Switzerland. Group Ecological Plant Protection: Activity Report Heinz Krebs—Results 2010 (Internal Report of Research Group for Ecological Plant Protection, in German). Unpublished work. 2010. [Google Scholar]
- Hansen, J.G.; Andersson, B.; Bain, R.; Ritchie, G.; Kildea, S.; Cooke, L.; Filippov, A.; Hannukkala, A.; Hausladen, H.; Hausvater, E.; et al. The development and control of Late Blight (Phytophthora infestans) in Europe in 2012. PPO Spec. Rep. 2014, 16, 11–26. [Google Scholar]
- Forrer, H.R.; Hecker, A.; Musa, T.; Schwab, F.; Bucheli, T.D.; Wettstein, F.E.; Vogelgsang, S. Fusarium head blight control and prevention of mycotoxin contamination in wheat with botanicals and tannic acid. Toxins 2014, 6, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Vogelgsang, S.; Bänziger, I.; Krebs, H.; Legro, R.J.; Sanchez-Sava, V.; Forrer, H.R. Control of Microdochium majus in winter wheat with botanicals—From laboratory to the field. Plant Pathol. 2013, 62, 1020–1029. [Google Scholar] [CrossRef]
- Freeman, B.C.; Beattie, G.A. An overview of plant defenses against pathogens and herbivores. Plant Health Instr. 2008. [Google Scholar] [CrossRef]
- Tamm, L.; Thürig, B.; Fliessbach, A.; Goltlieb, A.E.; Karavani, S.; Cohen, Y. Elicitors and soil management to induce resistance against fungal plant diseases. NJAS Wagening. J. Life Sci. 2011, 58, 131–137. [Google Scholar] [CrossRef]
- Borza, T.; Schofield, A.; Sakthivel, G.; Bergese, J.; Gao, X.; Rand, J.; Wang-Pruski, G. Ion chromatography analysis of phosphite uptake and translocation by potato plants: Dose-dependent uptake and inhibition of Phytophthora infestans development. Crop Prot. 2014, 56, 74–81. [Google Scholar] [CrossRef]
- Kromann, P.; Pérez, W.G.; Taipe, A.; Schulte-Geldermann, E.; Sharma, B.P.; Andrade-Piedra, J.L.; Forbes, G.A. Use of phosphonate to manage foliar potato late blight in developing countries. Plant Dis. 2012, 96, 1008–1015. [Google Scholar] [CrossRef]
- Guest, D.; Grant, B. The complex action of phosphonates as antifungal agents. Biol. Rev. 1991, 66, 159–187. [Google Scholar] [CrossRef]
- Machinandiarena, M.F.; Lobato, M.C.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B. Potassium phosphite primes defense responses in potato against Phytophthora infestans. J. Plant Physiol. 2012, 169, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Borza, T.; Peters, R.D.; Wu, Y.; Schofield, A.; Rand, J.; Ganga, Z.; Al-Mughrabi, K.I.; Coffin, R.H.; Wang-Pruski, G. Phosphite uptake and distribution in potato tubers following foliar and postharvest applications of phosphite-based fungicides for late blight control. Ann. Appl. Biol. 2017, 170, 127–139. [Google Scholar] [CrossRef]
- Liljeroth, E.; Lankinen, A.; Wiik, L.; Burra, D.D.; Alexandersson, E.; Andreasson, E. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Prot. 2016, 86, 42–55. [Google Scholar] [CrossRef]
- Wang-Pruski, G.; Coffin, R.H.; Peters, R.D.; Al-Mughrabi, K.I.; Platt, H.W.; Pinto, D.; Veenhuis-MacNeill, S.; Hardy, W.; Lim, S.; Astatkie, T. Phosphorous acid for late blight suppression in potato leaves. Am. J. Plant Sci. Biotechnol. 2010, 4, 25–29. [Google Scholar]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance potassium phosphonates. EFSA J. 2012, 10, 2963. Available online: https://www.efsa.europa.eu/de/efsajournal/pub/2963 (accessed on 20 October 2017).
