Evaluation of Left Ventricular Function Using Four-Dimensional Flow Cardiovascular Magnetic Resonance: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Quantitative Assessment
3. Results
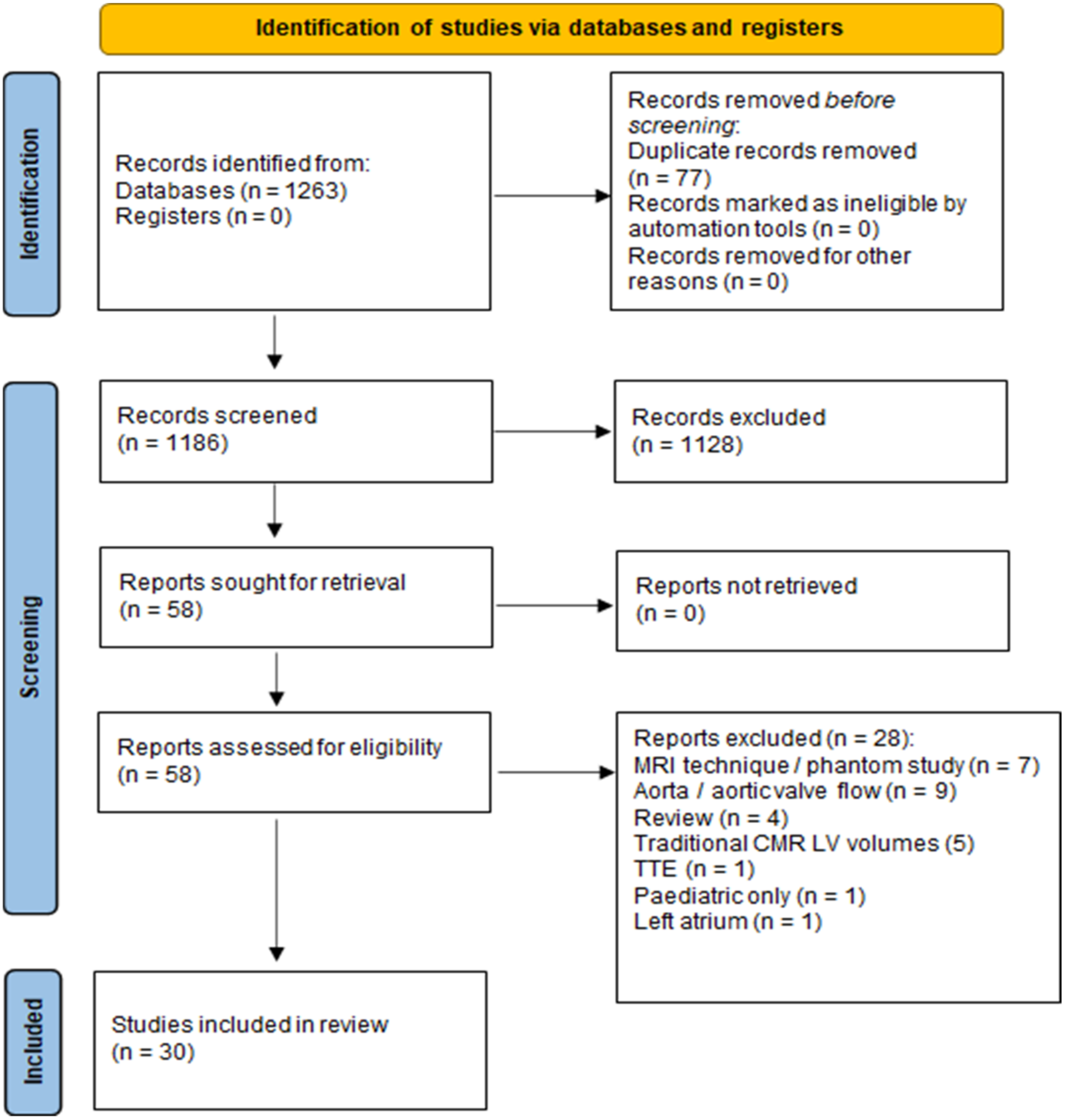
3.1. Search Strategy
3.2. Description of the Included Studies
3.3. Study Population
3.4. 4D-Flow MR Pulse Sequence Parameters
3.5. Image Analysis Tools and Methodologies
3.6. Scan–Rescan Reproducibility
3.7. Intra-/Inter-Observer Reproducibility
3.8. Validation Methods
3.9. Novel Flow Parameters
3.9.1. Ventricular Kinetic Energy
3.9.2. Flow Components
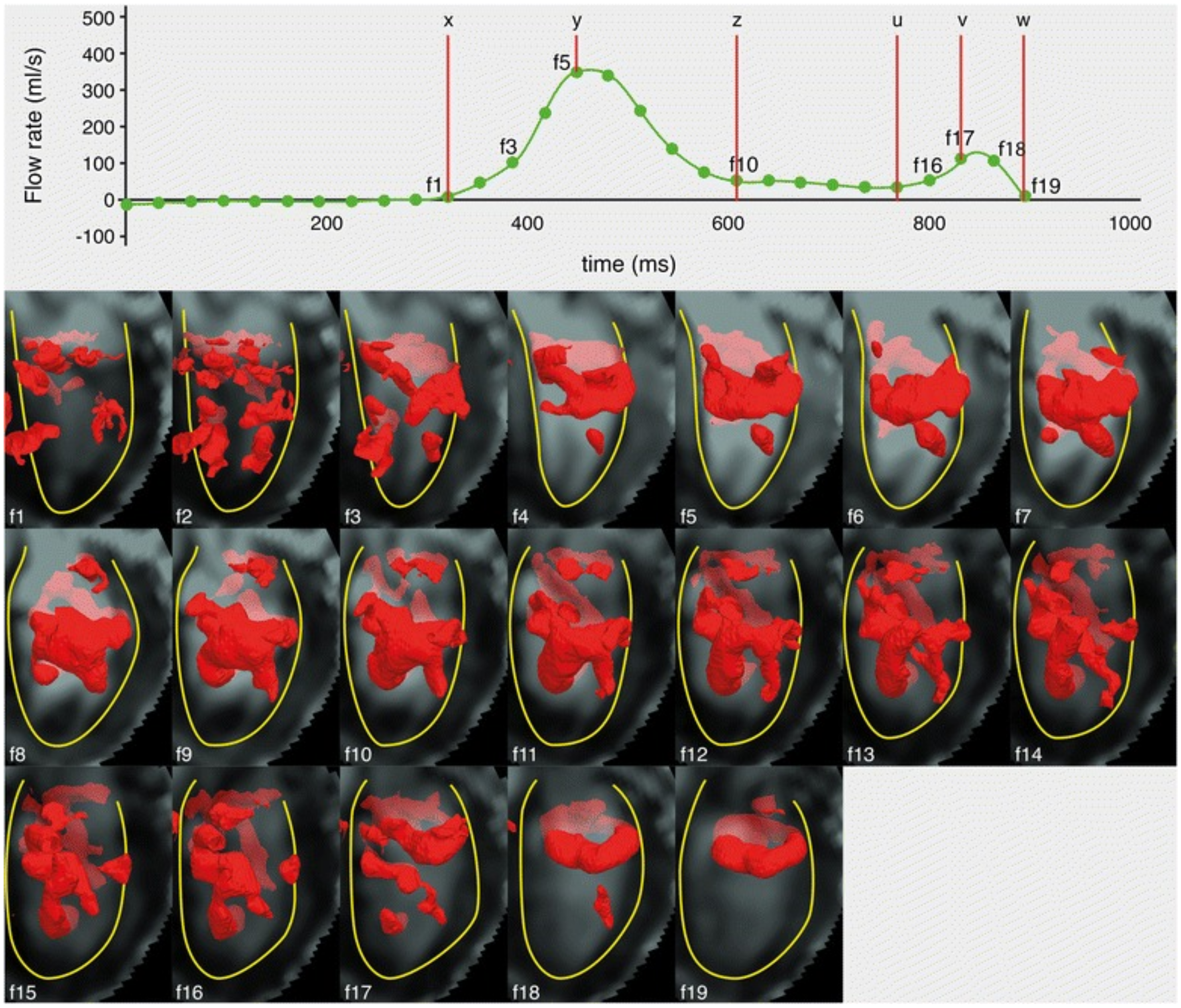
3.9.3. LV Vortex Morphology
3.9.4. LV Vorticity
3.9.5. Viscous Energy Loss
3.9.6. LV Haemodynamic Forces
3.9.7. Residence Time Distribution
3.9.8. Turbulent Kinetic Energy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Sirajuddin, A.; Zhao, S.; Lu, M. The role of 4D flow MRI for clinical applications in cardiovascular disease: Current status and future perspectives. Quant. Imaging Med. Surg. 2021, 11, 9. Available online: https://qims.amegroups.com/article/view/71234 (accessed on 8 June 2022). [CrossRef] [PubMed]
- Costello, B.T.; Qadri, M.; Price, B.; Papapostolou, S.; Thompson, M.; Hare, J.L.; La Gerche, A.; Rudman, M.; Taylor, A.J. The ventricular residence time distribution derived from 4D flow particle tracing: A novel marker of myocardial dysfunction. Int. J. Cardiovasc. Imaging 2018, 34, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Dorosz, J.L.; Lezotte, D.C.; Weitzenkamp, D.A.; Allen, L.A.; Salcedo, E.E. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1799–1808. [Google Scholar] [CrossRef]
- Ohyama, H.; Hosomi, N.; Takahashi, T.; Mizushige, K.; Osaka, K.; Kohno, M.; Koziol, J.A. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke 2003, 34, 2436–2439. [Google Scholar] [CrossRef]
- Markl, M.; Geiger, J.; Arnold, R.; Stroh, A.; Damjanovic, D.; Föll, D.; Beyersdorf, F. Comprehensive 4-dimensional magnetic resonance flow analysis after successful heart transplantation resolves controversial intraoperative findings and reveals complex hemodynamic alterations. Circulation 2011, 123, e381–e383. [Google Scholar] [CrossRef]
- Azarine, A.; Garçon, P.; Stansal, A.; Canepa, N.; Angelopoulos, G.; Silvera, S.; Sidi, D.; Marteau, V.; Zins, M. Four-dimensional Flow MRI: Principles and Cardiovascular Applications. Radiographics 2019, 39, 632–648. [Google Scholar] [CrossRef]
- Svalbring, E.; Fredriksson, A.; Eriksson, J.; Dyverfeldt, P.; Ebbers, T.; Bolger, A.F.; Engvall, J.; Carlhäll, C.-J. Altered diastolic flow patterns and kinetic energy in subtle left ventricular remodeling and dysfunction detected by 4D flow MRI. PLoS ONE 2016, 11, e0161391. [Google Scholar] [CrossRef]
