Transcatheter Aortic Valve Implantation and Conduction Disturbances: Focus on Clinical Implications
Abstract
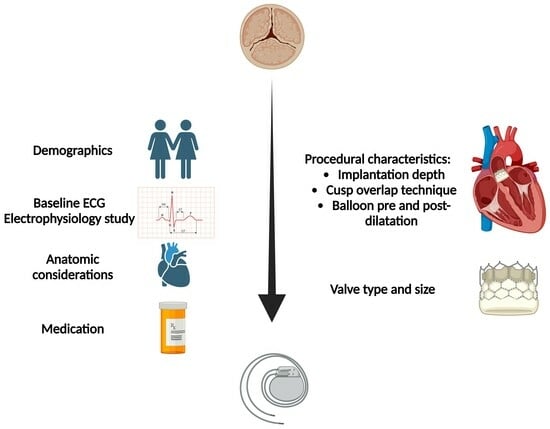
:1. Introduction
2. Incidence of New Onset CA and PPM Implantation Post TAVI
3. Risk Factors for CA Post TAVI
3.1. Anatomic Factors
3.2. Baseline Electrocardiography (ECG)
3.3. Demographic Characteristics
3.4. Transcatheter Aortic device
3.5. Implantation Depth
3.6. Medication
4. Impact of New LBBB, New CA and PPM Implantation
5. Current and Future Strategies to Address CA and the Need for PPM Implantation
6. Conclusions
7. Key Points
- The occurrence of new-onset LBBB and conduction abnormalities, including high-grade atrioventricular block requiring permanent pacemaker implantation, remain the most common complications following TAVI.
- The Heart Team’s choice between SAVR and TAVI should also weigh the risk of a temporary pacemaker, especially in young patients.
- The existence of RBBB is the strongest predictor of permanent pacemaker implantation post TAVI.
- The risk of new-onset conduction abnormalities requiring pacemaker implantation is related to the pre-procedural, intra-procedural and device characteristics.
- Post-procedural care requires a stepwise approach regarding temporary pacemaker presence duration, arrhythmic monitoring and permanent pacemaker implantation decisions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Otto, C.M.; Prendergast, B. Aortic-valve stenosis—From patients at risk to severe valve obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef]
- Ancona, R.; Pinto, S.C. Epidemiology of aortic valve stenosis (AS) and of aortic valve incompetence (AI): Is the prevalence of AS/AI similar in different parts of the world? Am. J. Cardiol. 2020, 10, 10–12. [Google Scholar]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef]
- Mangieri, A.; Montalto, C.; Pagnesi, M.; Lanzillo, G.; Demir, O.; Testa, L.; Colombo, A.; Latib, A. TAVI and Post Procedural Cardiac Conduction Abnormalities. Front. Cardiovasc. Med. 2018, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodes-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef]
- van Rosendael, P.J.; Delgado, V.; Bax, J.J. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: A systematic review. Eur. Heart J. 2018, 39, 2003–2013. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntane-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jorgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef]
- Rodes-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntane-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated With Transcatheter Aortic Valve Replacement: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]
- Varc-3 Writing, C.; Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Muntane-Carol, G.; Okoh, A.K.; Chen, C.; Nault, I.; Kassotis, J.; Mohammadi, S.; Coromilas, J.; Lee, L.Y.; Alperi, A.; Philippon, F.; et al. Ambulatory Electrocardiographic Monitoring Following Minimalist Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Guetta, V.; Klempfner, R.; Konen, E.; Raanani, E.; Glikson, M.; Goitein, O.; Segev, A.; Barbash, I.; Fefer, P.; et al. Inverse Relationship Between Membranous Septal Length and the Risk of Atrioventricular Block in Patients Undergoing Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2015, 8, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Nassar, M.; Schwammenthal, E.; Perlman, G.; Arow, Z.; Lessick, J.; Kerner, A.; Barsheshet, A.; Assa, H.V.; Assali, A.; et al. Short membranous septum length in bicuspid aortic valve stenosis increases the risk of conduction disturbances. J. Cardiovasc. Comput. Tomogr. 2021, 15, 339–347. [Google Scholar] [CrossRef]
