Fluoroscopy Time as a New Predictor of Short-Term Outcomes after Transcatheter Aortic Valve Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural Characteristics
3.3. Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; Kappetein, A.P.; Wolner, E.; Nataf, P.; Thomas, M.; Schächinger, V.; De Bruyne, B.; Eltchaninoff, H.; Thielmann, M.; Himbert, D.; et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur. Heart J. 2011, 32, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Eltchaninoff, H.; Prat, A.; Gilard, M.; Leguerrier, A.; Blanchard, D.; Fournial, G.; Iung, B.; Donzeau-Gouge, P.; Tribouilloy, C.; Debrux, J.L.; et al. Transcatheter aortic valve implantation: Early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur. Heart J. 2011, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Schymik, G.; Walther, T.; Himbert, D.; Lefèvre, T.; Treede, H.; Eggebrecht, H.; Rubino, P.; Michev, I.; Lange, R.; et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010, 122, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Webb, J.G.; Cheung, A.; Ye, J.; Dumont, E.; Feindel, C.M.; Osten, M.; Natarajan, M.K.; Velianou, J.L.; Martucci, G.; et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: Acute and late outcomes of the multicenter Canadian experience. J. Am. Coll. Cardiol. 2010, 55, 1080–1090. [Google Scholar] [CrossRef]
- Petronio, A.S.; De Carlo, M.; Bedogni, F.; Marzocchi, A.; Klugmann, S.; Maisano, F.; Ramondo, A.; Ussia, G.P.; Ettori, F.; Poli, A.; et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ. Cardiovasc. Interv. 2010, 3, 359–366. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Himbert, D.; Descoutures, F.; Al-Attar, N.; Iung, B.; Ducrocq, G.; Détaint, D.; Brochet, E.; Messika-Zeitoun, D.; Francis, F.; Ibrahim, H.; et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 303–311. [Google Scholar] [CrossRef]
- Piazza, N.; Grube, E.; Gerckens, U.; den Heijer, P.; Linke, A.; Luha, O.; Ramondo, A.; Ussia, G.; Wenaweser, P.; Windecker, S.; et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: Results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention 2008, 4, 242–249. [Google Scholar] [CrossRef]