- Kamphuis, V.P.; Westenberg, J.J.M.; van der Palen, R.L.F.; Blom, N.A.; de Roos, A.; van der Geest, R.; Elbaz, M.S.M.; Roest, A.A.W. Unravelling cardiovascular disease using four dimensional flow cardiovascular magnetic resonance. Int. J. Cardiovasc. Imaging 2017, 33, 1069–1081. [Google Scholar] [CrossRef]
- Plein, S.; Kozerke, S. Are we there yet? JACC Cardiovasc. Imaging 2021, 14, 1755–1757. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, n71. [Google Scholar] [CrossRef]
- Elsayed, A.; Gilbert, K.; Scadeng, M.; Cowan, B.R.; Pushparajah, K.; Young, A.A. Four-dimensional flow cardiovascular magnetic resonance in tetralogy of Fallot: A systematic review. J. Cardiovasc. Magn. Reson. 2021, 23, 59. [Google Scholar] [CrossRef]
- Corrado, P.A.; Macdonald, J.A.; François, C.J.; Aggarwal, N.R.; Weinsaft, J.W.; Wieben, O. Reduced regional flow in the left ventricle after anterior acute myocardial infarction: A case control study using 4D flow MRI. BMC Med. Imaging 2019, 19, 101. [Google Scholar] [CrossRef]
- Miyajima, K.; Urushida, T.; Ito, K.; Kin, F.; Okazaki, A.; Takashima, Y.; Watanabe, T.; Kawaguchi, Y.; Wakabayashi, Y.; Takehara, Y.; et al. Four-dimensional flow magnetic resonance imaging visualizes reverse vortex pattern and energy loss increase in left bundle branch block. EP Europace 2021, 24, 1284–1290. [Google Scholar] [CrossRef]
- Wong, J.; Chabiniok, R.; deVecchi, A.; Dedieu, N.; Sammut, E.; Schaeffter, T.; Razavi, R. Age-related changes in intraventricular kinetic energy: A physiological or pathological adaptation? Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H747–H755. [Google Scholar] [CrossRef]
- Arvidsson, P.M.; Töger, J.; Pedrizzetti, G.; Heiberg, E.; Borgquist, R.; Carlsson, M.; Arheden, H. Hemodynamic forces using four-dimensional flow MRI: An independent biomarker of cardiac function in heart failure with left ventricular dyssynchrony? Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1627–H1639. [Google Scholar] [CrossRef]
- Garg, P.; van der Geest, R.J.; Swoboda, P.P.; Crandon, S.; Fent, G.J.; Foley, J.R.J.; Dobson, L.E.; Al Musa, T.; Onciul, S.; Vijayan, S.; et al. Left ventricular thrombus formation in myocardial infarction is associated with altered left ventricular blood flow energetics. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 108–117. [Google Scholar] [CrossRef]
- Suwa, K.; Saitoh, T.; Takehara, Y.; Sano, M.; Saotome, M.; Urushida, T.; Katoh, H.; Satoh, H.; Sugiyama, M.; Wakayama, T.; et al. Intra-left ventricular flow dynamics in patients with preserved and impaired left ventricular function: Analysis with 3D cine phase contrast MRI (4D-Flow). J. Magn. Reson. Imaging 2016, 44, 1493–1503. [Google Scholar] [CrossRef]