- Tretter, J.T.; Mori, S.; Anderson, R.H.; Taylor, M.D.; Ollberding, N.; Truong, V.; Choo, J.; Kereiakes, D.; Mazur, W. Anatomical predictors of conduction damage after transcatheter implantation of the aortic valve. Open Heart 2019, 6, e000972. [Google Scholar] [CrossRef]
- Toggweiler, S.; Kobza, R. Pacemaker implantation after transcatheter aortic valve: Why is this still happening? J. Thorac. Dis. 2018, 10, S3614–S3619. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Patel, J.N.; Loc, B.L.; Vipparthy, S.C.; Divecha, C.; Barzallo, P.X.; Kim, M.; Baman, T.; Barzallo, M.; Mungee, S. Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: A Cost Analysis. Cureus 2019, 11, e5005. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Ramanan, T.; Sawant, A.C.; Sinibaldi, E.; Pham, M.; Khan, S.; Qureshi, R.; Agrawal, N.; Khalil, C.; Hansen, R.; et al. Quality of life outcomes in transcatheter aortic valve replacement patients requiring pacemaker implantation. J. Arrhythm. 2018, 34, 441–449. [Google Scholar] [CrossRef]
- Yagel, O.; Belhassen, B.; Planer, D.; Amir, O.; Elbaz-Greener, G. The R-wave amplitude in V1 on baseline electrocardiogram correlates with the occurrence of high-degree atrioventricular block following left bundle branch block after transcatheter aortic valve replacement. EP Eur. 2023, 25, euad066. [Google Scholar] [CrossRef]
- Novelli, L.; Jamie, G.; Regazzoli, D.; Reimers, B.; Frontera, A.; Mangieri, A. How to predict conduction disturbances after transcatheter aortic valve replacement. Kardiol. Pol. 2023, 81, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Elchinova, E.; Nozica, N.; Bartkowiak, J.; Ryffel, C.; Bernhard, B.; Elsmaan, M.; Asatryan, B.; Branca, M.; Okuno, T.; Lanz, J.; et al. Permanent pacemaker implantation late after transcatheter aortic valve implantation. Heart Rhythm. 2021, 18, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Spring, A.M.; Catalano, M.A.; Prasad, V.; Rutkin, B.; Koss, E.; Hartman, A.; Yu, P.J. Evaluating the Validity of Risk Scoring in Predicting Pacemaker Rates following Transcatheter Aortic Valve Replacement. J. Interv. Cardiol. 2020, 2020, 1807909. [Google Scholar] [CrossRef] [PubMed]
- Sammour, Y.; Krishnaswamy, A.; Kumar, A.; Puri, R.; Tarakji, K.G.; Bazarbashi, N.; Harb, S.; Griffin, B.; Svensson, L.; Wazni, O.; et al. Incidence, Predictors, and Implications of Permanent Pacemaker Requirement After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 115–134. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Zhao, Z.; Du, R.; Staniloae, C.; Saric, M.; Neuburger, P.J.; Querijero, M.; Vainrib, A.; Hisamoto, K.; Ibrahim, H.; et al. Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1796–1807. [Google Scholar] [CrossRef]
- Kawashima, T.; Sasaki, H. A macroscopic anatomical investigation of atrioventricular bundle locational variation relative to the membranous part of the ventricular septum in elderly human hearts. Surg. Radiol. Anat. 2005, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.N.; Daou, L.; Saliba, Z. Device Closure of Perimembranous Ventricular Septal Defect: Choosing Between Amplatzer Occluders. Front. Pediatr. 2019, 7, 300. [Google Scholar] [CrossRef]