- Grube, E.; Buellesfeld, L.; Mueller, R.; Sauren, B.; Zickmann, B.; Nair, D.; Beucher, H.; Felderhoff, T.; Iversen, S.; Gerckens, U. Progress and current status of percutaneous aortic valve replacement: Results of three device generations of the CoreValve Revalving system. Circ. Cardiovasc. Interv. 2008, 1, 167–175. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- D’Errigo, P.; Barbanti, M.; Ranucci, M.; Onorati, F.; Covello, R.D.; Rosato, S.; Tamburino, C.; Santini, F.; Santoro, G.; Seccareccia, F. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis: Results from an intermediate risk propensity-matched population of the Italian OBSERVANT study. Int. J. Cardiol. 2013, 167, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: 1-Year Results from the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Chambers, C.E.; Fetterly, K.A.; Holzer, R.; Lin, P.J.; Blankenship, J.C.; Balter, S.; Laskey, W.K. Radiation safety program for the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 2011, 77, 546–556. [Google Scholar] [CrossRef]
- Little, M.P. Risks associated with ionizing radiation. Br. Med. Bull. 2003, 68, 259–275. [Google Scholar] [CrossRef]
- Sharma, D.; Ramsewak, A.; O’Conaire, S.; Manoharan, G.; Spence, M.S. Reducing radiation exposure during transcatheter aortic valve implantation (TAVI). Catheter. Cardiovasc. Interv. 2015, 85, 1256–1261. [Google Scholar] [CrossRef]
- Daneault, B.; Balter, S.; Kodali, S.K.; Williams, M.R.; Généreux, P.; Reiss, G.R.; Paradis, J.M.; Green, P.; Kirtane, A.J.; Smith, C.; et al. Patient radiation exposure during transcatheter aortic valve replacement procedures. EuroIntervention 2012, 8, 679–684. [Google Scholar] [CrossRef]
- Michel, J.M.; Hashorva, D.; Kretschmer, A.; Alvarez-Covarrubias, H.A.; Mayr, N.P.; Pellegrini, C.; Rheude, T.; Frangieh, A.H.; Giacoppo, D.; Kastrati, A.; et al. Evaluation of a Low-Dose Radiation Protocol during Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 139, 71–78. [Google Scholar] [CrossRef]
- Madder, R.D.; VanOosterhout, S.; Mulder, A.; Ten Brock, T.; Clarey, A.T.; Parker, J.L.; Jacoby, M.E. Patient Body Mass Index and Physician Radiation Dose during Coronary Angiography. Circ. Cardiovasc. Interv. 2019, 12, e006823. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Nikolsky, E.; Pucelikova, T.; Mehran, R.; Balter, S.; Kaufman, L.; Fahy, M.; Lansky, A.J.; Leon, M.B.; Moses, J.W.; Stone, G.W.; et al. An evaluation of fluoroscopy time and correlation with outcomes after percutaneous coronary intervention. J. Invasive Cardiol. 2007, 19, 208–213. [Google Scholar] [PubMed]