- Kamphuis, V.P.; Westenberg, J.J.M.; van der Palen, R.L.F.; van den Boogaard, P.J.; van der Geest, R.J.; de Roos, A.; Blom, N.A.; Roest, A.A.W.; Elbaz, M.S.M. Scan-rescan reproducibility of diastolic left ventricular kinetic energy, viscous energy loss and vorticity assessment using 4D flow MRI: Analysis in healthy subjects. Int. J. Cardiovasc. Imaging 2018, 34, 905–920. [Google Scholar] [CrossRef]
- Stoll, V.M.; Loudon, M.; Eriksson, J.; Bissell, M.M.; Dyverfeldt, P.; Ebbers, T.; Myerson, S.G.; Neubauer, S.; Carlhäll, C.-J.; Hess, A.T. Test-retest variability of left ventricular 4D flow cardiovascular magnetic resonance measurements in healthy subjects. J. Cardiovasc. Magn. Reson. 2018, 20, 15. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, M.; Heiberg, E.; Toger, J.; Arheden, H. Quantification of left and right ventricular kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H893–H900. [Google Scholar] [CrossRef]
- Nakaji, K.; Itatani, K.; Tamaki, N.; Morichi, H.; Nakanishi, N.; Takigami, M.; Yamagishi, M.; Yaku, H.; Yamada, K. Assessment of biventricular hemodynamics and energy dynamics using lumen-tracking 4D flow MRI without contrast medium. J. Cardiol. 2021, 78, 79–87. [Google Scholar] [CrossRef]
- Crandon, S.; Westenberg, J.J.M.; Swoboda, P.P.; Fent, G.J.; Foley, J.R.J.; Chew, P.G.; Brown, L.A.E.; Saunderson, C.; Al-Mohammad, A.; Greenwood, J.P.; et al. Impact of Age and Diastolic Function on Novel, 4D flow CMR Biomarkers of Left Ventricular Blood Flow Kinetic Energy. Sci. Rep. 2018, 8, 14436. [Google Scholar] [CrossRef]
- Garg, P.; Crandon, S.; Swoboda, P.P.; Fent, G.J.; Foley, J.R.J.; Chew, P.G.; Brown, L.A.E.; Vijayan, S.; Hassell, M.E.C.J.; Nijveldt, R.; et al. Left ventricular blood flow kinetic energy after myocardial infarction—Insights from 4D flow cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 61. [Google Scholar] [CrossRef]
- Sundin, J.; Engvall, J.; Nylander, E.; Ebbers, T.; Bolger, A.F.; Carlhäll, C.-J. Improved efficiency of intraventricular blood flow transit under cardiac stress: A 4D flow dobutamine CMR study. Front. Cardiovasc. Med. 2020, 7, 581495. [Google Scholar] [CrossRef]
- Elbaz, M.S.M.; Calkoen, E.E.; Westenberg, J.J.M.; Lelieveldt, B.P.F.; Roest, A.A.W.; van der Geest, R.J. Vortex flow during early and late left ventricular filling in normal subjects: Quantitative characterization using retrospectively-gated 4D flow cardiovascular magnetic resonance and three-dimensional vortex core analysis. J. Cardiovasc. Magn. Reson. 2014, 16, 78. [Google Scholar] [CrossRef]