- Dhoble, A.; Zhao, Y.; Vejpongsa, P.; Loghin, C.; Smalling, R.W.; Estrera, A.; Nguyen, T.C. National 10-year trends and outcomes of isolated and concomitant tricuspid valve surgery. J. Cardiovasc. Surg. 2019, 60, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ravaux, J.M.; Di Mauro, M.; Vernooy, K.; Kats, S.; Mariani, S.; Ronco, D.; Actis Dato, G.; Simons, J.; Hof, A.W.V.; Maessen, J.G.; et al. Permanent pacemaker implantation following transcatheter aortic valve implantation using self-expandable, balloon-expandable, or mechanically expandable devices: A network meta-analysis. EP Eur. 2021, 23, 1998–2009. [Google Scholar] [CrossRef]
- Koliastasis, L.; Doundoulakis, I.; Kokkinidis, D.G.; Milkas, A.; Kostopoulos, G.; Drakopoulou, M.; Latsios, G.; Synetos, A.; Benetos, G.; Lampropoulos, K.; et al. Study Level Meta-Analysis of Transcatheter Aortic Valve Implantation With the ACURATE neo Self-Expanding Transcatheter Heart Valve. Cardiol. Rev. 2023, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Kandregula, K.; Sankaramangalam, K.; Anumandla, A.; Kumar, A.; Parikh, P.; Kerrigan, J.; Khubber, S.; Krishnaswamy, A.; Mick, S.; et al. Meta-analysis of the Impact of Avoiding Balloon Predilation in Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 477–482. [Google Scholar] [CrossRef]
- Ochiai, T.; Yamanaka, F.; Shishido, K.; Moriyama, N.; Komatsu, I.; Yokoyama, H.; Miyashita, H.; Sato, D.; Sugiyama, Y.; Hayashi, T.; et al. Impact of High Implantation of Transcatheter Aortic Valve on Subsequent Conduction Disturbances and Coronary Access. JACC Cardiovasc. Interv. 2023, 16, 1192–1204. [Google Scholar] [CrossRef]
- Wienemann, H.; Maier, O.; Beyer, M.; Portratz, M.; Tanaka, T.; Mauri, V.; Ernst, A.; Waldschmidt, L.; Kuhn, E.; Bleiziffer, S.; et al. Cusp overlap versus standard three-cusp technique for self-expanding Evolut transcatheter aortic valves. EuroIntervention 2023, 19, e176–e187. [Google Scholar] [CrossRef] [PubMed]
- Grubb, K.J.; Gada, H.; Mittal, S.; Nazif, T.; Rodes-Cabau, J.; Fraser, D.G.W.; Lin, L.; Rovin, J.D.; Khalil, R.; Sultan, I.; et al. Clinical Impact of Standardized TAVR Technique and Care Pathway: Insights From the Optimize PRO Study. JACC Cardiovasc. Interv. 2023, 16, 558–570. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. Corrigendum to: 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC): With the special contribution of the European Heart Rhythm Association (EHRA). EP Eur. 2022, 24, 699. [Google Scholar] [CrossRef]
- Miyasaka, M.; Tada, N.; Taguri, M.; Kato, S.; Enta, Y.; Otomo, T.; Hata, M.; Watanabe, Y.; Naganuma, T.; Araki, M.; et al. Incidence, Predictors, and Clinical Impact of Prosthesis-Patient Mismatch Following Transcatheter Aortic Valve Replacement in Asian Patients: The OCEAN-TAVI Registry. JACC Cardiovasc. Interv. 2018, 11, 771–780. [Google Scholar] [CrossRef]
- Younis, A.; Orvin, K.; Nof, E.; Barabash, I.M.; Segev, A.; Berkovitch, A.; Guetta, V.; Assali, A.; Kornowski, R.; Beinart, R. The effect of periprocedural beta blocker withdrawal on arrhythmic risk following transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2019, 93, 1361–1366. [Google Scholar] [CrossRef]