- Tajti, P.; Ayoub, M.; Nuehrenberg, T.; Ferenc, M.; Behnes, M.; Buettner, H.J.; Neumann, F.J.; Mashayekhi, K. Association of Prolonged Fluoroscopy Time with Procedural Success of Percutaneous Coronary Intervention for Stable Coronary Artery Disease with and without Chronic Total Occlusion. J. Clin. Med. 2021, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Sakakura, K.; Asada, S.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Seguchi, M.; Jinnouchi, H.; Wada, H.; Fujita, H. Clinical Factors Associated with Long Fluoroscopy Time in Percutaneous Coronary Interventions to the Culprit Lesion of Non-ST-Segment Elevation Myocardial Infarction. Int. Heart J. 2021, 62, 282–289. [Google Scholar] [CrossRef]
- Geisler, D.; Rudziński, P.N.; Hasan, W.; Andreas, M.; Hasimbegovic, E.; Adlbrecht, C.; Winkler, B.; Weiss, G.; Strouhal, A.; Delle-Karth, G.; et al. Identifying Patients without a Survival Benefit following Transfemoral and Transapical Transcatheter Aortic Valve Replacement. J. Clin. Med. 2021, 10, 4911. [Google Scholar] [CrossRef]
- Iacovelli, F.; Loizzi, F.; Cafaro, A.; Burattini, O.; Salemme, L.; Cioppa, A.; Rizzo, F.; Palmitessa, C.; D’Alessandro, M.; De Feo, D.; et al. Surgical Mortality Risk Scores in Transcatheter Aortic Valve Implantation: Is Their Early Predictive Value Still Strong? J. Cardiovasc. Dev. Dis. 2023, 10, 244. [Google Scholar] [CrossRef]
- Rudzinski, P.N.; Leipsic, J.A.; Schoepf, U.J.; Dudek, D.; Schwarz, F.; Andreas, M.; Zlahoda-Huzior, A.; Thilo, C.; Renker, M.; Burt, J.R.; et al. CT in Transcatheter-delivered Treatment of Valvular Heart Disease. Radiology 2022, 304, 4–17. [Google Scholar] [CrossRef]
- Palmerini, T.; Saia, F.; Kim, W.K.; Renker, M.; Iadanza, A.; Fineschi, M.; Bruno, A.G.; Ghetti, G.; Vanhaverbeke, M.; Søndergaard, L.; et al. Vascular Access in Patients with Peripheral Arterial Disease Undergoing TAVR: The Hostile Registry. JACC Cardiovasc. Interv. 2023, 16, 396–411. [Google Scholar] [CrossRef]

| Variable | All | Fluoroscopy Time | |||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | p | ||
| Patients characteristics | |||||
| Age (years) | 80.86 ± 5.71 | 80.72 ± 5.59 | 80.61 ± 6.13 | 81.17 ± 5.48 | 0.403 |
| Male | 785/1797 (43.68%) | 206/491 (41.95%) | 210/438 (47.94%) | 220/463 (47.52%) | 0.118 |
| Body mass index (kg/m2) | 27.34 ± 4.73 | 27.34 ± 4.77 | 27.20 ± 4.32 | 27.48 ± 5.07 | 0.802 |
| Hypertension | 1690/1785 (94.68%) | 453/486 (93.21%) | 409/437 (93.59%) | 433/457 (94.75%) | 0.597 |