- Schäfer, M.; Browning, J.; Schroeder, J.D.; Shandas, R.; Kheyfets, V.O.; Buckner, J.K.; Hunter, K.S.; Hertzberg, J.R.; Fenster, B.E. Vorticity is a marker of diastolic ventricular interdependency in pulmonary hypertension. Pulm. Circ. 2016, 6, 46–54. [Google Scholar] [CrossRef]
- Steding-Ehrenborg, K.; Arvidsson, P.M.; Töger, J.; Rydberg, M.; Heiberg, E.; Carlsson, M.; Arheden, H. Determinants of kinetic energy of blood flow in the four-chambered heart in athletes and sedentary controls. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H113–H122. [Google Scholar] [CrossRef]
- Park, J.-H.; Marwick, T.H. Use and limitations of e/e’ to assess left ventricular filling pressure by echocardiography. J. Cardiovasc. Ultrasound 2011, 19, 169–173. [Google Scholar] [CrossRef]
- Kanski, M.; Arvidsson, P.M.; Töger, J.; Borgquist, R.; Heiberg, E.; Carlsson, M.; Arheden, H. Left ventricular fluid kinetic energy time curves in heart failure from cardiovascular magnetic resonance 4D flow data. J. Cardiovasc. Magn. Reson. 2015, 17, 111. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, D.R.; Barton, G.P.; François, C.J.; Aggarwal, N.; Roldán-Alzate, A. Sex differences in cardiac flow dynamics of healthy volunteers. Radiol. Cardiothorac. Imaging 2020, 2, e190058. [Google Scholar] [CrossRef] [PubMed]
- Pewowaruk, R.; Rutkowski, D.; Johnson, C.; Wolfinger, A.; Roldán-Alzate, A. Assessment of sex differences in ventricular-vascular coupling of left ventricular and aortic flow derived from 4D flow MRI in healthy, young adults. J. Biomech. 2021, 117, 110276. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Hastings, J.L.; Bhella, P.S.; Shibata, S.; Gandhi, N.K.; Carrick-Ranson, G.; Palmer, D.; Levine, B.D. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J. Physiol. 2012, 590, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.D.; Gore, J.; Yarzebski, J.; Spencer, F.; Lessard, D.; Goldberg, R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Ugander, M.; Ekmehag, B.; Arheden, H. The relationship between left ventricular ejection fraction and infarct size assessed by MRI. Scand. Cardiovasc. J. 2008, 42, 137–145. [Google Scholar] [CrossRef]
- Xing, X.; Li, D.; Chen, S.; Wang, L.; Li, Z.; He, L. Evaluation of left ventricular systolic function in patients with different types of ischemic heart disease by two-dimensional speckle tracking imaging. J. Cardiothorac. Surg. 2020, 15, 325. [Google Scholar] [CrossRef]
- Bolger, A.F.; Heiberg, E.; Karlsson, M.; Wigström, L.; Engvall, J.; Sigfridsson, A.; Ebbers, T.; Kvitting, J.-P.E.; Carlhäll, C.J.; Wranne, B. Transit of blood flow through the human left ventricle mapped by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2007, 9, 741–747. [Google Scholar] [CrossRef]