- Mayton, H.; Myers, K.; Fry, W.E. Potato late blight in tubers—The role of foliar phosphonate applications in suppressing pre-harvest tuber infections. Crop Prot. 2008, 27, 943–950. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Statement on the dietary risk assessment for proposed temporary maximum residue levels (t-MRLs) for fosetyl-Al in certain crops. EFSA J. 2014, 12, 3695. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2014.3695/epdf (accessed on 20 October 2017).
- Schroetter, S.; Angeler-Wedler, D.; Kreuzig, R.; Schnug, E. Effects of phosphite on phosphorus supply and growth of corn (Zea mays). Landbauforsch. Völkenrode 2006, 56, 87–99. [Google Scholar]


| Product Group/Agents | Abbreviation | Main Component | Provider |
|---|---|---|---|
| Botanical (Name of Plant/Name of Drug) | |||
| Frangula alnus/Frangula cortex | FA | powder 2 of alder buckthorn bark | Hänseler AG (CH) |
| Rheum palmatum/Rhei radix | RP | powder of RP roots | Hänseler AG (CH) |
| Galla chinensis 1 | GC | powder of GC galls | Berg Apotheke Zürich (CH) |
| Phosphonate | |||
| Phosfik® 3.27.18 | Ph | 3% N, 27% P2O5, 18% K2O 3 | Biolchim Hannover (DE) |
| Fungicides | |||
| Kocide DF® | KoDF | synthetic, 40% copper | Bayer (Schweiz) AG (CH) |
| Kocide Opti™ | Ko | synthetic, 30% copper | Bayer (Schweiz) AG (CH) |
| Additive | |||
| Nu-Film 17 | NuF | resin of American pine | Intrachem Bio (Int.) SA (CH) |
| Year | 2010 | 2011 | 2012 | 2013 * | |||
|---|---|---|---|---|---|---|---|
| Location (Tänikon & Reckenholz) | Tän. | Tän. | Rec. | Tän. | Rec. | Tän. | Rec. |
| Standards | |||||||
| Untreated | x | x | x | x | x | x | x |
| KoDF 200 g/ha Cu | x | ||||||
| KoDF 300 g/ha Cu | x | x | x | x | |||
| KoOpt (Ko) ** | x | x | |||||
| Botanicals and phosphonate | |||||||
| Frangula alnus 4% (FA) | x | x | x | x | x | ||
| Frangula alnus 4% (FA+) | x | ||||||
| Galla chinensis 4% (GC) | x | x | x | ||||
| Rheum palmatum 4% (RP) | x | x | x | ||||
| Phosfik® 1.5 L/ha (Ph) | x | x | x *** | x *** | |||
| Phosfik® 3.0 L/ha (Ph) | x | x | |||||
| 4× Ph 3.0 L/ha, then 4× FA (4%) | x | x | |||||
| 4× Ph 3.0 L/ha, then 4× KoDF **** | x | x | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forrer, H.-R.; Vogelgsang, S.; Musa, T. Botanicals and Phosphonate Show Potential to Replace Copper for Control of Potato Late Blight. J. Fungi 2017, 3, 65. https://doi.org/10.3390/jof3040065
Forrer H-R, Vogelgsang S, Musa T. Botanicals and Phosphonate Show Potential to Replace Copper for Control of Potato Late Blight. Journal of Fungi. 2017; 3(4):65. https://doi.org/10.3390/jof3040065
Chicago/Turabian StyleForrer, Hans-Rudolf, Susanne Vogelgsang, and Tomke Musa. 2017. "Botanicals and Phosphonate Show Potential to Replace Copper for Control of Potato Late Blight" Journal of Fungi 3, no. 4: 65. https://doi.org/10.3390/jof3040065