| Diabetes mellitus | 577/1788 (32.27%) | 160/488 (32.79%) | 149/437 (34.10%) | 154/458 (33.62%) | 0.912 |
| Insulin | 238/1759 (13.53%) | 65/483 (13.46%) | 71/424 (16.74%) | 72/447 (16.11%) | 0.339 |
| Dyslipidemia | 1170/1786 (65.51%) | 320/487 (65.71%) | 296/437 (67.73%) | 304/457 (66.52%) | 0.807 |
| Smoking | 122/1744 (6.99%) | 40/483 (8.28%) | 23/421 (5.46%) | 28/436 (6.42%) | 0.227 |
| Anemia | 977/1782 (54.83%) | 254/490 (51.84%) | 239/435 (54.94%) | 267/459 (58.17%) | 0.147 |
| COPD | 452/1786 (25.31%) | 122/487 (25.01%) | 111/437 (25.40%) | 138/458 (30.13%) | 0.151 |
| Neurological dysfunction | 146/1759 (8.30%) | 36/483 (7.45%) | 35/427 (8.20%) | 35/451 (7.76%) | 0.916 |
| Severe liver disease | 41/1783 (2.30%) | 13/488 (%) | 10/435 (%) | 10/457 (%) | 0.882 |
| PAD | 421/1758 (23.95%) | 128/483 (26.50%) | 90/428 (21.03%) | 109/451 (24.17%) | 0.155 |
| Carotid stenosis ≥ 50% | 45/1354 (3.23%) | 6/297 (%) | 10/338 (%) | 15/345 (%) | 0.235 |
| Critical preoperative state | 66/1779 (3.71%) | 18/485 (3.71%) | 23/435 (5.29%) | 18/456 (3.95%) | 0.454 |
| CAD history | 448/1784 (25.11%) | 125/488 (25.61%) | 109/436 (25.00%) | 130/456 (28.51%) | 0.441 |
| Prior myocardial infarction | 255/1786 (14.28%) | 76/488 (15.57%) | 64/436 (14.68%) | 86/457 (18.82%) | 0.208 |
| Prior cardiac surgery | 253/1787 (14.16%) | 68/489 (13.91%) | 68/437 (15.56%) | 72/458 (15.72%) | 0.687 |
| Prior myocardial revascularization | 406/1791 (22.68%) | 119/489 (24.33%) | 95/437 (21.74%) | 118/459 (25.71%) | 0.370 |
| PCI | 248/1791 (13.85%) | 76/489 (15.54%) | 48/437 (10.98%) | 74/459 (16.12%) | 0.056 |
| CABG | 92/1791 (5.14%) | 28/489 (5.73%) | 27/437 (6.18%) | 26/459 (5.66%) | 0.938 |
| PCI + CABG | 66/1791 (3.69%) | 15/489 (3.07%) | 20/437 (4.58%) | 18/459 (3.92%) | 0.486 |
| Myocardial revascularization close to TAVR | 271/1791 (15.13%) | 67/486 (13.79%) | 54/437 (12.36%) | 65/463 (14.04%) | 0.728 |
| PCI | 266/1791 (14.85%) | 66/486 (13.58%) | 53/437 (12.13%) | 62/463 (13.39%) | 0.781 |
| CABG | 4/1791 (0.22%) | 1/486 (0.21%) | 1/437 (0.23%) | 2/463 (0.43%) | 0.778 |
| PCI + CABG | 1/1791 (0.05%) | 0/486 (0.00%) | 0/437 (0.00%) | 1/463 (0.22%) | 0.369 |
| Residual significant CAD during TAVR | 214/1782 (12.01%) | 60/483 (12.42%) | 48/437 (12.34%) | 49/459 (10.67%) | 0.666 |
| Prior PM/ICD/CRT implantation | 222/1772 (12.53%) | 57/485 (11.75%) | 49/433 (11.32%) | 62/454 (13.66%) | 0.522 |