- Zajac, J.; Eriksson, J.; Alehagen, U.; Ebbers, T.; Bolger, A.F.; Carlhäll, C.-J. Mechanical dyssynchrony alters left ventricular flow energetics in failing hearts with LBBB: A 4D flow CMR pilot study. Int. J. Cardiovasc. Imaging 2018, 34, 587–596. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Lim, T.T.; Nickels, T.B. Vortex Rings. In Fluid Vortices; Green, S.I., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 95–153. [Google Scholar]
- Akhmetov, D.G. Introduction. In Vortex Rings; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–3. [Google Scholar]
- Kheradvar, A.; Pedrizzetti, G. Vortex formation in the heart. In Vortex Formation in the Cardiovascular System; Springer: London, UK, 2012; p. 30. [Google Scholar]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A test in context: E/A and e/e’ to assess diastolic dysfunction and LV filling pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef]
- Calkoen, E.E.; Elbaz, M.S.M.; Westenberg, J.J.M.; Kroft, L.J.M.; Hazekamp, M.G.; Roest, A.A.W.; van der Geest, R.J. Altered left ventricular vortex ring formation by 4-dimensional flow magnetic resonance imaging after repair of atrioventricular septal defects. J. Thorac. Cardiovasc. Surg. 2015, 150, 1233–1240.e1. [Google Scholar] [CrossRef]
- Schäfer, M.; Humphries, S.; Stenmark, K.R.; Kheyfets, V.O.; Buckner, J.K.; Hunter, K.S.; Fenster, B.E. 4D-flow cardiac magnetic resonance-derived vorticity is sensitive marker of left ventricular diastolic dysfunction in patients with mild-to-moderate chronic obstructive pulmonary disease. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 415–424. [Google Scholar] [CrossRef]
- Elbaz, M.S.M.; van der Geest, R.J.; Calkoen, E.E.; de Roos, A.; Lelieveldt, B.P.F.; Roest, A.A.W.; Westenberg, J.J.M. Assessment of viscous energy loss and the association with three-dimensional vortex ring formation in left ventricular inflow: In vivo evaluation using four-dimensional flow MRI. Magn. Reson. Med. 2017, 77, 794–805. [Google Scholar] [CrossRef]
- Arvidsson, P.M.; Töger, J.; Carlsson, M.; Steding-Ehrenborg, K.; Pedrizzetti, G.; Heiberg, E.; Arheden, H. Left and right ventricular hemodynamic forces in healthy volunteers and elite athletes assessed with 4D flow magnetic resonance imaging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H314–H328. [Google Scholar] [CrossRef]
- Eriksson, J.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Assessment of left ventricular hemodynamic forces in healthy subjects and patients with dilated cardiomyopathy using 4D flow MRI. Physiol. Rep. 2016, 4, e12685. [Google Scholar] [CrossRef]