- Maeno, Y.; Abramowitz, Y.; Kawamori, H.; Kazuno, Y.; Kubo, S.; Takahashi, N.; Mangat, G.; Okuyama, K.; Kashif, M.; Chakravarty, T.; et al. A Highly Predictive Risk Model for Pacemaker Implantation After TAVR. JACC Cardiovasc. Imaging 2017, 10, 1139–1147. [Google Scholar] [CrossRef]
- Ruck, A.; Saleh, N.; Glaser, N. Outcomes Following Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: SWEDEHEART Observational Study. JACC Cardiovasc. Interv. 2021, 14, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Chamandi, C.; Barbanti, M.; Munoz-Garcia, A.; Latib, A.; Nombela-Franco, L.; Gutierrez-Ibanez, E.; Veiga-Fernandez, G.; Cheema, A.N.; Cruz-Gonzalez, I.; Serra, V.; et al. Long-Term Outcomes in Patients With New-Onset Persistent Left Bundle Branch Block Following TAVR. JACC Cardiovasc. Interv. 2019, 12, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, T.H.; De Backer, O.; Gerds, T.A.; Bieliauskas, G.; Svendsen, J.H.; Sondergaard, L. Mortality and Heart Failure Hospitalization in Patients With Conduction Abnormalities After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Persson, M.; Dalen, M.; Sartipy, U. Long-term Outcomes Associated With Permanent Pacemaker Implantation After Surgical Aortic Valve Replacement. JAMA Netw. Open 2021, 4, e2116564. [Google Scholar] [CrossRef]
- Tomii, D.; Okuno, T.; Heg, D.; Pilgrim, T.; Windecker, S. Long-term outcomes of new-onset conduction abnormalities following transcatheter aortic valve implantation. Arch. Cardiovasc. Dis. 2022, 115, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Fadahunsi, O.O.; Olowoyeye, A.; Ukaigwe, A.; Li, Z.; Vora, A.N.; Vemulapalli, S.; Elgin, E.; Donato, A. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement: Analysis From the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc. Interv. 2016, 9, 2189–2199. [Google Scholar] [CrossRef]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J. Am. Coll. Cardiol. 2009, 54, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Mulla, W.; Etzion, S.; Elyagon, S.; Gillis, R.; Murninkas, M.; Konstantino, Y.; Mannhardt, I.; Eschenhagen, T.; Liel-Cohen, N.; Etzion, Y. Prominent differences in left ventricular performance and myocardial properties between right ventricular and left ventricular-based pacing modes in rats. Sci. Rep. 2017, 7, 5931. [Google Scholar] [CrossRef]
- Hochstadt, A.; Merdler, I.; Meridor, Y.; Schwartz, A.L.; Ingbir, M.; Ghantous, E.; Havakuk, O.; Mazo, A.; Steinvil, A.; Finkelstein, A.; et al. Effect of pacemaker implantation after transcatheter aortic valve replacement on long- and mid-term mortality. Heart Rhythm. 2021, 18, 199–206. [Google Scholar] [CrossRef]
- Tomii, D.; Okuno, T.; Praz, F.; Heg, D.; Wild, M.G.; Lanz, J.; Stortecky, S.; Reineke, D.; Windecker, S.; Pilgrim, T. Potential Candidates for Transcatheter Tricuspid Valve Intervention After Transcatheter Aortic Valve Replacement: Predictors and Prognosis. JACC Cardiovasc. Interv. 2021, 14, 2246–2256. [Google Scholar] [CrossRef]
- Lilly, S.M.; Deshmukh, A.J.; Epstein, A.E.; Ricciardi, M.J.; Shreenivas, S.; Velagapudi, P.; Wyman, J.F. 2020 ACC Expert Consensus Decision Pathway on Management of Conduction Disturbances in Patients Undergoing Transcatheter Aortic Valve Replacement: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Badertscher, P.; Knecht, S.; Zeljkovic, I.; Sticherling, C.; de Asmundis, C.; Conte, G.; Barra, S.; Jedrzej, K.; Kuhne, M.; Boveda, S. Management of conduction disorders after transcatheter aortic valve implantation: Results of the EHRA survey. EP Eur. 2022, 24, 1179–1185. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Sammour, Y.; Mangieri, A.; Kadri, A.; Karrthik, A.; Banerjee, K.; Kaur, M.; Giannini, F.; Pagliaro, B.; Ancona, M.; et al. The Utility of Rapid Atrial Pacing Immediately Post-TAVR to Predict the Need for Pacemaker Implantation. JACC Cardiovasc. Interv. 2020, 13, 1046–1054. [Google Scholar] [CrossRef]