| NYHA functional class III-IV | 1475/1786 (82.59%) | 402/487 (82.55%) | 355/437 (81.24%) | 356/457 (77.90%) | 0.181 |
| CKD | 743/1797 (41.35%) | 212/491 (43.18%) | 178/438 (40.64%) | 200/463 (43.20%) | 0.671 |
| Electrocardiography | |||||
| Sinus rhythm | 1462/1788 (81.77%) | 390/488 (79.92%) | 354/437 (81.01%) | 383/458 (83.62%) | 0.325 |
| Atrial fibrillation/flutter | 326/1788 (18.23%) | 98/488 (20.08%) | 83/437 (18.99%) | 75/458 (16.38%) | 0.325 |
| PM-induced rhythm | 94/1788 (5.26%) | 31/488 (6.35%) | 24/437 (5.49%) | 24/458 (5.24%) | 0.741 |
| Echocardiography | |||||
| LVEF (%) | 53.345 ± 10.21 | 54.22 ± 10.81 | 52.88 ± 10.47 | 52.33 ± 9.77 | <0.001 |
| Maximum aortic gradient (mmHg) | 75.67 ± 21.22 | 73.43 ± 20.45 | 77.38 ± 20.00 | 76.33 ± 22.69 | 0.032 |
| Mean aortic gradient (mmHg) | 46.40 ± 14.33 | 45.27 ± 14.29 | 47.21 ± 13.23 | 46.92 ± 14.93 | 0.074 |
| Moderate-to-severe mitral regurgitation | 679/1678 (40.46%) | 175/455 (38.46%) | 179/407 (43.98%) | 194/429 (45.22%) | 0.095 |
| Pulmonary arterial systolic pressure (mmHg) | 40.22 ± 13.37 | 40.23 ± 12.47 | 39.72 ± 12.84 | 40.07 ± 12.83 | 0.834 |
| Mortality risk scores | |||||
| Logistic EuroSCORE | 16.14 ± 12.31 | 16.04 ± 12.76 | 15.94 ± 12.18 | 17.49 ± 13.55 | 0.068 |
| EuroSCORE II | 5.79 ± 12.75 | 5.61 ± 5.84 | 5.26 ± 5.35 | 6.04 ± 6.79 | 0.283 |
| STS-PROM | 4.60 ± 3.55 | 4.70 ± 3.60 | 4.47 ± 3.45 | 4.96 ± 4.32 | 0.077 |
| STS-PROM ≥ 8 | 176/1779 (9.90%) | 45/486 (9.26%) | 44/435 (10.11%) | 60/453 (13.24%) | 0.122 |
| Variable | All | Fluoroscopy Time | |||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | p | ||
| Procedural details | |||||
| Transfemoral access route | 1703/1797 (94.77%) | 444/491 (90.43%) | 416/438 (94.98%) | 440/463 (95.03%) | 0.005 |
| Other access routes | 94/1797 (5.23%) | 47/491 (9.57%) | 22/438 (5.02%) | 23/463 (4.97%) | 0.005 |
| Trans-subclavian | 27/1797 (1.57%) | 9/491(1.83%) | 6/438 (1.37%) | 12/463 (2.59%) | 0.404 |
| Transapical | 57/1797 (3.17%) | 36/491 (7.33%) | 10/438 (2.28%) | 9/463 (1.94%) | <0.001 |
| Direct aortic | 8/1797 (0.44%) | 1/491(0.20%) | 6/438 (1.37%) | 1/463 (0.22%) | 0.029 |
| Orotracheal intubation | 233/1796 (12.97%) | 73/491 (14.87%) | 66/437 (15.10%) | 90/463 19.44%) | 0.107 |
| Valve-in-valve | 73/1794 (4.07%) | 16/491 (3.26%) | 20/437 (4.58%) | 27/461 (5.86%) | 0.157 |
| Predilatation | 827/1784 (46.36%) | 186/489 (38.04%) | 248/436 (56.88%) | 282/456 (61.84%) | <0.001 |
| Valve kind | |||||