- Eriksson, J.; Zajac, J.; Alehagen, U.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Left ventricular hemodynamic forces as a marker of mechanical dyssynchrony in heart failure patients with left bundle branch block. Sci. Rep. 2017, 7, 2971. [Google Scholar] [CrossRef]
- Zajac, J.; Eriksson, J.; Dyverfeldt, P.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Turbulent kinetic energy in normal and myopathic left ventricles. J. Magn. Reson. Imaging 2015, 41, 1021–1029. [Google Scholar] [CrossRef]
- Kaur, H.; Assadi, H.; Alabed, S.; Cameron, D.; Vassiliou, V.S.; Westenberg, J.J.M.; van der Geest, R.; Zhong, L.; Dastidar, A.; Swift, A.J.; et al. Left ventricular blood flow kinetic energy assessment by 4D flow cardiovascular magnetic resonance: A systematic review of the clinical relevance. J. Cardiovasc. Dev. Dis. 2020, 7, 37. [Google Scholar] [CrossRef]
- Carlhäll, C.J.; Bolger, A. Passing strange: Flow in the failing ventricle. Circ. Heart Fail. 2010, 3, 326–331. [Google Scholar] [CrossRef]
- Crandon, S.; Elbaz, M.S.M.; Westenberg, J.J.M.; van der Geest, R.J.; Plein, S.; Garg, P. Clinical applications of intra-cardiac four-dimensional flow cardiovascular magnetic resonance: A systematic review. Int. J. Cardiol. 2017, 249, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Raman, B.; Banerjee, R.; Milanesi, M. Novel Insights into Complex Cardiovascular Pathologies using 4D Flow Analysis by Cardiovascular Magnetic Resonance Imaging. Curr. Pharm. Des. 2017, 23, 3262–3267. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Schrauben, E.M.; Garcia, J.; Uribe, S.; Grieve, S.M.; Elbaz, M.S.; Barker, A.J.; Geiger, J.; Nordmeyer, S.; Marsden, A. Intracardiac 4D flow MRI in congenital heart disease: Recommendations on behalf of the ISMRM flow & motion study group. J. Magn. Reson. Imaging 2019, 50, 677–681. [Google Scholar] [PubMed]
| Study | Intra-/Inter-Observer Reproducibility Methodology | Reproducibility Results |
|---|---|---|
| Costello et al., 2018 [3] | ICC of LV and RV RTDc | Intra-observer: LV 0.901 (p < 0.001), RV 0.768 (p = 0.004); Inter-observer: LV 0.881 (p < 0.001), RV 0.728 (p = 0.008) |
| Crandon et al., 2018 [23] | CV; 10 cases for intra- and 20 cases for inter-observer | Average CV for all variables: 6 ± 2%; Intra-observer CV: global KE 3.5%, systolic KE 3.9%, diastolic KE 6.0%, peak E-wave KE 4.4%, peak A-wave KE 5.5%; Inter-observer CV: global KE 7%, systolic KE 11%, diastolic KE 6.4%, peak E-wave KE 6.6%, peak A-wave KE 6.3% |
| Elbaz et al., 2014 [26] | Intra-observer: repeat measurements by same observer one week apart; Inter-observer: two observers independently assessed same database; ICC of C, L, R coordinates and orientation of vortex ring cores | Intra-observer: ICC = 0.97, p < 0.001, CV 1–3%; Inter-observer: ICC = 0.96, p < 0.001, CV 1–8% |