- Tan, B.E.; Hashem, A.; Boppana, L.K.T.; Mohamed, M.S.; Abbas, S.F.; Faisaluddin, M.; Thakkar, S.; Ahmed, A.K.; Hall, C.; Abtahian, F.; et al. Utility of rapid atrial pacing before and after TAVR with balloon-expandable valve in predicting permanent pacemaker implantation. Catheter. Cardiovasc. Interv. 2023, 102, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Devraj, M.; Thomaides, A.; Steinvil, A.; Lipinski, M.J.; Buchanan, K.D.; Alraies, M.C.; Koifman, E.; Gai, J.; Torguson, R.; et al. Utility of Invasive Electrophysiology Studies in Patients With Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 121, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Schaer, B.; Reichlin, T.; Spies, F.; Madaffari, A.; Vischer, A.; Fahrni, G.; Jeger, R.; Kaiser, C.; Osswald, S.; et al. Electrophysiology Testing to Stratify Patients With Left Bundle Branch Block After Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2020, 9, e014446. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Ingallina, G.; Ancona, F.; Biondi, F.; Margonato, D.; Barki, M.; Tavernese, A.; Belli, M.; Stella, S. Evolution of interventional imaging in structural heart disease. Eur. Heart J. Suppl. 2023, 25, C189–C199. [Google Scholar] [CrossRef] [PubMed]

| Clinical Trial | Studied Valve Type | STS Score, (%) | Age, (Years) | 30-Day Pacemaker Rate, (%) |
|---|---|---|---|---|
| US CoreValve | CoreValve | 7.3 ± 3.0 | 83.2 ± 7.1 | 19.8 |
| SURTAVI | CoreValve, Evolut R | 4.4 ± 1.5 | 79.9 ± 6.2 | 25.9 |
| Evolut Low Risk | CoreValve, Evolut R/PRO | 1.9 ± 0.7 | 74.0 ± 5.9 | 17.4 |
| Notion | CoreValve | 2.9 ± 1.6 | 79.2 ± 4.9 | 34.1 |
| Scope I | ACURATE neo | 3.7 (2.5–4.9) | 82.6 ± 4.3 | 10 |
| Scope II | ACURATE neo | 4.6 (3.0) | 83.4 (4.2) | 11 |
| Partner | Sapien | 11.8 ± 3.3 | 83.6 ± 6.8 | 3.8 |
| Partner 2 | Sapien XT | 5.8 ± 2.1 | 81.5 ± 6.7 | 8.5 |
| Partner 3 | Sapien 3 | 1.9 ± 0.7 | 73.3 ± 5.8 | 6.6 |
| Portico IDE | Portico | 6.4 (3.4) | 83.0 (7.6) | 27.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halapas, A.; Koliastasis, L.; Doundoulakis, I.; Antoniou, C.-K.; Stefanadis, C.; Tsiachris, D. Transcatheter Aortic Valve Implantation and Conduction Disturbances: Focus on Clinical Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 469. https://doi.org/10.3390/jcdd10110469
Halapas A, Koliastasis L, Doundoulakis I, Antoniou C-K, Stefanadis C, Tsiachris D. Transcatheter Aortic Valve Implantation and Conduction Disturbances: Focus on Clinical Implications. Journal of Cardiovascular Development and Disease. 2023; 10(11):469. https://doi.org/10.3390/jcdd10110469
Chicago/Turabian StyleHalapas, Antonios, Leonidas Koliastasis, Ioannis Doundoulakis, Christos-Konstantinos Antoniou, Christodoulos Stefanadis, and Dimitrios Tsiachris. 2023. "Transcatheter Aortic Valve Implantation and Conduction Disturbances: Focus on Clinical Implications" Journal of Cardiovascular Development and Disease 10, no. 11: 469. https://doi.org/10.3390/jcdd10110469