| Balloon-expandable | 551/1797 (30.66%) | 190/491 (38.70%) | 153/438 (34.93%) | 130/463 (28.08%) | 0.002 |
| Self-expanding | 1124/1797 (62.55%) | 266/491 (54.17%) | 248/438 (56.62%) | 294/463 (63.50%) | 0.011 |
| Others | 122/1797 (6.79%) | 35/491 (7.13%) | 37/438 (8.45%) | 39/463 (8.42%) | 0.691 |
| Valve Size > 26 mm | 722/1793 (40.27%) | 175/489 (35.79%) | 166/438 (37.90%) | 200/461 (43.38%) | 0.048 |
| Postdilatation | 479/1795 (26.68%) | 97/491 (19.76%) | 112/436 (25.69%) | 145/463 (31.32%) | <0.001 |
| CM volume (mL) | 149.97 ± 76.36 | 130.431 ± 54.03 | 161.14 ± 67.22 | 197.61 ± 96.94 | <0.001 |
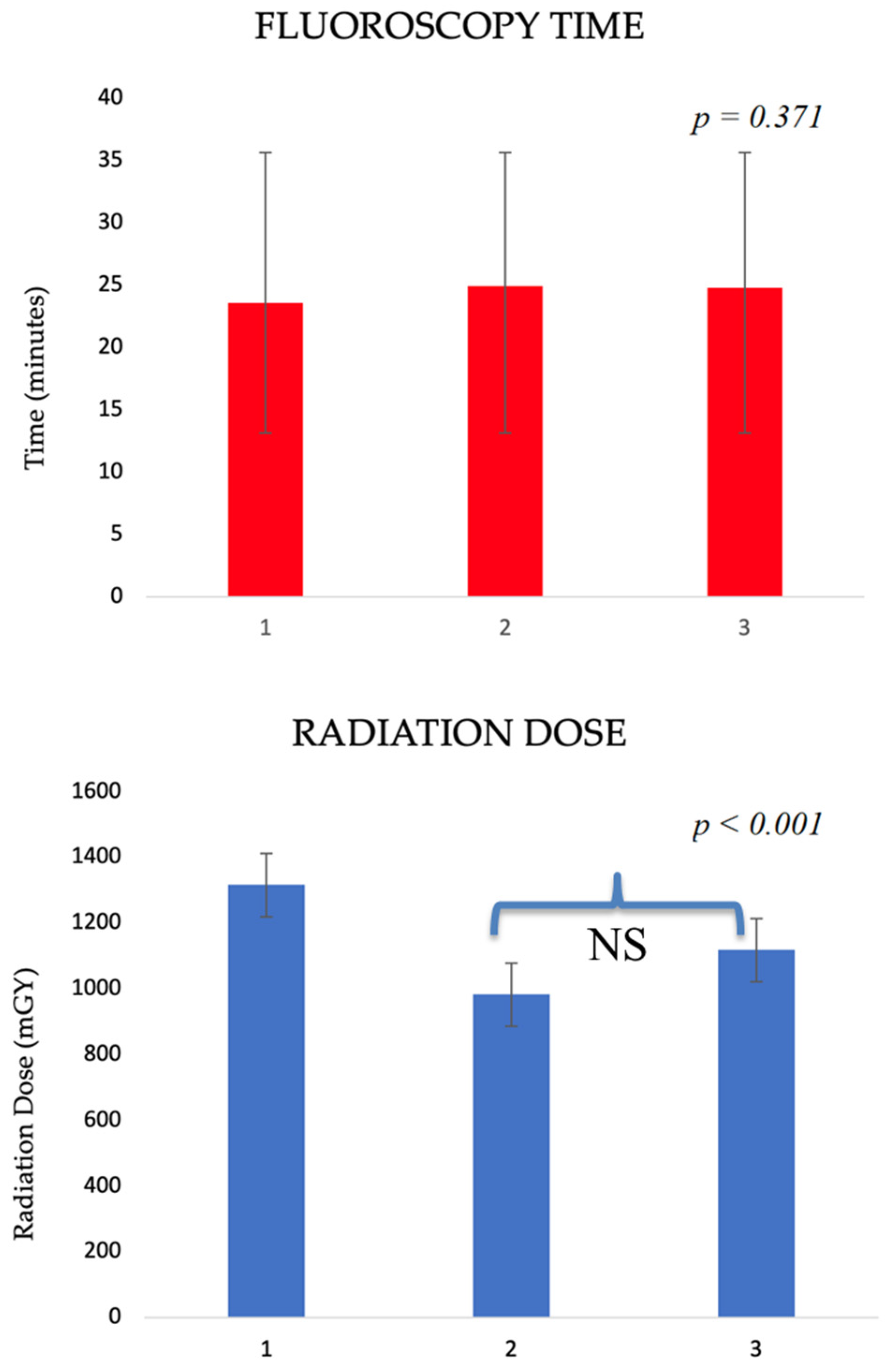
| Radiation dose (mGy) | 1366.18 ± 1241.57 | 1070.68 ± 1051.40 | 1381.24 ± 1070.11 | 2112.15 ± 1748.60 | <0.001 |
| Complications and outcomes (VARC-3) | |||||
| AKI | 272/1714 (15.87%) | 70/472 (14.83%) | 50/420 (11.90%) | 72/433 (16.63%) | 0.142 |
| CVVH | 41/1730 (2.37%) | 10/476 (2.10%) | 8/420 (1.90%) | 15/438 (3.42%) | 0.288 |
| Chronic hemodialysis | 9/1667 (0.54%) | 3/460 (0.65%) | 2/409 (0.49%) | 4/423 (0.95%) | 0.724 |
| Bleeding (VARC-3) | 588/1399 (48.03%) | 124/343 (36.15%) | 139/313 (44.41%) | 214/362 (59.12%) | <0.001 |
| Type 1 | 192/ (13.72%) | 34/343 (9.91%) | 55/313 (17.57%) | 72/362 (19.89%) | <0.001 |
| Type 2 | 307/1399 (21.94%) | 67/343 (19.53%) | 67/313 (21.41%) | 102/362 (28.18%) | 0.017 |
| Type 3–5 | 89/1399 (6.36%) | 23/343 (6.71%) | 17/313 (5.43%) | 40/362 (11.05%) | 0.016 |
| BARC ≥ 3 | 561/1773 (32.37%) | 124/477 (26.00%) | 117/424 (27.59%) | 208/449 (46.32%) | <0.001 |
| Need of transfusion | 298/1721 (17.31%) | 61/475 (12.84%) | 65/419 (15.51%) | 108/442 (24.43%) | <0.001 |
| 1 unit | 140/1721 (8.13%) | 30/475 (6.32%) | 31/419 (7.40%) | 52/442 (11.76%) | 0.008 |
| 2 units | 106/1721 (6.16%) | 23/475 (4.84%) | 23/419 (5.49%) | 38/442 (8.60%) | 0.046 |
| >2 units | 52/1721 (3.02%) | 8/475 (1.68%) | 11/419 (2.62%) | 18/442 (4.07%) | 0.086 |
| Vascular complications | 286/1765 (16.20%) | 59/484 (12.19%) | 57/436 (13.07%) | 118/455 (25.93%) | <0.001 |
| minor | 170/1765 (9.63%) | 41/484 (8.47%) | 34/436 (7.80%) | 65/455 (14.29%) | 0.002 |
| major | 116/1765 (6.57%) | 18/484 (3.72%) | 23/436 (5.27%) | 53/455 (11.65%) | <0.001 |
| Access-site related vascular complications | 224/339 (66.08%) | 22/69 (31.88%) | 43/86 (50.00%) | 47/137 (34.31%) | 0.029 |
| PCD failure | 101/1556 (6.49%) | 20/404 (4.72%) | 22/373 (5.90%) | 40/387 (10.34%) | 0.005 |