| Garg et al., 2018 [24] | Inter-observer: ICC of KE computed from short-axis LV contours segmented by two observers independently; Intra-observer: ICC of KE computed from short axis LV contours segmented by same observer 3 months apart | Overall ICC for all global KE parameters: average 0.99, p > 0.9; TD to peak E-wave KE ICC = 0.94, 95% CI 0.88 to 0.97 Intra-observer: mean bias for KEiEDV 3 ± 9%; Inter-observer: mean bias for KEiEDV 2 ± 9%; |
| Garg et al., 2019 [17] | Inter-observer: ICC of KE computed from short-axis LV contours segmented by two observers independently; Intra-observer: ICC of KE computed from short axis LV contours segmented by same observer 3 months apart; Inter-rater reliability κ of KE and TD; | Intra-observer: global KE parameters bias 2%, precision −16%; Inter-observer: global KE parameters bias 4%, precision −20%; Inter-rater reliability weighted kappa: in-plane KE κ = 1, distal A-wave KE drop κ = 0.63, and TD from base to mid κ = 0.67 |
| Miyajima et al., 2021 [14] | Inter-observer: κ of inflow pattern analysis | κ = 0.68 |
| Nakaji et al., 2021 [22] | Qualitative assessment of segmentation process by radiologist, cardiac surgeon, masters student in more than 3 cases | Small qualitative differences |
| Schäfer et al., 2016 [27] | Inter-observer: ICC of LV vorticity | E-wave vorticity ICC = 0.94; A-wave vorticity ICC = 0.93 |
| Stoll et al., 2018 [20] | Intra-observer: two blinded assessments by same observer one month apart; Inter-observer: two observers performed assessment on same dataset independently | Intra-observer: CV of flow components 3.6–6.1%; Inter-observer: CV of flow components: 2.6–5.7% |
| Sundin et al., 2020 [25] | ICC of LV flow component | Intra-observer: LV flow component with dobutamine ICC = 0.99; Inter-observe: LV flow component with dobutamine ICC = 0.80–0.91 |
| Suwa et al., 2016 [18] | Inter-observer: κ or CV for the determination of the presence of an intra-LV vortex and vortex features | Determination of presence of vortex: κ = 0.867; Estimation of vortex features: CV distance to vortex core, 0.83; vortex area, 0.85; sphericity index of the vortex, 0.94; p < 0.01 |
| Novel Flow Parameter | Definition | Number of Studies | Clinical Applications | Current Limitations | Metrics Most Likely to Have Clinical Potential |
|---|---|---|---|---|---|
| Kinetic energy | Work performed on blood to accelerate it from stationary to a specific speed | 12 | Ageing; ischaemic heart disease; heart failure; sex differences; athletes | Reported variably as raw value or indexed values, and as averaged or at specific cardiac phases | KE indexed to LVEDV at peak E- and A-filling |