| At least moderate residual aortic Regurgitation | 129/1562 (8.26%) | 26/412 (6.31%) | 30/377 (7.96%) | 38/398 (9.55%) | 0.223 |
| Permanent PM implantation | 226/1572 (14.38%) | 51/419 (12.17%) | 64/484 (16.67%) | 60/381 (15.75%) | 0.163 |
| ECM/cardiac arrest | 66/1713 (3.85%) | 15/473 (3.17%) | 7/425 (1.65%) | 34/440 (7.73%) | <0.001 |
| New-onset atrial fibrillation/flutter | 124/1412 (8.78%) | 33/375 (8.80%) | 25/343 (7.29%) | 33/376 (8.78%) | 0.707 |
| Acute myocardial infarction | 19/1774 (1.07%) | 2/486 (0.41%) | 3/437 (0.69%) | 11/454 (2.42%) | 0.009 |
| Stroke/TIA | 34/1773 (1.92%) | 6/487 (1.23%) | 8/437 (1.83%) | 7/453 (1.54%) | 0.759 |
| Hospital length of stay (days) | 5.73 ± 9.63 | 4.99 ± 3.52 | 5.53 ± 3.93 | 6.34 ± 4.46 | <0.001 |
| Hospital length of stay > 5 days | 627/1722 (36.41%) | 139/472 (29.45%) | 160/424 (37.74%) | 194/431 (45.01%) | <0.001 |
| Technical success | 1612/1752 (92.01%) | 456/480 (95.00%) | 406/431 (94.20%) | 382/453 (84.33%) | <0.001 |
| Device success | 1562/1764 (88.55%) | 442/485 (91.13%) | 383/431 (88.86%) | 385/451 (85.37%) | 0.021 |
| Periprocedural mortality | 43/1734 (2.48%) | 10/475 (2.10%) | 10/423 (2.36%) | 19/452 (4.21%) | 0.118 |
| Mortality at one year (F-U) | 59/1686 (3.50%) | 19/465 (4.09%) | 10/413 (2.42%) | 20/432 (4.63%) | 0.212 |
| Early safety absence (VARC-2) | 222/1732 (12.82%) | 42/477 (8.80%) | 43/426 (10.09%) | 87/449 (19.38%) | <0.001 |
| Early safety absence (VARC-3) | 559/1732 (32.27%) | 141/477 (29.56%) | 115/426 (26.99%) | 161/449 (35.86%) | 0.013 |
| Postprocedural complications (VARC-2 and VARC-3) | 427/839 (50.89%) | 91/203 (44.83%) | 100/188 (53.19%) | 146/250 (58.40%) | 0.016 |
| Complication time delay (days) from TAVR | 3.44 ± 39.39 | 3.13 ± 18.55 | 6.99 ± 19.80 | 2.55 ± 11.97 | 0.049 |
| Variable | Technical Success | p | T-Statistic | |
| Yes | No | |||
| Fluoroscopy time min (unmatched) | 23.82 ± 2.32 | 37.99 ± 2.32 | <0.001 | 2.58 |
| Fluoroscopy time min (matched after PSM) | 22.01 ± 4.23 | 37.99 ± 4.23 | 0.001 | 1.90 |
| Device Success (VARC-3) | p | T-statistic | ||
| Yes | No | |||
| Fluoroscopy time min (unmatched) | 23.70 ± 1.93 | 31.95 ± 1.93 | <0.001 | 4.27 |
| Fluoroscopy time min (matched after PSM) | 22.83 ± 2.76 | 32.23 ± 2.76 | 0.007 | 3.41 |
| Early Safety (VARC-2) | p | T-statistic | ||
| Yes | No | |||
| Fluoroscopy time min (unmatched) | 23.72 ± 3.05 | 33.72 ± 2.05 | <0.001 | 4.87 |
| Fluoroscopy time min (matched after PSM) | 22.11 ± 3.10 | 33.72 ± 3.10 | 0.046 | 3.74 |
| Early Safety (VARC-3) | p | T-statistic | ||
| Yes | No | |||
| Fluoroscopy Time min (unmatched) | 23.58 ± 1.80 | 28.23 ± 1.80 | <0.001 | 2.58 |
| Fluoroscopy Time min (matched after PSM) | 24.09 ± 3.17 | 28.23 ± 3.17 | 0.035 | 1.90 |
| AUC ± DeLong Standard Error | 95% CI | Asymptotic Significance | Cut-Off | Youden Index | Sensitivity (%) | Specificity (%) | Accuracy (%) | LR−/LR+ | Adjusted R-Square | Slope | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No technical success | 0.680 ± 0.028 | 0.654–0.704 | <0.001 | 27.8 ± 0.04 | 0.305 | 54.17 | 76.21 | 74.27% | 0.60–2.27 | 0.046 | 0.886 |
| No device success (VARC-2) | 0.590 ± 0.024 | 0.564–0.616 | <0.001 | 22 ± 0.04 | 0.158 | 58.60 | 56.28 | 56.55% | 0.73–1.34 | 0.009 | 0.866 |
| No device success (VARC-3) | 0.608 ± 0.021 | 0.581–0.633 | <0.001 | 22 ± 0.03 | 0.195 | 60.56 | 57.89 | 58.40% | 0.78–1.44 | 0.026 | 0.948 |
| No early safety (VARC-2) | 0.628 ± 0.024 | 0.601–0.654 | <0.001 | 30.1 ± 0.03 | 0.229 | 41.28 | 81.53 | 76.41% | 0.72–2.23 | 0.032 | 0.929 |
| No early safety (VARC-3) | 0.545 ± 0.017 | 0.518–0.571 | 0.008 | 30.00 ± 0.02 | 0.108 | 29.50 | 80.86 | 65.01% | 0.87–1.54 | 0.011 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafaro, A.; Spione, F.; Burattini, O.; De Feo, D.; Xhelo, A.; Palmitessa, C.; D’Alessandro, M.; Amendola, V.P.; Rimmaudo, F.; Guaricci, A.I.; et al. Fluoroscopy Time as a New Predictor of Short-Term Outcomes after Transcatheter Aortic Valve Replacement. J. Cardiovasc. Dev. Dis. 2023, 10, 459. https://doi.org/10.3390/jcdd10110459
Cafaro A, Spione F, Burattini O, De Feo D, Xhelo A, Palmitessa C, D’Alessandro M, Amendola VP, Rimmaudo F, Guaricci AI, et al. Fluoroscopy Time as a New Predictor of Short-Term Outcomes after Transcatheter Aortic Valve Replacement. Journal of Cardiovascular Development and Disease. 2023; 10(11):459. https://doi.org/10.3390/jcdd10110459
Chicago/Turabian StyleCafaro, Alessandro, Francesco Spione, Osvaldo Burattini, Daniele De Feo, Alessandro Xhelo, Chiara Palmitessa, Maurizio D’Alessandro, Vincenzo Pio Amendola, Flavio Rimmaudo, Andrea Igoren Guaricci, and et al. 2023. "Fluoroscopy Time as a New Predictor of Short-Term Outcomes after Transcatheter Aortic Valve Replacement" Journal of Cardiovascular Development and Disease 10, no. 11: 459. https://doi.org/10.3390/jcdd10110459