| Flow components | Blood components with different flow paths over a cardiac cycle | 5 | Ischaemic heart disease; heart failure; LV dyssynchrony; dobutamine stress | Reported variably as volume proportional to total flow volume or LVEDV; limited studies quantifying component KE | Flow component volume as a proportion of total flow volume or LVEDV; component KE indexed to LVEDV |
| Vortex morphology | Shape, location, orientation and dimension of the LV diastolic vortex | 4 | AVSD; heart failure; diastolic dysfunction; LV dyssynchrony | Limited studies; no standardised measurement approach | Vortex location relative to MV; vortex dimensions |
| Vorticity | Tendency for a fluid to rotate | 4 | Diastolic dysfunction associated with COPD and pulmonary hypertension; sex differences | Limited studies; uncertain clinical utility | Vorticity |
| Viscous energy loss | Loss of mechanical energy due to fluid viscosity and friction | 4 | AVSD; LV dyssynchrony | Limited studies; reported variably as indexed to SV, EDV or BSA; uncertain value as a standalone metric | EL indexed to SV, EDV or BSA |
| Haemodynamic forces | Forces exchanged between the myocardium and blood | 4 | Dilated cardiomyopathy; LV dyssynchrony | Limited studies; uncertain clinical utility | Haemodynamic force ratio |
| Residence time distribution | Cumulative distribution of time it takes for blood to transit through a heart chamber and exit | 1 | Dilated cardiomyopathy | Limited studies, uncertain clinical utility | Residence time distribution constant |
| Turbulent kinetic energy | Kinetic energy that counts for variations in the magnitude of blood flow velocity that leads to the appearance of vortices | 1 | Dilated cardiomyopathy | Limited studies, uncertain clinical utility | Turbulent kinetic energy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.J.; Indja, B.; Gholipour, A.; Gök, M.; Grieve, S.M. Evaluation of Left Ventricular Function Using Four-Dimensional Flow Cardiovascular Magnetic Resonance: A Systematic Review. J. Cardiovasc. Dev. Dis. 2022, 9, 304. https://doi.org/10.3390/jcdd9090304
Qin JJ, Indja B, Gholipour A, Gök M, Grieve SM. Evaluation of Left Ventricular Function Using Four-Dimensional Flow Cardiovascular Magnetic Resonance: A Systematic Review. Journal of Cardiovascular Development and Disease. 2022; 9(9):304. https://doi.org/10.3390/jcdd9090304
Chicago/Turabian StyleQin, Jiaxing Jason, Ben Indja, Alireza Gholipour, Mustafa Gök, and Stuart M. Grieve. 2022. "Evaluation of Left Ventricular Function Using Four-Dimensional Flow Cardiovascular Magnetic Resonance: A Systematic Review" Journal of Cardiovascular Development and Disease 9, no. 9: 304. https://doi.org/10.3390/jcdd9090304






